Abstract
Background
Patients with subocclusive Crohn’s disease (CD) who received azathioprine (AZA) therapy had lower re-hospitalization rates due to all causes and for surgical management of CD compared to those treated with mesalazine during a 3-year period. We investigated whether AZA also was effective for prevention of recurrent bowel obstruction.
Material/Methods
Rates of recurrent bowel occlusion were compared between patients treated with AZA and those treated with mesalazine. We assessed the time interval-off intestinal obstruction as well as the occlusion-free survival for both groups.
Results
There was a significantly lower cumulative rate of patients with recurrent subocclusion in the AZA group (56%) compared with the mesalazine group (79%; OR 3.34, 95% CI 1.67–8.6; P=0.003), with the number needed to treat in order to prevent 1 subocclusion episode of 3.7 favoring AZA. The occlusion-free time interval was longer in the AZA group compared with the mesalazine group (28.8 vs. 18.3 months; P=0.000). The occlusion-free survival at 12, 24, and 36 months was significantly higher in the AZA group (91%, 81%, and 72%, respectively) than in the mesalazine group (64.7%, 35.3%, and 23.5%, respectively; P<0.05 for all comparisons).
Conclusions
In an exploratory analysis of patients with subocclusive ileocecal CD, maintenance therapy with AZA is more effective than mesalazine for eliminating or postponing recurrent intestinal obstruction during 3 years of therapy.
MeSH Keywords: Azathioprine, Crohn Disease, Intestinal Obstruction, Mesalamine
Background
Crohn’s disease (CD) encompasses a broad array of clinical scenarios. The spectrum is diverse because of different phenotypes that can cause distinctive symptoms, and because CD can occur virtually anywhere in the gastrointestinal tract. Additionally, CD varies greatly in its severity – it may be severe or mild, and can be clinically disabling or altogether tolerable.
The occurrence of strictures as a complication of Crohn’s disease is a significant clinical problem [1]. Despite many recent advances in the management of patients with CD, occlusive or subocclusive symptoms are observed mainly on the fibrostenosing phenotype, particularly in the presence of clinically significant strictures, and they remain a challenging clinical problem. Although it is clear that surgical intervention is indicated for an obstructing ileocecal stricture that fails to respond to medical therapy, the optimal clinical approach for CD-associated intestinal subocclusion remains controversial [2].
Retrospective studies show that the impact of stricturing CD in referral centers is significant [3,4]. Whereas the majority of patients (80%) present with inflammatory, non-stricturing and non-penetrating disease at diagnosis, it is striking that almost 30% of individuals develop a stricturing behavior within 10 years, especially those with isolated small bowel disease [3]. The natural history of ileocecal CD after the first episode of intestinal subocclusion resolved without surgery has not been well established, but increasing evidence suggests a stepwise progression towards irreversible intestinal occlusion, ultimately requiring surgical resection [5,6]. Although surgery is necessary in the most of these patients with CD [7], symptomatic strictures tend to return after surgically-induced remission, and this frequently leads to repeated bowel resections and eventually to short bowel syndrome and consequent intestinal failure in later life [6].
Based on these concerns, medical therapy that potentially controls intestinal inflammation and perhaps postpones or prevents the outcome of fixed intestinal obstruction is a logical approach. Effective medical management is challenging in the prevention of recurrent bowel obstruction, including thiopurines therapy, a subject of debate and little-evaluated. Thiopurines have been used for many auto-immune and inflammatory diseases with successful rates of disease control and prevention of complications [8–10]. Some data obtained from a case series [11] and a retrospective cohort study [12] suggest that some patients with stricturing CD, including those presenting with small-bowel obstruction relieved without surgery, may experience a partial or complete response to medical therapy in the short- and medium-term. However, no controlled studies evaluating medical therapies in this clinical setting have been published.
Previously, we found that patients with subocclusive CD who received azathioprine (AZA) therapy had lower re-hospitalization rates due to all causes and for surgical management of CD compared to those treated with mesalazine during a 3-year period [12]. Here, we evaluated the effectiveness of AZA when compared with mesalazine in preventing/delaying recurrent intestinal obstruction in patients with distal ileum and/or right colon CD who had successful conservative treatment for the first intestinal subocclusion episode.
Material and Methods
Study design
Data for this post hoc analysis were drawn from a 3-year, randomized, blind-investigator, controlled, 2-center maintenance trial of AZA or mesalazine in CD limited to the distal ileum and/or right colon, presenting with first episode of small-bowel subocclusion that relented without surgery within 72 h of the onset of symptoms.
In calculating the sample size, we assumed an approximately 70% cumulative probability of recurrent small bowel subocclusion over a 3-year period for patients with distal ileum and/or right colon CD presenting the initial episode of small-bowel obstruction that relented without surgery [12]. To demonstrate a decrease in recurrent obstruction rate from 70% to 35% in those individuals taking AZA with 90% power and a 5% significance level (2-sided) based on a 2-group χ2 test (continuity corrected), 36 patients per treatment group would have to be evaluated. This calculation is based on an intention-to-treat population.
Briefly, eligible patients were 18–65 years old with a confirmed diagnosis of CD restricted to the ileocecal region, and first episode of intestinal subocclusion relented without surgery. Small bowel evaluation through examination or computed tomography enterography and ileocolonoscopy were performed in all patients within the first 2 weeks following the resolution of the occlusive episode, ensuring that disease was confined to the distal ileum and/or right colon. Subjects had to be treatment-naïve to immunosuppressant and anti-TNF. Prior 5-aminosalicylate therapy was permitted if the dosage had been stable for 4 weeks before entry into the trial.
Patients presented with the following criteria were excluded: under 18 or over 65 years of age; presented intestinal obstruction refractory to medical treatment in the first 72 h; needed urgent surgery for CD-related complications, multiple intestinal stenosis, internal fistulas, systemic infections, evidence of intra-abdominal abscess, previous intolerance of or contraindications to the use of AZA or MSZ, or use of corticosteroids within the 4 weeks prior to study entry; history of steroid-dependent disease; and previous use of anti-TNFα therapy, thalidomide, or immunosuppressants. Patients were also excluded if they had previous or current history of malignancies, surgeries in the abdomen and/or pelvis, severe infections in the last 3 months, alcohol use (daily alcohol consumption above 40 g), drug addiction, or disabling chronic organ failure. Pregnant women, nursing mothers, and women who wanted to become pregnant during the study were not selected. Women with childbearing potential were subjected to pregnancy tests and instructed to use contraception during the study.
At hospital admission, all patients were medically managed in a similar manner, including: supportive or resuscitative therapy with fluid and electrolytes, nil per oral, nasogastric tube insertion, and intravenous hydrocortisone (100 mg t.i.d.) during a maximal period of 72 h. Patients refractory to medical treatment over this time interval underwent surgical management and were not included, and those responders who satisfied inclusion criteria were scheduled for randomization. Patients who were successfully treated conservatively were instructed to consume a low-fiber diet and were transitioned to an equivalent oral regimen of steroids and discharged. Thus, after resolution of the index episode of intestinal obstruction, all individuals were treated with prednisone 40 mg per day orally for 2 weeks. Then, the dose was gradually tapered until withdrawal, by 5 mg every week, over a period of 8 weeks. All patients were randomized to receive AZA (2–3 mg/kg per day) or mesalazine (3.2 g per day; Chron Asa 5; EMS Laboratories, Brazil) as soon as they were able to resume oral feeding and continued the maintenance treatment for 36 months or until study withdrawal. Patients visited the Outpatient Clinic at intervals of 1–3 months throughout the study extension and whenever they had a clinical complaint. If emergency room visits or hospitalizations occurred at an outside facility, medical records were obtained to document the presence/absence of bowel occlusion.
Concomitant use of the following drugs was not allowed during the trial: systemic corticosteroids (with the exception during the medical treatment of bowel subocclusion), antibiotics (for an extended period of more than 14 days), nonsteroidal anti-inflammatory drugs (for a cumulative duration of more than 7 days), anti-tumor necrosis factor α agents, thalidomide, or other immunosuppressive drugs. Institutional Ethics Committee approval was obtained from the 2 study centers, and written informed consent was obtained from all patients prior to enrollment.
Patient sample and clinical assessments
Randomized patients who were receiving AZA or mesalazine during the trial were included in this analysis. We assessed the proportion of patients with recurrent bowel occlusion at the end of 12, 24, and 36 months after commencement of AZA or mesalazine, as well as the time interval-off intestinal obstruction during follow-up. This analysis was based on patients who received at least 1 dose of the study drug (ie, intention-to-treat population).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS, Chicago, IL, USA). Continuous variables are presented as medians and ranges, and categorical variables are expressed as percentages. Statistical comparisons for the differences in recurrent subocclusion rates between the AZA and mesalazine-treated patients were based on the chi-squared test. The duration of intestinal obstruction in the 2 treatment arms was compared using the t-test. Cumulative subocclusion-free survival rates were compared between groups by life-table analysis according to the method of Kaplan-Meier and 95% confidence intervals (CI). The differences in curves were tested using the log-rank test. For comparison, the level of statistical significance was set at P<0.05 and all reported P values are 2-tailed.
Results
Patient characteristics and disposition
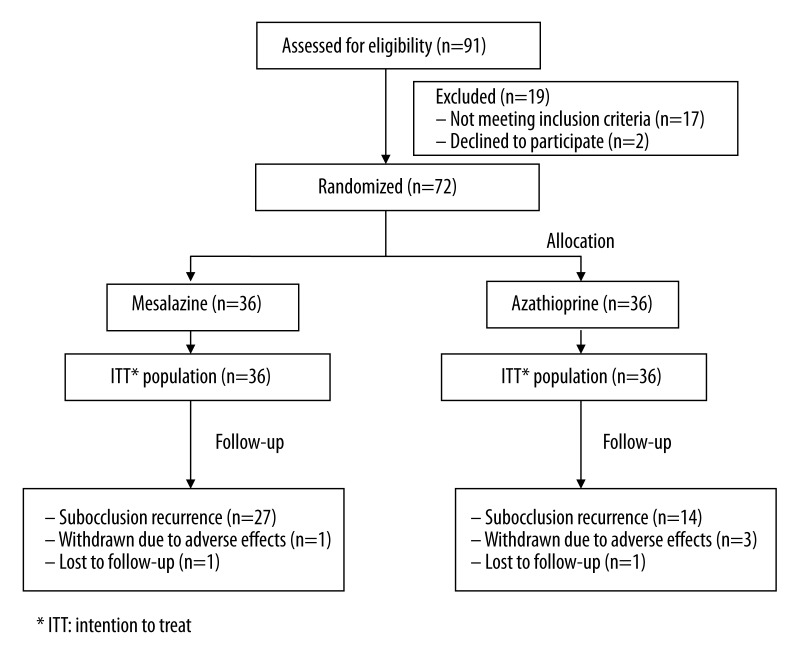
Demographics for the overall trial patient population are shown in Table 1. In summary, of 91 CD patients screened for the study, 19 individuals (21%) were not enrolled because they presented some of the exclusion criteria (n=17) or refused to participate after reading the informed consent (n=2). Thus, 72 patients (79%) of the eligible population (35 males, 37 females, mean age 37±12.5 years (range 19–61) were randomized and analyzed, 36 to AZA and 36 to mesalazine (Figure 1).
Table 1.
Baseline demographics, smoking status, and laboratorial data in patients with subocclusive ileocecal Crohn’s disease according to therapy with azathioprine or mesalazine.
| Characteristics | Azathioprine group (n=36) | Mesalazine group (n = 36) | P value |
|---|---|---|---|
| Gender (F/M) (n) | 18/18 | 19/17 | 0.81 |
| Age (yr)* | 36±12 | 38±12.6 | 0.49 |
| Age <40 yr (n) | 25 | 19 | 0.14 |
| Smokers (n) | 5 | 4 | 0.87 |
| Disease duration (yr)* | 5.8±2.9 | 5.9±2.7 | 0.91 |
| C-reactive protein (mg/L)* | 20±17.9 | 18.6±17.3 | 0.74 |
| ESR** (mm/h)* | 10.4±4.5 | 10.1±4 | 0.73 |
| Platelet count (mm3)* | 308,732±69,070 | 301,583±71,334 | 0.67 |
mean ±SD;
ESR – erythrocyte sedimentation rate.
Figure 1.
Flow chart illustrating the progress of Crohn’s patients throughout the trial (36 months).
Intestinal occlusion relapse
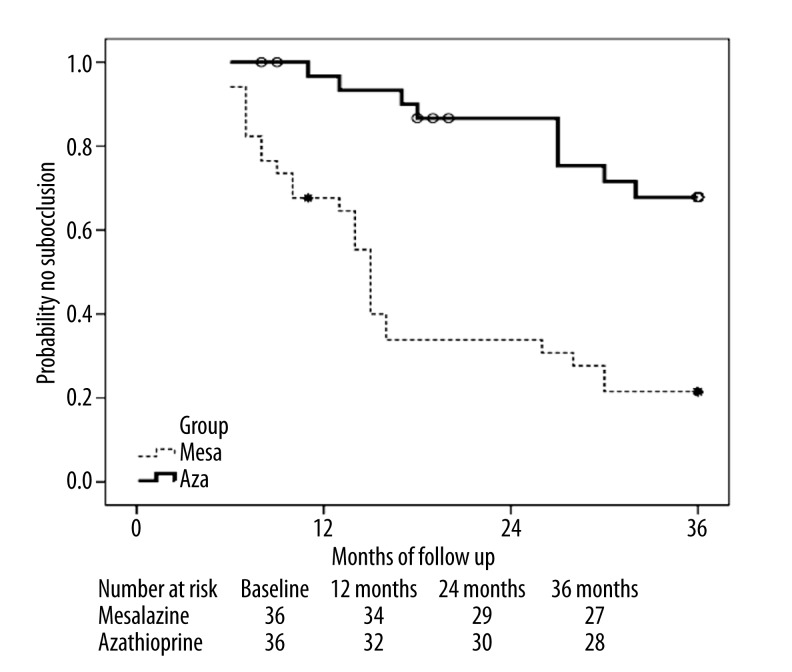
Overall, during 3 years of follow-up, there was a significant decrease in the rate of subocclusion in the AZA group (OR=3.34, 95% CI 1.67–8.6) compared with mesalazine (43.8% vs. 79.4%, respectively, P=0.003), with the number needed to treat in order to prevent 1 subocclusion episode of 3.7, favoring AZA. The intestinal obstruction-free time interval in AZA patients was significantly higher than in those on mesalazine (28.8±11.4 vs. 18.3±9.7 months; P=0.000). As shown in Figure 2, the occlusion-free survival at 12, 24, and 36 months was significantly higher in the AZA group (91%, 81%, and 72%, respectively) than in the mesalazine arm (64.7%, 35.3%, and 23.5%, respectively; P<0.05 for all comparisons).
Figure 2.
Kaplan-Meier plot showing probability of subocclusion-free survival in Crohn’s disease patients related to time after randomization to azathioprine or mesalazine therapy. AZA patients showed significantly higher occlusion-free survival at 12, 24, and 36 months (log rank at 12 months: 0.01; at 24 months: 0.000; at 36 months: 0.001).
Discussion
In this post hoc analysis that evaluated the use of AZA or mesalazine in patients who have undergone successful medical treatment for subocclusive ileocecal CD, AZA was more effective than mesalazine for eliminating or postponing recurrent intestinal obstruction during a 3-year follow-up period. It must be emphasized that in the original trial, all CD patients had undergone an initial concomitant course of corticosteroids to treat the possible inflammatory component of the stricture. This initial induction strategy with steroids may be important, because AZA has a significantly delayed onset of action, with several studies demonstrating clinical efficacy after 2–3 months of treatment [13,14].
The current study provides good-quality evidence for recommending subsequent treatment with AZA for patients with terminal ileum or ileocecal CD with a clinically resolved initial episode of intestinal subocclusion. AZA therapy, compared with mesalazine, reduced recurrent obstruction rate throughout 3 years of therapy. This finding is thought-provoking and suggests that AZA therapy initiated immediately after the initial subocclusion episode has been dealt with by clinical management may be a preferred approach and potentially slows disease progression and to some extent alters the natural history of the terminal ileum or ileocecal subocclusive CD in the medium-term [15]. Arguably, the initiation of AZA treatment might lead to both more effective mucosal immunomodulation and enhanced control of ongoing inflammation, thus allowing the inflammatory lesions to heal before the establishment of irreversible fibrotic wall-thickening in a certain proportion of individuals [16]. Accumulating evidence suggests a potential benefit of thiopurines to help change the course of CD over time. Indeed, several recent studies have shown that thiopurines may change the natural course of CD by decreasing the need for first surgery [15,17,18] and surgical re-resections [19]. Similarly, a recent single-center retrospective study focusing on the long-term follow-up evaluation of AZA responders in CD showed that AZA responders required significantly fewer surgical procedures than controls (adjusted OR, 0.69; 95% CI, 0.52–0.91) [15]. In addition, in a recent systematic review, thiopurines therapy was associated with a 40% lower risk of first surgical resection in patients with CD [20]. It is interesting that prolonged therapy with thiopurines was found to reduce the likelihood of surgery whereby more than 12 months of this therapy reduces the risk of first intestinal surgery by 2-fold, although early initiation of therapy with thiopurines offered no apparent additional benefit [21]. Hence, it is possible that long-term therapy with thiopurines may be needed to change the natural course of CD.
However, therapy with AZA that is initiated immediately after subocclusion resolution does not, unfortunately, guarantee success and prevention of obstruction recurrence for all patients. Thus, it is clear that recurrent intestinal obstruction remains a major problem in an important subset of patients with fibrostenosing ileocecal CD, irrespective of the suggested improvement in medium-term outcome during AZA therapy. Indeed, from 32 individuals on AZA (excluding the analysis 1 patient lost to follow-up and 3 due to adverse effects), 14 (44%) of the patients experienced at least 1 recurrent subocclusion episode during the trial period. For most of these patients presenting probable irreversible fibrotic strictures, use AZA or other therapeutic agents does not have any disease-modifying potential, and ileocolic resection may be a better alternative to long-term medical therapy.
Although this study is an exploratory analysis of a randomized clinical trial that has long-term follow-up data, there are some limitations that warrant discussion. First is the lack of assessment if obstructive symptoms were due to fibrotic strictures or acute inflammatory stenosis. It is possible that differentiation of these 2 types of pathological mechanisms that result in the narrowing of the bowel lumen could optimize the appropriate approach. Indeed, fibrotic stenosis ordinarily is treated surgically, whereas inflammatory stenosis may be initially managed medically [22]. Some studies evaluating CD patient populations have found that imaging, including magnetic resonance enterography [23] and PET/CT enterography [24], may help to identify patients with strictures containing severe inflammation, thereby stratifying them to initial medical treatment rather than surgical therapy. However, these promising preliminary reports must be interpreted with caution. Indeed, it should be acknowledged that the majority of fibrotic strictures contain variable degrees of inflammatory component, resulting in significant overlap between the 2 histological subtypes [25]. Despite this limitation, our analysis included only CD patients presenting the first episode of intestinal subocclusion relieved rapidly without surgery (i.e., individuals more likely having predominantly inflammatory strictures. Furthermore, patients presenting evidence of prestenotic small-bowel dilatation (i.e., subjects with predominantly fibrotic strictures) were excluded from the original trial.
A second issue is blinding – neither the study investigators nor the patients were blinded to the treatment allocation; however, because the exploratory analysis was highly objective (recurrent bowel occlusion), we do not think a double-blinded approach would have impacted the results significantly.
Despite these reservations, from the clinician’s point of view, the present study provides an important contribution for clinicians on the optimal pharmacological approach to prevent/delay recurrent bowel occlusion in patients with ileocecal CD who have undergone successful medical treatment for the initial episode of intestinal subocclusion. To our knowledge this is the first study to demonstrate a decrease of recurrent occlusion rate for up to 3 years, and perhaps, a change in the medium-term natural history of subocclusive ileocecal CD in a subpopulation of patients receiving AZA in a clinical trial setting.
Although the optimal medical management of terminal ileum or ileocecal fibrostenosing CD remains a challenging problem without a clear best answer for an important subgroup of patients, there is hope that ongoing studies will define other potential therapeutic avenues for modifying the inexorable progression of this phenotype of illness. Further research through randomized controlled trials is needed to compare medical (including anti-TNF agents) and surgical therapy in subocclusive ileocecal CD patients [26]. In particular, it is important that these studies include issues regarding long-term efficacy, patient preference, long-term quality of life, and overall costs of the medical and surgical strategy.
Conclusions
In ileum terminal or ileocecal CD patients presenting with a clinically resolved initial episode of small-bowel subocclusion, subsequent maintenance therapy with AZA compared with mesalazine shows higher efficacy in eliminating or postponing recurrent obstruction through-out 3 years of therapy. The data obtained from this exploratory analysis suggest that to reduce the risk of recurrence of bowel obstruction in patients with ileocecal CD, AZA must be given after the first subocclusive episode has been dealt with by clinical therapy.
Footnotes
Source of support: Julio Maria Fonseca Chebli is the recipient of a grant from CNPq, Brazil; this study was partly supported by a clinical research grant from CNPq and FAPEMIG, Brazil
Conflicts of interest and source of funding
J.M.F. Chebli has served as a speaker for Abbott and Janssen. The remaining authors declare they have no relevant conflicts of interest. The authors confirm that this article content has no conflicts of interest.
References
- 1.Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–84. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohn’s Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–82. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–50. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Hanauer SB, Sandborn WJ Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–83. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 6.Blum E, Katz JA. Postoperative therapy for Crohn’s disease. Inflamm Bowel Dis. 2009;15:463–72. doi: 10.1002/ibd.20741. [DOI] [PubMed] [Google Scholar]
- 7.Sieb JP. Myasthenia gravis: an update for the clinician. Clin Exp Immunol. 2014;175:408–18. doi: 10.1111/cei.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruocco E, Wolf R, Ruocco V, et al. Pemphigus: associations and management guidelines: facts and controversies. Clin Dermatol. 2013;31:382–90. doi: 10.1016/j.clindermatol.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58–72. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe BH, Korelitz BI. Prognosis for nonoperative management of small-bowel obstruction in Crohn’s disease. J Clin Gastroenterol. 1983;5:211–15. doi: 10.1097/00004836-198306000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Samimi R, Flasar MH, Kavic S, et al. Outcome of medical treatment of stricturing and penetrating Crohn’s disease: a retrospective study. Inflamm Bowel Dis. 2010;16:1187–94. doi: 10.1002/ibd.21160. [DOI] [PubMed] [Google Scholar]
- 12.de Souza GS, Vidigal FM, Chebli LA, et al. Effect of azathioprine or mesalazine therapy on incidence of re-hospitalization in subocclusive ileocecal Crohn’s disease patients. Med Sci Monit. 2013;19:716–22. doi: 10.12659/MSM.889196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto AL, Chebli LA, Ribeiro MS, et al. Azathioprine therapy in steroid-dependent patients with Crohn disease: results of a 10-year longitudinal follow-up study. Med Sci Monit. 2009;15(5):PI19–26. [PubMed] [Google Scholar]
- 14.Chebli LA, Chaves LD, Pimentel FF, et al. Azathioprine maintains long-term steroid-free remission through 3 years in patients with steroid-dependent ulcerative colitis. Inflamm Bowel Dis. 2010;16:613–19. doi: 10.1002/ibd.21083. [DOI] [PubMed] [Google Scholar]
- 15.Camus M, Seksik P, Bourrier A, et al. Long-term outcome of patients with Crohn’s disease who respond to azathioprine. Clin Gastroenterol Hepatol. 2013;11:389–94. doi: 10.1016/j.cgh.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 16.D’Haens G, Geboes K, Ponette E, et al. Healing of severe recurrent ileitis with azathioprine therapy in patients with Crohn’s disease. Gastroenterology. 1997;112:1475–81. doi: 10.1016/s0016-5085(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 17.Ramadas AV, Gunesh S, Thomas GA, et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–6. doi: 10.1136/gut.2009.202101. [DOI] [PubMed] [Google Scholar]
- 18.Peyrin-Biroulet L, Oussalah A, Williet N, et al. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut. 2011;60:930–36. doi: 10.1136/gut.2010.227884. [DOI] [PubMed] [Google Scholar]
- 19.van Loo ES, Vosseberg NW, van der Heide F, et al. Thiopurines are associated with a reduction in surgical re-resections in patients with Crohn’s disease: a long-term follow-up study in a regional and academic cohort. Inflamm Bowel Dis. 2013;19:2801–8. doi: 10.1097/01.MIB.0000435758.97952.a8. [DOI] [PubMed] [Google Scholar]
- 20.Chatu S, Subramanian V, Saxena S, Pollok RC. The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:23–34. doi: 10.1038/ajg.2013.402. [DOI] [PubMed] [Google Scholar]
- 21.Chatu S, Saxena S, Subramanian V, et al. The Impact of Timing and Duration of Thiopurine Treatment on First Intestinal Resection in Crohn’s Disease: National UK Population-Based Study 1989–2010. Am J Gastroenterol. 2014;109:409–16. doi: 10.1038/ajg.2013.462. [DOI] [PubMed] [Google Scholar]
- 22.Spinelli A, Correale C, Szabo H, Montorsi M. Intestinal fibrosis in Crohn’s disease: medical treatment or surgery? Curr Drug Targets. 2010;11:242–48. doi: 10.2174/138945010790309984. [DOI] [PubMed] [Google Scholar]
- 23.Pozza A, Scarpa M, Lacognata C, et al. Magnetic resonance enterography for Crohn’s disease: what the surgeon can take home. J Gastrointest Surg. 2011;15:1689–98. doi: 10.1007/s11605-011-1622-7. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadi A, Li Q, Muller K, et al. Diagnostic value of noninvasive combined fluorine-18 labeled fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography enterography in active Crohn’s disease. Inflamm Bowel Dis. 2010;16:974–81. doi: 10.1002/ibd.21153. [DOI] [PubMed] [Google Scholar]
- 25.Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–61. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 26.Chebli JM, Gaburri PD, Chebli LA, et al. A guide to prepare patients with inflammatory bowel diseases for anti-TNF-α therapy. Med Sci Monit. 2014;20:487–98. doi: 10.12659/MSM.890331. [DOI] [PMC free article] [PubMed] [Google Scholar]