Abstract
Patients who develop end-stage renal disease (ESRD) associated with Type I Diabetes Mellitus may receive kidney alone (KA) transplantation, simultaneous pancreas-kidney (SPK) transplantation, or a pancreas after kidney (PAK) transplantation. The goal of this study is to examine the long-term impact of pancreas transplantation on kidney graft and patient survival rates. A total of 85 transplantation cases, consisting of 30 that received living donor KA, 21 that received SPK, and 34 that received PAK, from 2003–2010 at Akdeniz University Organ Transplantation Institute were retrospectively screened. There was a graft loss in 4 cases from the KA group, and in 1 case from each of the SPK and PAK groups. The five-year kidney graft survival rates were 86.7% in KA, 95.2% in SPK, and 97.1% in PAK. There was a single patient loss in both KA and SPK. The kidney survival percentages were higher in SPK and PAK groups compared to the KA group. Therefore, SPK should be the primary preference in these patients; however, for the cases that have a living donor, pancreas transplantation should be considered after kidney transplantation, or the patients can be followed-up on with close blood sugar control.
Key words: Kidney, Pancreas, Transplantation, Kidney survival, Patient survival
The discovery of insulin in 1921 enabled the transition from diabetic ketoacidosis and diabetic coma to an increasing number of patients with prolonged life expectancies in the clinical course of diabetes mellitus (DM). However, with prolonged lifetime, increases in the neurological, ocular, and renal complications of DM have become evident. With a 40% rate, DM is the leading cause of end-stage renal disease (ESRD) in the United States.1 In patients with type I DM-related kidney failure, kidney transplant is highly more preferable in terms of the negative effects of long-term dialysis on the patient survival and quality of life compared with the benefits of kidney transplants.2 In patients who develop type I DM-related kidney failure, kidney-alone transplantation (KA) from a living donor or a cadaver, simultaneous pancreas-kidney transplantation (SPK), or pancreas-after-kidney transplantation (PAK) are among the transplantation alternatives. The 10-year life expectancy in patients receiving hemodialysis for ESRD, and in those undergoing living donor or a cadaveric renal transplantation, was reported to be 4.4, 32.9, and 59.3% in the United States, respectively.3 Similarly, while the average life expectancy for diabetes patients waiting for kidney transplantation was 8 years, the average life expectancy after kidney transplantation was determined to be 22 years.2 When pancreas transplantation is added to kidney transplantation, prolonged kidney and patient survival rates can be attained along with other benefits, such as protection from the secondary effects of diabetes and an increase in patients' quality of life. While the 4-year mortality rate in the selected dialysis patients on the waitlist for pancreas-kidney was 40%, it was 10% in patients who received SPK transplantation.4 The goal of this study is to compare the impact of the KA, SPK, and PAK transplantation methods on kidney graft and patient survival rates in patients with ESRD associated with type I diabetes.
Patients and Methods
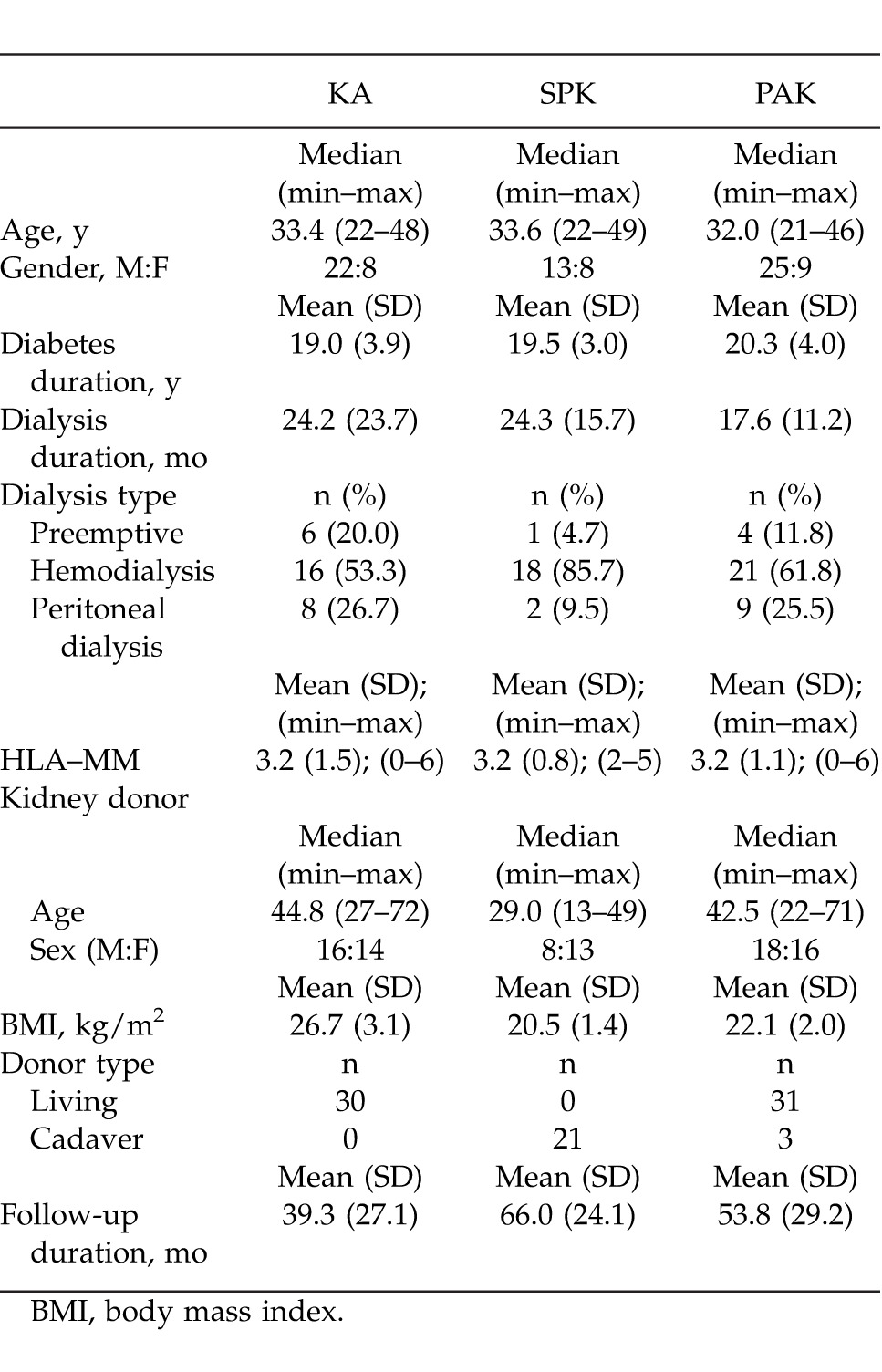
A total of 85 patients who underwent type I DM-related kidney and pancreas-kidney transplantation at the Akdeniz University Organ Transplantation Institute between February 2003 and June 2010 were included in this study. Patients were divided into three groups: KA, SPK, and PAK. The donor and recipient demographics were collected through retrospective screening of the medical records (Table 1). SPK and PAK patients were given antithymocite globulin (ATG), 2.5 mg/kg/day, as induction therapy during the early postoperative stage (days 5 through 14). Following the induction therapy, tacrolimus (0.15 mg/kg/day), 2 g/day double dose of mycophenolate mofetil (MMF), and steroid protocol were applied. In the live donor KA cases, there were patients who used 6 to 8 mg/kg/day of cyclosporine in addition to the same protocol. For infection prophylaxis in the early postoperative period, 13.5 g/day of tazocin and 200 mg/day of fluconazole were given to SPK and PAK patients, and 1 g/single dose of 3rd generation cephalosporin was given to KA patients. Afterward, all patients were administered with oral valganciclovir and co-trimoxazole treatment for 6 months. Iliac vessels were used for vascular anastomoses in all patients. While low-molecular-weight heparin was used for thrombosis prophylaxis in the early postoperative period; 100 mg/day of aspirin was prescribed after the discharge. Acute and chronic rejection diagnoses were made based on kidney biopsy in all patients.
Table 1.
Demographics features of patients
Statistical Analysis
Statistical analysis was conducted using computer software (SPSS version 15.0; SPSS Inc., Chicago, IL). Numeric, ordinal, and categorical variables are expressed as mean (standard deviation), median (minimum-maximum), and n (%), respectively. Student's t-test and the Mann-Whitney U test were used for analysis of variables with normal and abnormal distribution, respectively. The χ2 test was used for categorical variables. Tests of normalcy were conducted using the Kolmogorov Smirnov test. All hypotheses were two-sided and the alpha level of significance was set at 0.05.
Results
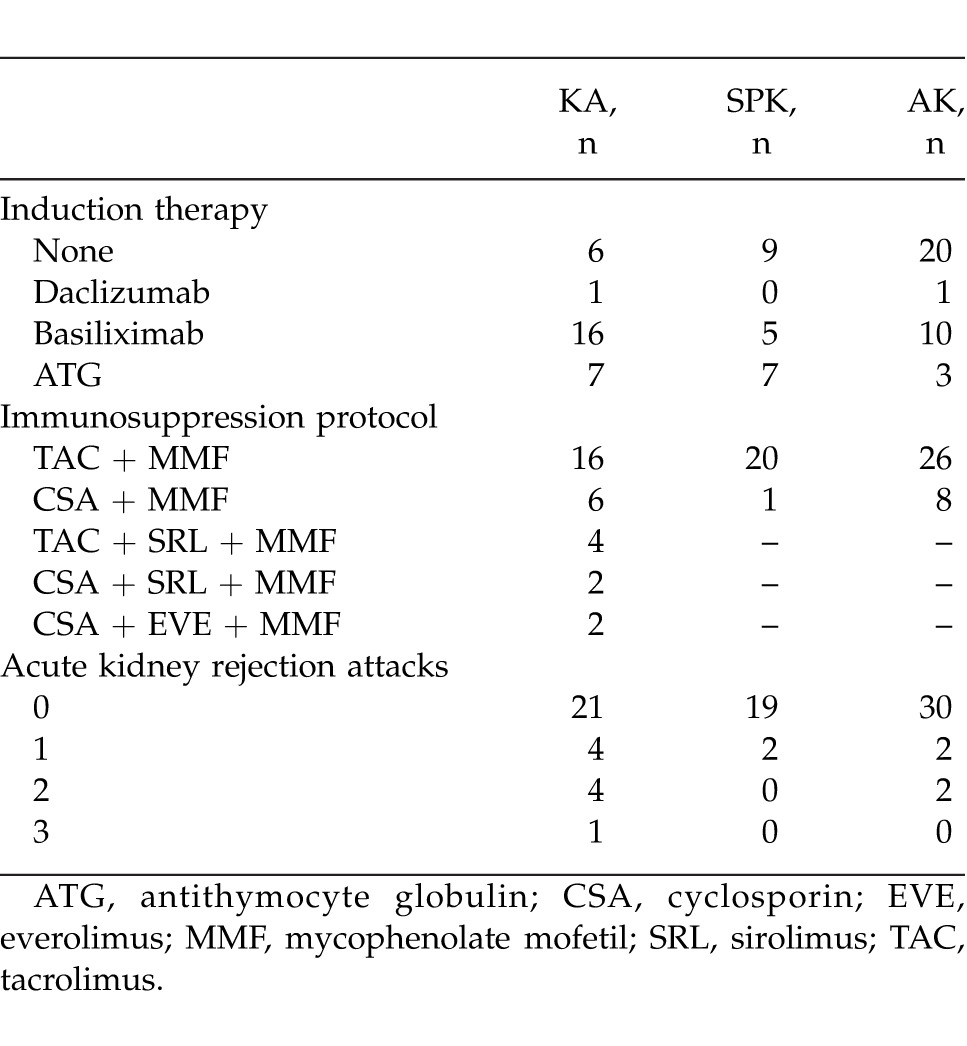
Of all the cases, 30 (35.2%) were in the KA group, 21 (24.8%) in the SPK group, and 34 (40%) were in the PAK group. Median (min–max) age was 33.4 (22–48), 33.6 (22–49), and 32.0 (21–46) years, respectively. There were 22 (73.3%) males and 8 (26.7%) females in the KA group, 13 (61.9%) males and 8 (38.1%) females in the SPK group, and 25 (73.5%) males and 9 (26.5%) females in the PAK group. Mean (SD) duration of diabetes was 19.9 (3.9) years in KA patients, 19.5 (3.0) years in SPK patients, and 20.3 (4.0) years in PAK patients. Median (min–max) donor ages in the groups were 44.8 (27–72), 29.0 (13–49), 42.5 (22–72) years, respectively. Iliac vessels were used for all arterial and venous anastomoses, except for 1 patient in the PAK group with pancreatic venous drainage into the portal system. The exocrine drainage was done as bladder exocrine drainage in 7 and as enteric exocrine drainage in 14 SPK patients, as bladder exocrine drainage in 6 and as enteric anastomosis in 28 PAK patients. All patients received low-molecular-weight heparin for postoperative heparinization. One PAK case developed renal artery thrombosis postoperatively that was corrected with reconstruction. Thrombosis was observed in the vascular anastomoses of the pancreatic graft of 3 SPK and 7 PAK patients. Of these cases, the flow was reinstated in one of the PAK patients as a result of early intervention reconstruction; however, graft pancreatectomy was performed in all of the other cases. The surgical and systemic complications observed in all three groups are displayed in Table 2, and Table 3 displays the applied induction therapy and maintenance immunosuppression protocol. Postoperative length of hospital stay was 7.6 (3.4) days in KA, 18.3 (8.9) days in SPK, and 19.5 (9.7) days in the PAK groups. The median follow-up duration for all patients was 55 months. Acute kidney rejection was observed at least once in 9 KA patients, 2 SPK patients, and 4 PAK patients (Table 3). When the acute rejection incidences were compared across groups, the difference between KA and SPK (P = 0.037), and the difference between KA and PAK (P = 0.035) was statistically significant. A total of 5 KA patients, 1 SPK patient, and 2 PAK patients received pulse steroid treatment, and the other patients were treated with ATG (2.5 mg/kg/day). At the end of the 5-year follow-up, kidney loss occurred in 4 KA, 1 SPK, and 1 PAK patients, while the kidney graft survival rate was 86.7%, 95.2%, and 97.1%, respectively, in the groups (P = 0.25). The fact that kidney graft survival percentages were higher in SPK and PAK patients when compared with KA patients, but not statistically significant, was thought to be due to the small number of patients. One patient from each of the KA and SPK groups had died by the end of the 5-year follow-up. In both cases, the cause of death was cardiovascular problems. There was no patient loss in the PAK group. The 5-year patient survival rate was 96.7%, 95.2%, and 100%, respectively, in all groups; there was no statistical difference between the groups (P = 0.34).
Table 2.
Surgical and systemic complications, n (%)
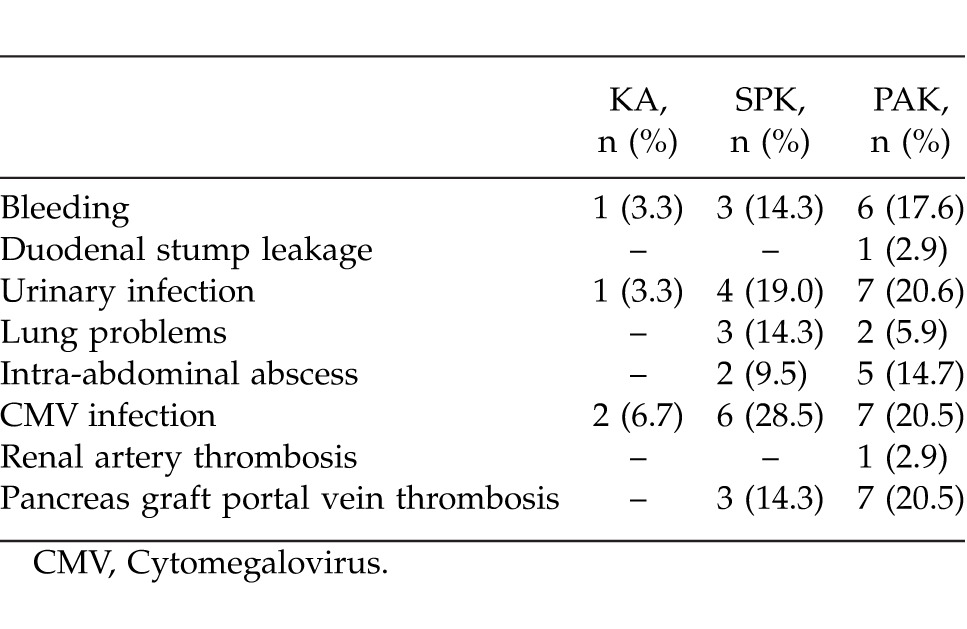
Table 3.
Immunosuppression protocols and rejection attack
Discussion
Adding pancreas transplantation to kidney transplantation in type I diabetes patients should be evaluated by considering the key points regarding the prolonged patient and kidney life expectancy, in addition to the secondary benefits like diminished secondary effects of diabetes and increased quality of life.5 Improved results and lower rates of complications and rejections have been reported in pancreas transplantation by means of new surgical techniques and advances in immunosuppressive therapy.6 In addition to improvements obtained in neuropathy, early retinopathy, and blood lipid-cholesterol profiles, development of nephropathy in kidney transplant can also be prevented via strict control of blood glucose levels in the long-term follow-ups postpancreas-transplantation.7 However, in addition to these important benefits of pancreas transplantation, many early period complications are observed. In our study, there were significantly less postoperative complications in KA when compared with SPK and PAK procedures (Table 2). We believe that because living-donor kidney alone (LDKA) is planned and the patients have an appointment system that they can prepare for, the cold ischemia time is lower; moreover, because it is an easier surgical intervention than SPK and PAK, postoperative complications are encountered less frequently and the kidney graft starts to function quicker. Acute kidney rejection attacks are known to have an important effect on patient and kidney survival rates.8 In this study, acute rejection attack incidences were highest in the KA group. This difference was statistically significant in comparison with the groups that received SPK and PAK procedures. The reason for this difference across groups with similar mean values of HLA-MM may be due to the effect of primarily using ATG in the induction therapy of patients who have pancreas transplantation. Considering the effect of acute rejection attacks on kidney graft survival rate, we observed at least 1 acute rejection attack in 5 of the 6 patients who had kidney graft loss, and the relationship between the acute rejection attacks and the kidney graft loss was statistically significant (P < 0.001). Considering the patient survival rates, as a result of the retrospective analysis, compared with kidney alone transplants, SPK has a 2% higher postoperative mortality rate in the first 90 days; and SPK and LDKA's 1-year mortality ratios were found to be 5% to 2 to 3%.9,10 In a study by Young et al, the 1-year patient survival rate in SPK, LDKA, and DDKA (deceased-donor kidney alone) was 95%, 97%, and 93%, while the survival of kidney graft was 93, 95, and 89%, respectively.11 In terms of 6-year patient survival rates, this was more in favor of SPK than LDKA (85%, 80%), while the kidney graft survival rates were found to be equal (72%, 72%). Through a 10-year follow-up in a study by Morath et al SPK was found to be better than LDKA and DDKA in terms of post-transplant kidney survival.12 While Kleinclauss et al did not find any difference between LDKA and PAK patients regarding patient and kidney survival rates, they observed that HbA1c and glomerular filtration rate (GFR) to be better in PAK cases.13 In a study by Sampaio et al on the comparison of LDKA and PAK patients, both patient survival rate (75 versus 85%) and kidney survival rate (62 versus 75%) were determined to be better in the PAK group at the end of the 8-year follow-up.14 In this study, kidney graft survival rates in KA, SPK, and PSK groups were 86.7, 95.2, and 97.1%, and patient survival rates were 96.7, 95.2, and 100%, respectively. Our findings revealed better kidney graft survival rates in patients with kidney-pancreas transplantation, with no significant differences with respect to patient survival rates between transplantation methods during long-term follow-up.
Conclusion
Consequently, based on our findings as well as related literature concerning long-term follow-ups, kidney-pancreas transplantation seems to be associated with better clinical results compared with LDKA, mainly due to better glycemic control and more prominent reduction in cardiovascular risk, making it more preferable in terms of early-period complications and risks than pancreas transplantation. This study shows that kidney survival rate was higher in SPK and PAK groups compared with the KA group. However, there were no differences observed in terms of patient survival rates. Therefore, SPK should be the primary preference in patients with ESRD associated with type I DM; however, for cases that have a living donor, pancreas transplantation should be considered only after kidney transplantation and blood glucose levels have been closely monitored via follow-up.
References
- 1.Odorico JS, Sollinger HW. Technical and immunosuppressive advances in transplantation for insulin-dependent diabetes mellitus. World J Surg. 2002;26(2):194–211. doi: 10.1007/s00268-001-0207-0. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.USRDS 1999 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health; US Renal Data System. National Institute of Diabetes and Digestive and Kidney Diseases, 1999, E.52, E.68, E.84. [Google Scholar]
- 4.Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant. 2004;4(12):2018–2026. doi: 10.1111/j.1600-6143.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman AC. The role of kidney-pancreas transplantation in diabetic kidney disease. Curr Diab Rep. 2010;10(5):385–391. doi: 10.1007/s11892-010-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakupoglu YK, Dinckan A, Gurkan A, Tuncer M, Erdogan O, Altunbas H, et al. Kidney-Pancreas transplantation: single-center experience at a university hospital in Turkey. Transplant Proc. 2005;37(7):3205–3208. doi: 10.1016/j.transproceed.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Smets YF, Westendorp RG, van der Pijl JW, de Charro FT, Ringers J, de Fijter JW, et al. Effect of simultaneous pancreas-kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet. 1999;353(9168):1915–1919. doi: 10.1016/S0140-6736(98)07513-8. [DOI] [PubMed] [Google Scholar]
- 8.Rangel EB, Melaragno CS, Gonzalez AM, Linhares MM, de Sá JR, Salzedas A, et al. Delayed kidney allograft function after simultaneous pancreas-kidney transplantation. Transplant Proc. 2010;42(9):3655–3659. doi: 10.1016/j.transproceed.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Gruessner AC, Sutherland DE, Gruessner RW. Pancreas transplantation in the United States: a review. Curr Opin Organ Transplant. 2010;15(1):93–101. doi: 10.1097/MOT.0b013e32833552d2. [DOI] [PubMed] [Google Scholar]
- 10.Martins L, Henriques AC, Dias L, Pedroso S, Almeida M, Santos J, et al. One hundred eleven simultaneous pancreas-kidney transplantation: 10-year experience from a single center in Portugal. Transplant Proc. 2011;43(1):205–208. doi: 10.1016/j.transproceed.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Young BY, Gill J, Huang E, Takemoto SK, Anastasi B, Shah T, et al. Living donor kidney versus simultaneous pancreas-kidney transplant in type I diabetics: an analysis of the OPTN/UNOS database. Clin J Am Soc Nephrol. 2009;4(4):845–852. doi: 10.2215/CJN.02250508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morath C, Zeier M, Döhler B, Schmidt J, Nawroth PP, Opelz G. Metabolic control improves long-term renal allograft and patient survival in type 1 diabetes. J Am Soc Nephrol. 2008;19(8):1557–1563. doi: 10.1681/ASN.2007070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinclauss F, Fauda M, Sutherland DE, Kleinclauss C, Gruessner RW, Matas AJ, et al. Pancreas after living donor kidney transplants in diabetic patients: impact on long-term kidney graft function. Clin Transplant. 2009;23(4):437–446. doi: 10.1111/j.1399-0012.2009.00998.x. [DOI] [PubMed] [Google Scholar]
- 14.Sampaio MS, Poommipanit N, Cho YW, Shah T, Bunnapradist S. Transplantation with pancreas after living donor kidney vs. living donor kidney alone in type 1 diabetes mellitus recipients. Clin Transplant. 2010;24(6):812–820. doi: 10.1111/j.1399-0012.2009.01195.x. [DOI] [PubMed] [Google Scholar]


