Abstract
Background: Wide distribution and low half-life of acyclovir has led to a high dose consumption of the drug. Recent studies have shown that encapsulation of acyclovir in nano-carriers can increase effectiveness and decrease its side effects. We investigated the inhibitory effect of acyclovir loaded nano-niosomes against herpes simplex virus type-1 (HSV-1) in cell culture.
Methods: In-vitro cytotoxicity study of empty niosomes (E-N), acyclovir loaded niosomes (ACV-N) and ACV as a free drug against HeLa cell line was performed by MTT assay and the viral titers was tested by TCID50 assay.
Results: The results indicated that a significant higher antiviral activity for acyclovir loaded nano-niosomes of about 3 times in comparison with free drug.
Conclusion: The results of this study revealed ACV-N have a higher antiviral activity compared with free drug; it could be a suitable carrier for delivery of acyclovir in the treatment of HSV-1 infections.
Keywords: Nano-niosomes, Herpes simplex virus, Cytotoxicity
Introduction
Herpes simplex virus types 1&2 belong to Alpha- herpesvirinae sub-family of Herpesviridae family. HSV virus genome has a double stranded DNA which codes over 70 gene products. HSV infection is the most common viral infections in human and causes an extended range of diseases (1,2). There are several antiviral drugs which are effective against HSV infections that most of them inhibit viral DNA synthesis. Acyclovir (ACV), a synthetic analogue of 2 deoxiguanosine , is the drug of choice against HSV infections (3). Recently, physicians and researchers are faced with the problem of elongated treatment with acyclovir due to the formation of drug resistant mutants and toxicity of the drug (4,5). Treatment with acyclovir has many limitations. The oral absorption has low bioavailability ranging from 10% to 30%. The mean plasma half-life of acyclovir is reported to be 2 to 3 h, so repetitive high dose of acyclovir for treatment is necessary (6,7). Niosomes are non-ionic surfactant vesicles that formed a bilayer structure (8). These structures are similar to liposomes that can serve as drug carriers (9). Niosomes are preferred compared to traditional liposomes because they are biodegradable, biocompatible and also have more chemical and physical stability, low toxicity and cost (10,11). Recently niosomes were investigated for the delivery of drug, and also other bioactive molecules (12). The main objective of this study was to determine the inhibitory effect of acyclovir loaded nano-niosomes against herpes simplex virus type-1 (HSV-1) in cell culture.
Methods
Preparation of acyclovir loaded nano–niosomes
Span60/Cho/DCP (65:30:5), Span60/Cho/TPGS (55:30:15) and span60/Choniosomeswere prepared by thin film hydration method. Briefly, the lipid mixture consisted of cholesterol, span, DCP or TPGS were dissolved in chloroform and added to a 250-ml round bottom flask. The mixture was placed in a rotary vacuum evaporator at 150 rpm to evaporate the solvent at 60˚C. A thin film of dried lipids was hydrated with solution of acyclovir in phosphate buffer saline and continued for 70 min. Then prepared niosomal dispersion was sonicated at 60˚C for 60 min. After sonication, the niosomes were kept at room temperature for 60 min to form the vesicles. The prepared niosomes were characterized in terms of encapsulation efficiency (EE), particle size and in-vitro drug release.
Cell culture and viruses
HeLa cells were grown in disposable plastic dishes or in 24 well plates and incubated in Dulbeccos Modified Eagles (DMEM) supplemented with 8% fetal bovine serum (FBS), 100 IV/ml of penicillin and 100 ug/ml of streptomycin. Herpes simplex virus type 1 (HSV-1) was isolated from a patient and identified by specific monoclonal anti HSV-1 antibodies.
Virus titration
Virus titration was performed by both TCID50 procedures in HeLa cells. For TCID50 cells were grown in 24 wells tissue culture dishes and the test was performed according to the Reed &Muench method.
Cell viability assay
We used MTT method to evaluate the cytotoxicity effect of ACV-N, E-N and ACV against HeLa cells. MTT assay was performed according to the standard method described by Mosmann (13). HeLa cells were seeded in 96-well plates at a density of 5×103 / well. Then incubated with ACV-N, E-N and ACV for 48 h at 37 ˚C. Then 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to the wells and incubated for 2 h at 37 ˚C to allow the conversion of MTT to Formazan by the mitochondrial dehydrogenase. Formazan crystals were dissolved in DMSO and its absorbansy was determined at 570 nm using a 96-well plates Reader (MRX, Dynex, USA). The viability of each formulation was calculated and presented as a percentage, by comparing with the untreated cells.
Evaluation of antiviral activity
The titer of the virus was 1×106 / ml, as determined by The TCID 50 method according to The Reed &Muench procedure. Approximately 1 × 105HeLa cells were seeded in 96-well plates and incubated at 37 ˚C and 5% CO2. When the cells reached 75% of confluency, they were infected with HSV-1(MOI 0.01 pfu/cell) and incubated at 37 ˚C for 1 h to allow viral adsorption. The medium was then removed and replaced with fresh medium containing different concentrations of ACV-N, E-N and ACV. After 24 h incubation, the antiviral activity of ACV-N, E-N and ACV against HSV-1 was determined. The end-point of the test was the inhibitory concentration of drug that decreased virus yield by 50% in HeLa cells.
Results
Cellular toxicity
The toxicity of all formulations was studied by MTT method. The viability values were depicted in percentage compared to the non-treated cells. Figure 1 shows no considerable toxicity of different formulations at concentration of up to 100 µM after 48 h of incubation.
Fig. 1 .
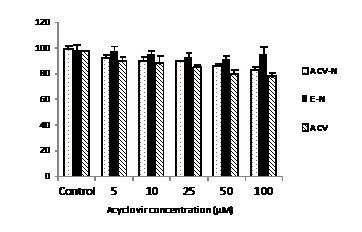
Cytotoxicity of different acyclovir formulations against non-infected Hela cells for 48 hours.Each point represent the Mean±SD (n=3).
Antiviral activity of acyclovir loaded niosomes
The antiviral activity of ACV-N and ACV against HSV-1 were evaluated by TCID50 method. Figure 2 shows the effects of different concentration of ACV-N and ACV on the HSV-1 replication at 24 h. The efficient concentration to inhibit 50% of virus replication (TCID50) for ACV-N and the drug solution was 1 mM and 3 mM respectively. These results show that the niosomes containing acyclovir has 3 times greater antiviral activity than free drug. Whereas niosomes without acyclovir shows no antiviral activity (data not shown).
Fig. 2 .
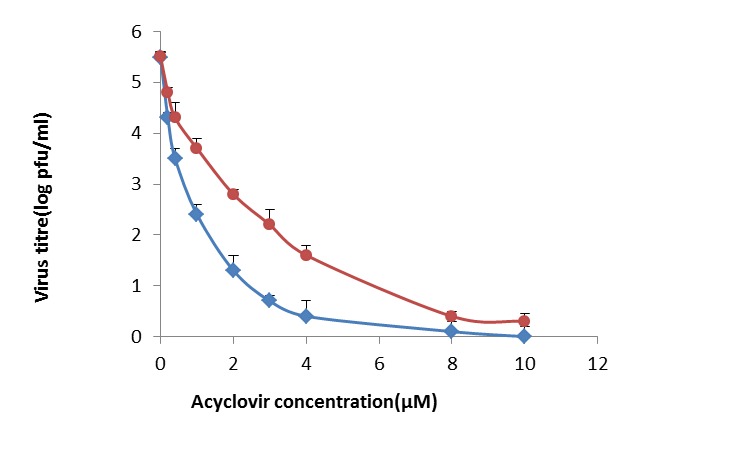
Effect of different concentration of acyclovir- niosomes and acyclovir on HSV replication at 24 h
Discussion
Viruses are intracellular parasites that depend on the host cell for their replication. So, limited number of viral replication steps can be targeted by antiviral drugs. Generally, these targets are viral proteins and these are vital for viral replication and pathogenesis. Moreover, most of viral functions are specific for each virus and make it hard to develop antiviral materials. There is a need to search for new and more effective antiviral agents. For example, plant derivatives occupy an important place in the field of chemotherapeutic research in recent years (14). Also, the extracts of some medicinal herbs were investigated as potential candidates for treating HSV infection (15). On the other hand, the development of new drug formulations changes the feature of antiviral molecules. More developments can be obtained through the use of new delivery systems for antiviral administration. For example, nanotechnology has helped us to develop nano delivery system by niosomes which was a good delivery tool comparing with the other drug delivery systems. Nano-technological strategy is useful to improve the design, formulation and delivery of antiviral drugs. This modern therapeutic nano-materials, also called nano-pharmaceuticals, shows especial distinctive role (16). Nano-particles can take small molecules, as well as proteins and nucleic acids, with a useful applications and target to specific sites where the antivirals are needed. Niosomes, non-ionic surfactant vesicles (NSVs), are the hydrated lipids containing non-ionic surfactants along with cholesterol or other lipids composing mainly of different classes of non-ionic surfactant and they can entrap both hydrophilic and hydrophobic drugs (17). Nowadays, niosomes are used as drug delivery systems, delivering drug to targeted site which are non-toxic, needs less production cost and steady over a longer period of time in different conditions (9, 18, 19). In this study, the toxicity of different concentrations of all formulations was studied by MTT method. The viability was showed in percent compared to non-treated cells. Figure 1 shows that at the highest acyclovir concentration tested, 100 µM, the amount of toxicity was relatively low, at about 10%. However the toxicity decreased by reducing the acyclovir concentration. The lack of toxicity in non-treated cells was due to this fact that acyclovir is a pro-drug and need to be phosphorylated by viral thymidine kinase (20). The antiviral activity of ACV-N, E-N and ACV was determined by TCID50 method. The assays were tested in HeLa cells. Figure 2 shows the effect of different concentration of ACV-Nand ACVon HSV replication at 24 h. These results showed that, the ACV-N seemed to be more efficient than ACV, whereas E-N presented no antiviral activity (data not shown). The higher antiviral activity of ACV-N compared to ACV as a free drug may be attributed to the interactions of niosomes with cells, which have been reported to enter cells by fusion or endocytosis (21).
On the other hand, this higher antiviral activity might be attributed to the uptake of different colloidal carriers that is correlated to the membrane perturbation caused by the virus (22). We found that niosomes in transferring the acyclovir had 3 times greater antiviral activity than acyclovir. Therefore the niosomal formulation could be a promising drug delivery system capable of increasing the antiviral activity of acyclovir.
Conclusion
The results of this study revealed that ACV-N have a higher antiviral activity compared with the free drug. ACV-N did not show cytotoxic effects on Hela cells. The results suggest that niosomal formulation could be a promising drug delivery system for acyclovir. This study indicated that ACV-N have nearly 3-fold increase in antiviral activity against HSV-1 and could be more efficient than the free drug solution.
Acknowledgments
This investigation has been funded by Tehran University of Medical Sciences
Cite this article as: Monavari S.H, Mirzaei parsa M.J, Bolouri B, Ebrahimi S.A, Ataei-pirkooh A. The inhibitory effect of Acyclovir loaded nano-niosomes against herpes simplex virus type-1 in cell culture. Med J Islam Repub Iran 2014 (17 September). Vol. 28:99.
References
- 1. Roizman B, Furlong D. The replication of herpesviruses. In: Reproduction: Springer; 1974. pp. 229-403.
- 2.Brandi G, Rossi L, Schiavano GF, Millo E, Magnani M. A new homodimer of aciclovir as a prodrug with increased solubility and antiviral activity. International journal of antimicrobial agents. 2009;34:177–180. doi: 10.1016/j.ijantimicag.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 3.de Jalón E G, Blanco-Príeto Ma J, Ygartua P, Santoyo S. Topical application of acyclovir-loaded microparticles: quantification of the drug in porcine skin layers. Journal of controlled release. 2001;75:191–197. doi: 10.1016/s0168-3659(01)00395-9. [DOI] [PubMed] [Google Scholar]
- 4.Chatis PA, Crumpacker CS. Resistance of herpesviruses to antiviral drugs. Antimicrobial agents and chemotherapy. 1992;36:1589. doi: 10.1128/aac.36.8.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schinazi R, Peters J, Williams C, Chance D, Nahmias A. Effect of combinations of acyclovir with vidarabine or its 5'-monophosphate on herpes simplex viruses in cell culture and in mice. Antimicrobial agents and chemotherapy. 1982;22:499–507. doi: 10.1128/aac.22.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain SK, Jain RK, Chourasia MK, Jain AK, Chalasani KB, Soni V. et al. Design and development of multivesicular liposomal depot delivery system for controlled systemic delivery of acyclovir sodium. AAPS Pharm Sci Tech. 2005;6:E35–E41. doi: 10.1208/pt060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain SK, Gupta Y, Jain A, Rai K. Enhanced transdermal delivery of acyclovir sodium via elastic liposomes. Drug delivery. 2008;15:141–147. doi: 10.1080/10717540801952407. [DOI] [PubMed] [Google Scholar]
- 8.Azeem A, Anwer MK, Talegaonkar S. Niosomes in sustained and targeted drug delivery: some recent advances. Journal of drug targeting. 2009;17:671–689. doi: 10.3109/10611860903079454. [DOI] [PubMed] [Google Scholar]
- 9.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. International Journal of Pharmaceutics. 1998;172:33–70. [Google Scholar]
- 10.Bragagni M, Mennini N, Furlanetto S, Orlandini S, Ghelardini C, Mura P. Development and characterization of functionalized niosomes for brain targeting of dynorphin-B. European Journal of Pharmaceutics and Biopharmaceutics. 2014 doi: 10.1016/j.ejpb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Tavano L, Aiello R, Ioele G, Picci N, Muzzalupo R. Niosomes from glucuronic acid-based surfactant as new carriers for cancer therapy: preparation, characterization and biological properties. Colloids and Surfaces B: Biointerfaces. 2014 doi: 10.1016/j.colsurfb.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Biological and Pharmaceutical Bulletin. 2011;34:945. doi: 10.1248/bpb.34.945. [DOI] [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Vijayan P, Raghu C, Ashok G, Dhanaraj S, Suresh B. Antiviral activity of medicinal plants of Nilgiris. Indian Journal of medical research. 2004;120:24–29. [PubMed] [Google Scholar]
- 15.Monavari SH, Shahrabadi MS, Keyvani H, Bokharaei-Salim F. Evaluation of in vitro antiviral activity of Chelidonium majus L against herpes simplex virus type-1. African Journal of Microbiology Research. 2012;6:4360–4364. [Google Scholar]
- 16.Lembo D, Cavalli R. Nanoparticulate delivery systems for antiviral drugs. Antivir Chem Chemother. 2010;21 doi: 10.3851/IMP1684. [DOI] [PubMed] [Google Scholar]
- 17.Namdeo A, Jain N. Niosomes as drug carriers. Indian journal of pharmaceutical sciences. 1996;58:41. [Google Scholar]
- 18.Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine: nanotechnology, biology and medicine. 2005;1:22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 19. Sahin NO. Niosomes as nanocarrier systems. In: Nanomaterials and nanosystems for biomedical applications: Springer; 2007. pp. 67-81.
- 20.Bencini M, Ranucci E, Ferruti P, Trotta F, Donalisio M, Cornaglia M. et al. Preparation and in vitro evaluation of the antiviral activity of the acyclovir complex of a β-cyclodextrin/poly (amidoamine) copolymer. Journal of controlled release. 2008;126:17–25. doi: 10.1016/j.jconrel.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Mujoriya RZ, Bodla R. Niosomes–challenge in preparation for pharmaceutical scientist. Int J App Pharm. 2011;3:11–15. [Google Scholar]
- 22.Ropert C, Mishal Jr Z, Rodrigues J, Malvy C, Couvreur P. Retrovirus budding may constitute a port of entry for drug carriers. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1996;1310:53–59. doi: 10.1016/0167-4889(95)00140-9. [DOI] [PubMed] [Google Scholar]


