Abstract
Background
Bone marrow stromal cells (BMSCs) play an important role in ischemic limb angiogenesis. BMSCs cultured in vitro can be exposed to oxygen tension much higher than that experienced in vivo. This study assessed oxygen tension in bone marrow and ischemic muscle in vivo, and then identified an appropriate oxygen concentration for culturing BMSCs.
Material/Methods
Unilateral hind limb ischemia was surgically induced in 30 mice, and tissue oxygen tension in bilateral gastrocnemius muscles and femoral bone marrow was monitored in vivo using a micro-electrode at 24 hours, 1 week, 2 weeks, and 3 weeks after modeling. Media used for culturing normal marrow, muscle, and artery tissue were incubated with various oxygen concentrations, and O2 tension was continuously monitored. Oxygen tension in aortic arterial blood was monitored using a micro-electrode and blood gas analyzer, and the results were compared.
Results
Oxygen tension in ischemic gastrocnemius muscle reached a nadir at 1 week after ischemic modeling, when histological changes were most noticeable. Culture media incubated with 3%, 6%, and 14% oxygen (the normal oxygen levels of bone marrow, muscle, and arterial blood, respectively) required 9, 6, and 2 hours, respectively, to reach an equilibrated oxygen tension, and oxygen tension was elevated by 1.6-, 1.2-, and 0.4-fold, respectively, upon re-exposure of the media to air.
Conclusions
Physiological oxygen tension differs in different tissues. A 3% O2 concentration mimics the physiological O2 exposure experienced by BMSCs and represents the hypoxic concentration. Culture medium incubated under hypoxic conditions requires a prolonged period of time to regain equilibrated oxygen tension.
MeSH Keywords: Arterial Pressure, Cell Hypoxia, Mesenchymal Stromal Cells
Background
Bone marrow stromal cells (BMSCs) are self-renewing and have the potential to differentiate into multiple cell types [1], making them attractive for use in therapeutic angiogenesis. While BMSCs reside in the hypoxic environment of the bone marrow cavity [2–4], most in vitro studies using BMSCs are conducted in the atmosphere. However, the high oxygen tension may lead to oxidative stress and inflammatory reaction and cause damage to primary cells [5].
Recently, researchers have realized that oxygen can act as a signaling molecule, and affect the basic characteristics of stem cells [4,6,7]. Although studies examining the effects on hypoxia on BMSCs have been performed, their results are controversial [8–10], and may be due exposure to different oxygen concentrations and interrupted oxygen tension in the culture medium.
To better mimic the natural environment of BMSCs in vitro, a researcher must know the actual oxygen tension in a certain tissue, which reflects a balance between local oxygen supply and demand. While some experts have defined 1~10% O2 (pO2 7.6~76 mmHg) as physiological hypoxia and <1% O2 as pathological hypoxia [11], the physiological oxygen tension in different tissues may vary significantly. Dyson et al. reported that oxygen tension in different tissues varied 12.75~68.25 mmHg [12]; however, oxygen tension in bone marrow was not included.
Our current study was conducted to assess tissue oxygen tension (PtO2) in mouse femoral bone marrow and gastrocnemius muscle under physiological and limb ischemic states. Real oxygen tension in hypoxic culture was analyzed by continuous monitoring of oxygen tension in culture medium incubated in a physiologic hypoxic environment.
According to Henry’s law [13], the partial pressure of O2 in solution equals that in air at a state of equilibrium. Continuous monitoring of oxygen tension in endothelial basal medium-2 (EBM-2) was performed to determine the length of time required to reach equilibrium, and how it influences studies conducted under normoxic conditions.
Material and methods
Animal model
Thirty Balb/c mice (aged 3 months ±2 weeks; weight 18±2 g; from Vital River Laboratory Animal Technology CO., LTD, Beijing, China) fed a normal diet were anesthetized by intra-peritoneal injection with 10% chloral hydrate (0.3 mL/100 g), and an incision was made in the skin of the left proximal hind limb. After ligating the proximal end of the femoral artery, the distal portion of the saphenous artery was severed by electrocoagulation, and the artery and its side-branches were dissected free. The femoral artery and its attached side-branches were then excised by electrocoagulation [14], with care taken to avoid injury to the accompanying vein and nerve. The overlying skin was then closed using a surgical stapler. The operated mice were then randomly assigned to 4 different groups (6 mice per group) consisting of ischemia for 24 hours, 1 week, 2 weeks, and 3 weeks. Five healthy mice were assigned to a control group. This study was performed with the approval of the local ethics committee and all the experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue oxygen tension monitoring
Arterial blood oxygen tension by blood gas analyzer and micro-electrode
Oxygen tension in arterial blood was measured by 2 different methods, and their variance was determined. In method #1, a sample of arterial blood (0.5 mL) was obtained from the abdominal aorta of each mouse for analysis of gases (ABL800 FLEX, Radiometer, Denmark). In method #2, following intra-peritoneal anesthesia, arterial blood oxygen tension was monitored in vivo by inserting a micro-electrode with temperature correction (POG-301, Unique Medical Japan Co., Ltd.) into the abdominal aorta. Five measurements of O2 concentration and tension in were taken using each method.
PtO2 of bilateral gastrocnemius muscles and femoral marrow cavity
Measurements of tissue oxygen pressure (PtO2) in bilateral gastrocnemius muscle and the femoral marrow cavity were obtained using a polarographic assay and micro-electrodes. The oxygen electrode was calibrated at 150 mmHg in distilled water aerated with air. For measurements in gastrocnemius muscle, the mouse was anesthetized using 10% chloral hydrate (i.p.), and placed in a supine position. A tiny incision was made into the calf skin, and the O2 electrode was inserted into the gastrocnemius muscle (making sure to insert the electrode at the same point and depth). Readings of O2 levels were obtained following a stabilization time of 3–5 minutes. For O2 measurements in femoral bone marrow, an incision was made in the lateral thigh, and the proximal portion of the femur was drilled using a 6G needle. The O2 electrode was then inserted into the marrow cavity and stabilized O2 readings were recorded 3–5 minutes later.
Oxygen tension in culture medium
A 5 mL aliquot of EBM-2 complete culture medium (LONZA, America) in a 25-cm2 culture flask was incubated in a triple-gas incubator (HERAcell 150, Themo Fisher, Germany). The oxygen concentration in the incubator was set based on the results of tissue oxygen tension monitoring, and oxygen tension in the medium was continuously monitored for 24 hours. When the liquid medium reached equilibrium, the flask was removed and exposed to normoxic conditions for 30 minutes by gentle shaking to mimic laboratory procedures performed in normal air.
Histological analysis
Samples of gastrocnemius obtained at each time point were fixed in 10% formaldehyde, dehydrated, and embedded in paraffin. Tissue sections (4-μM thickness) were prepared and stained with hematoxylin-eosin (H&E).
Statistical analysis
Results are expressed as the mean ± standard error (SE). The paired t test was performed using SPSS for Windows, Version 16.0. Chicago, IL: SPSS Inc. P values <0.05 were considered statistically significant.
Results
Histological analysis
Gross observations showed that the ischemic hind limb of each operated mouse appeared swollen and cyanotic at 24 hours post-modeling, and necrosis of the hindlimb toes appeared after 1 week. Muscle atrophy was absolute at 2 to 3 weeks post-modeling.
Staining with hematoxylin-eosin (Figure 1) showed swollen gastrocnemius muscle fibers, abnormal muscle striation, and minor inflammatory cell infiltration after 24 hours of ischemia. After 1 week of ischemia, necrotic and inflammatory changes were obvious, and numerous necrotic fragments and inflammatory cells had accumulated in inter-muscular spaces. After 2–3 weeks of ischemia, the number of inflammatory cells had decreased and muscle fibers showed significant attenuation.
Figure 1.
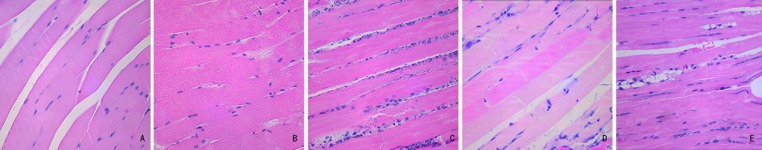
H&E staining of gastrocnemius muscle at different ischemic time points (10×40). (A) In the normal group, muscle fibers are in good order and cross striation is clear. (B) At 24 hours of hypoxia, gastrocnemius muscle fibers are swollen, abnormal striation is seen, and several inflammatory cells can be observed. (C) At 1 week of hypoxia, necrotic and inflammatory changes are most obvious. Numerous necrotic fragments and inflammatory cells have accumulated in intermuscular spaces. (D) At 2 to 3 weeks of ischemia, the numbers of inflammatory cells have decreased and muscle fibers are attenuated.
Arterial oxygen tension
Oxygen tension in blood obtained from the abdominal aorta was measured by 2 instruments. The mean value obtained using a blood gas analyzer was 20 mmHg higher than that obtained using a tissue oxygen tension analyzer (129±0.5 mmHg vs. 106±3.94 mmHg).
Tissue oxygen tension in gastrocnemius muscle and femoral bone marrow
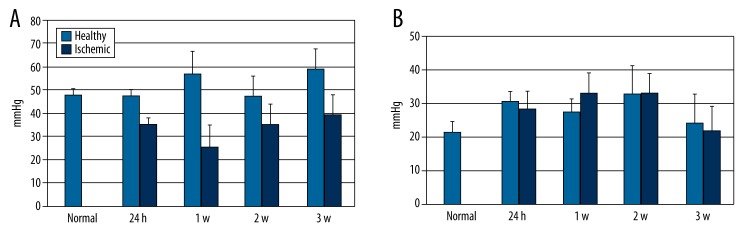
PtO2 readings of 47.78±4.37 mmHg, 35.38±2.61 mmHg, 25.54±8.57 mmHg, 35.38±8.51 mmHg, and 39.32±8.68 mmHg were recorded in normal gastrocnemius muscle at 24 hours, 1 week, 2 weeks, and 3 weeks, respectively, in ischemic muscle,. Additionally, PtO2 in the ischemic gastrocnemius muscle was significantly lower (P<0.05) compared to PtO2 in the contralateral normal gastrocnemius muscle all time points (Figure 2). PtO2 in ischemic muscle reached a nadir (25.54±8.57 mmHg) at 1 week after modeling. It then increased, but did not reach the levels shown in normal muscle (39.32±8.68 mmHg at 3 weeks post-modeling vs. 47.78±4.37 mmHg in normal muscle; P<0.05).
Figure 2.
Oxygen tension in gastrocnemius muscle and femoral bone marrow at different time points. Oxygen tension in ischemic limb gastrocnemius muscle (A) was significantly lower than in that in control group gastrocnemius muscle and contralateral healthy gastrocnemius muscle at every time point (P<0.05). Oxygen tension in femoral bone marrow (B) showed no significant change at any time point (P>0.05).
PtO2 in normal femoral bone marrow was 21.55±3.40 mmHg. In comparison, PtO2 in bilateral femoral bone marrow was slightly elevated at all time points following the modelling of 1 limb (27.44±3.07 mmHg, 27.34±5.96 mmHg, 24.58±8.34 mmHg, and 29.48±8.48 in non-ischemic side marrow and 30.44±5.36 mmHg, 27.46±12.34 mmHg, 28.76±11.45 mmHg, and 29.14±7.40 mmHg in ischemic side marrow, at 24 hours, 1 week, 2 weeks and 3 weeks post-modeling, respectively). However, these elevated levels were not significantly different from the levels in normal marrow (P>0.05).
Oxygen tension in culture medium
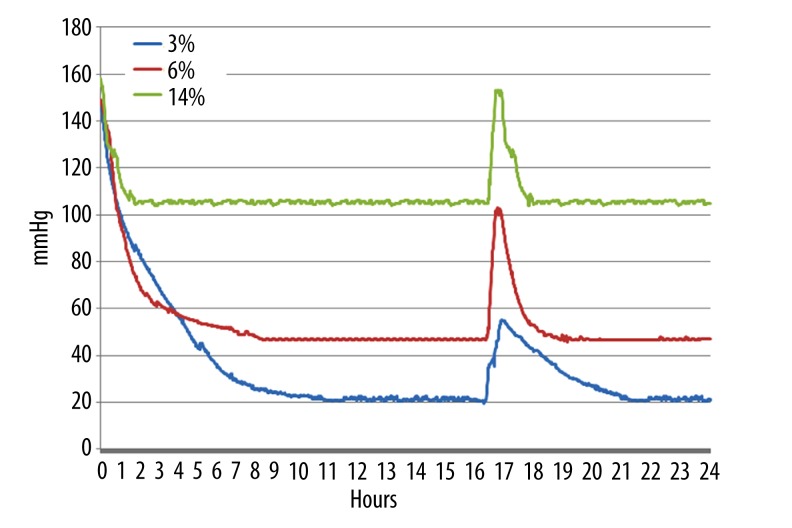
Oxygen tension was measured in 5-mL aliquots of EBM-2 complete culture medium contained in 25-cm3 culture flasks incubated under the following conditions: 37°C, 3% O2, 5% CO2, and 92% N2; 37°C, 6% O2, 5% CO2, and 89% N2; 37°C, 14% O2, 5% CO2, and 82% N2. The oxygen tension curves were traced and are shown in Figure 3. Oxygen tension in media incubated with 3%, 6%, and 14% O2 concentrations reached equilibrium at 9 hours, 6 hours, and 2 hours, respectively. When the flasks were removed after 15 hours of incubation and exposed to normoxic conditions for 30 minutes, oxygen tension was found to have increased ~1.6-fold (55 mmHg vs. 21 mmHg), 1.2-fold (103 mmHg vs. 47 mmHg), and 0.4-fold (153 mmHg vs. 106 mmHg) in media incubated with 3%, 6%, and 14% oxygen, respectively. Upon re-incubation at the controlled O2 levels, the media incubated with 3%, 6%, and 14% O2 returned to steady-state O2 tensions at 5 hours, 3 hours, and 2 hours, respectively.
Figure 3.
Culture media oxygen tension curves at different oxygen concentrations. The x-axis shows monitoring hours, and the Y-axis shows oxygen tension (mmHg). After 15 hours of incubation at each oxygen concentration, the flasks were removed and gently shaken in air for 30 minutes.
Discussion
Our study confirmed that the bone marrow cavity in which BMSCs reside is an extremely hypoxic environment (21.55±3.40 mmHg; ~3% oxygen). While PtO2 in bone marrow showed no significant change following surgical induction of hindlimb ischemia, PtO2 in gastrocnemius muscle reached a nadir that correlated with histological changes at 1 week post-modeling. A slight elevation in PtO2 was observed in bilateral bone marrow, and this may have been due to a systemic mobilization of oxygen in response to limb ischemia; however, this hypothesis requires further investigation.
When describing conditions of in vitro culture, the term hypoxic generally refers to an oxygen concentration <20% [15]. However, when air is inhaled into the lungs, enters the blood circulation, and finally reaches the interstitial space, oxygen tension has decreased to levels much lower than found in air [16]. Oxygen tension in different tissues differs greatly depending on their physiological state. Dyson et al. reported that oxygen tension in different tissues (muscle, liver, kidney, and bladder) of different species (human, rat, mouse, dog, and pig) ranged 12.75~68.25 mmHg [12]; however, oxygen tension in bone marrow where BMSCs reside was not reported. Following testing with a blood gas analyzer, Jonathan et al. reported an oxygen tension of 54.9±0.98 mmHg in iliac bone marrow obtained from healthy donors [16]. In our study, however, we found a femoral bone marrow oxygen tension of 21±3.40 mmHg, as determined by polarographic micro-electrode live monitoring in vivo. While the analytic principle of these 2 instruments is identical (both are Clark electrodes), the different results might be due to oxygen interfusion that occurs during aspiration of marrow. To minimize effects on the internal marrow environment, we measured oxygen tension using a micro-electrode with a diameter of only 0.4 mm. A comparison of methods used to analyze oxygen tension in arterial blood showed that values determined using a blood gas analyzer were 20 mmHg higher than those determined using a micro-electrode.
With our increasing knowledge concerning the impact of oxygen tension on stem/progenitor cell culture [2,4,11], a variety of oxygen concentrations have been used to study the effects of hypoxia on stem/progenitor cells. Some experts have hypothesized that short-term but sub-lethal hypoxic preconditioning (0.5–1% oxygen) might maintain BMSC viability, and also enhance their migration, angiogenic potential, and ability to promote tissue regeneration [17–20]. Other investigators have reported that hypoxic conditions (5–8% oxygen) accelerate BMSC differentiation [8,21], or that BMSCs remain quiescent in an environment containing 3% oxygen [22]. These findings are controversial, and we therefore selected a variety of oxygen concentrations for our studies. We found that the bone marrow cavity had an oxygen level of ~3%, which might explain why BMSCs are likely to remain quiescent when maintained in a culture environment containing 3% O2.
Oxygen levels of 1~10% (pO2 7.6~76 mmHg) and <1% have been previously regarded as physiologically hypoxic and pathologically hypoxic, respectively [11]. We measured variations in tissue oxygen tension at different ischemic time points and found that PtO2 reached a nadir (25.54±8.57 mmHg) at 1 week after surgery, when muscle cell necrosis was most apparent. However, normal bone marrow from non-operated mice showed a similar PtO2 level. In other words, a physiologically hypoxic oxygen tension for bone marrow was a pathologically hypoxic tension for muscle. Thus, a sweeping definition of physiological hypoxia might not be appropriate. Normal PtO2 levels can vary depending on the tissue examined, and can be much lower than those in air. Thus, a 21% oxygen concentration in an in vitro culture may not mimic the oxygen concentration a tissue experiences in a body’s microenvironment. To determine how long it takes to reach a steady state of oxygen tension when cultures are placed in a hypoxic environment, aliquots of culture media were incubated at different oxygen concentrations (3%, 6%, and 14%) with continuous monitoring for oxygen levels. Our results showed that culture medium incubated at lower O2 concentrations required longer time periods to reach a steady state. When the incubation flasks were re-exposed to normal air, media incubated in 3% oxygen showed the greatest change in oxygen tension (~1.6-fold) and required ~5 hours to return to a steady state. Thus, briefly exposing a hypoxic culture to normal air can greatly alter oxygen tension in the media. For studies which require a short-term hypoxic culture (for example, hypoxic pre-conditioning), the time required to reach a preset oxygen level might be much shorter than expected. Successful in vitro culturing of BMSCs might require a persistent and uninterrupted hypoxic environment.
Conclusions
Physiological oxygen levels for different tissues vary, and are much lower than the oxygen concentration in air. A ~ 3% oxygen concentration is physiologically hypoxic when cultivating BMSCs, and an uninterrupted appropriate oxygen concentration may be important when conducting in vitro studies with BMSCs. Further investigations on the biological characteristics of BMSCs cultured under different conditions of hypoxia need to be performed.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest regarding the design or outcomes of this study.
Source of support: Departmental sources
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Abdollahi H, Harris LJ, Zhang P, et al. The role of hypoxia in stem cell differentiation and therapeutics. J Surg Res. 2011;165:112–17. doi: 10.1016/j.jss.2009.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 4.Jing D, Wobus M, Poitz DM, et al. Oxygen tension plays a critical role in the hematopoietic microenvironment in vitro. Haematologica. 2012;97:331–39. doi: 10.3324/haematol.2011.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccone MM, Cortese F, Gesualdo M, et al. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. 2013;2013:782137. doi: 10.1155/2013/782137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito J, Zeng Z, Konopleva M, Wilson WR. Targeting hypoxia in the leukemia microenvironment. Int J Hematol Oncol. 2013;2:279–88. doi: 10.2217/IJH.13.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–53. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 9.Pattappa G, Thorpe SD, Jegard NC, et al. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods. 2013;19:68–79. doi: 10.1089/ten.TEC.2011.0734. [DOI] [PubMed] [Google Scholar]
- 10.Ren H, Cao Y, Zhao Q, et al. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12–21. doi: 10.1016/j.bbrc.2006.05.169. [DOI] [PubMed] [Google Scholar]
- 11.Hadjipanayi E, Brown RA, Mudera V, et al. Controlling physiological angiogenesis by hypoxia-induced signaling. J Control Release. 2010;146:309–17. doi: 10.1016/j.jconrel.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Dyson A, Singer M. Tissue oxygen tension monitoring: will it fill the void? Curr Opin Crit Care. 2011;17:281–89. doi: 10.1097/MCC.0b013e328344f1dc. [DOI] [PubMed] [Google Scholar]
- 13.Leng C, Kish JD, Kelley J, et al. Temperature-dependent Henry’s law constants of atmospheric organics of biogenic origin. J Phys Chem A. 2013;117:10359–67. doi: 10.1021/jp403603z. [DOI] [PubMed] [Google Scholar]
- 14.Couffinhal T, Silver M, Zheng LP, et al. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–79. [PMC free article] [PubMed] [Google Scholar]
- 15.He MC, Li J, Zhao CH. [Effect of hypoxia on mesenchymal stem cells – review] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:433–36. [in Chinese] [PubMed] [Google Scholar]
- 16.Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 17.Annabi B, Lee YT, Turcotte S, et al. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337–47. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 18.Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–82. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei L, Fraser JL, Lu ZY, et al. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–45. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Yin S, Zhang W, et al. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013;4:83. doi: 10.1186/scrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusuma S, Peijnenburg E, Patel P, Gerecht S. Low oxygen tension enhances endothelial fate of human pluripotent stem cells. Arterioscler Thromb Vasc Biol. 2014;34:913–20. doi: 10.1161/ATVBAHA.114.303274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–57. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]