Abstract
In the current study, we investigated whether anti-CD27 monoclonal antibody can enhance the antitumor efficacy of a dendritic cell–based vaccine in prostate cancer–bearing mice. The overall therapeutic effect of a dendritic cell–based vaccine for prostate cancer remains moderate. A prostate cancer model was established by subcutaneous injection of RM-1 tumor cells into male C57BL/6 mice on day 0. After 4 days, tumor-bearing mice were treated with RM-1 tumor lysate–pulsed dendritic cells (i.e., dendritic cell–based vaccine), anti-CD27 monoclonal antibody, or a combination of RM-1 tumor lysate–pulsed dendritic cells with anti-CD27 monoclonal antibody. Mice were killed at 21 days after tumor cell implantation. Tumor size was measured for assessment of antitumor effect. Spleens were collected for analysis of antitumor immune responses. The antitumor immune responses were evaluated by measuring the proliferation and activity of T cells, which have the ability to kill tumor cells. The combination therapy with RM-1 tumor lysate–pulsed dendritic cells and anti-CD27 antibody significantly enhanced T-cell proliferation and activity, and significantly reduced tumor growth, compared with monotherapy with RM-1 tumor lysate–pulsed dendritic cells or anti-CD27 antibody. Our results suggest that combined treatment can strengthen antitumor efficacy by improving T-cell proliferation and activity.
Key words: Anti-CD27 antibody, Dendritic cell vaccine, Prostate cancer, Mouse tumor model
Prostate cancer is the most frequently diagnosed cancer and the second leading cause of cancer-related death among men in the United States.1 Up to 20% to 40% of patients with primarily localized prostate cancer will experience disease recurrence after radical prostatectomy or radiation therapy.2–4 Although disease recurrence can be temporarily controlled with androgen deprivation therapy, recurrent prostate cancer inevitably progresses to metastatic castration-resistant prostate cancer.5,6 Patients with metastatic castration-resistant prostate cancer have traditionally been treated with chemotherapeutic agents. However, chemotherapy has a limited impact on overall survival and is associated with severe side effects.7 Therefore, novel therapeutic strategies for the management of metastatic castration-resistant prostate cancer are urgently needed.
Prostate cancer is regularly infiltrated by dendritic cells and T lymphocytes, and it is considered susceptible to immunotherapy.8,9 The recent trend in prostate cancer immunotherapy has been directed toward dendritic cell–based vaccines. Dendritic cells, the most potent antigen-presenting cells, have the capability to process and present antigens derived from tumor cells to CD4+ and CD8+ T cells.10 The interaction between dendritic cells and T cells leads to expansion and activation of T cells.11 Activated CD8+ T cells become cytotoxic T lymphocytes with the ability to destroy tumor cells expressing the same antigen on the cell surface.12 Activated CD4+ T cells have been shown to provide help for CD8+ T-cell activation by releasing cytokines, such as interferon-γ and interleukin-2.13 A total of 181 patients with metastatic castration-resistant prostate cancer were treated with dendritic cell–based vaccine in 17 clinical trials.9 Clinical responses to vaccine were evaluated according to the World Health Organization criteria: complete response was defined as complete disappearance of tumor; partial response was defined as ≥50% decrease in tumor size without the appearance of new metastases; stable disease was defined as <25% increase or <50% decrease in tumor size; and progressive disease was defined as ≥25% increase in tumor size or the appearance of a new metastasis.14 Of the 181 patients treated, 97 (54%) had a clinical response, with 1 complete response, 12 partial responses, 83 disease stabilizations, and 1 mixed response.9 The dendritic cell–based vaccine in these clinical trials was in general well tolerated, with minimal toxicity observed. Most adverse effects were local reactions at the injection site, fever, and flulike symptoms. Although dendritic cell–based vaccine therapy in patients with metastatic castration-resistant prostate cancer has encouraging results, the overall clinical benefit of this vaccine remains moderate. Methods to enhance the immune response induced by dendritic cell–based vaccine will potentially augment the therapeutic efficacy of this vaccine.
CD27, a member of the tumor necrosis factor receptor family, is expressed on most peripheral blood T cells.15 Upon T-cell activation, CD27 expression is strongly enhanced.16 Ligation of CD27 by anti-CD27 monoclonal antibody provides a costimulatory signal to T cells and promotes T-cell proliferation and activation.17 It has been reported that anti-CD27 antibody can mediate antitumor efficacy.18–21 We postulated that dendritic cell–based vaccine and anti-CD27 antibody may act as therapeutically synergistic partners against prostate cancer. The reason is as follows. Dendritic cell–based vaccine activates T cells by presenting tumor antigen to T cells. Activated T cells up-regulate CD27 expression. Subsequent addition of anti-CD27 antibody further enhances T-cell proliferation and activation via ligation of CD27 on T cells, thereby potentiating antitumor efficacy. To test this hypothesis, we investigated the combined action of dendritic cell–based vaccine with anti-CD27 antibody in mouse prostate cancer model.
Materials and Methods
Animals
Male C57BL/6 mice, ages 6 to 8 weeks, were purchased from Shanghai Laboratory Animal Center (Shanghai City, China). They were kept under specific pathogen-free conditions. Sterilized food and water were available ad libitum. All animal experiments followed a protocol that was approved by the ethics committee on animal research at our college. We followed appropriate care and use guidelines for experimental animals as published by the US National Institutes of Health.
Preparation of dendritic cell–based vaccine
Culture of dendritic cell–based vaccine was established as described by Lim et al.22 In brief, bone marrow single-cell suspensions of C57BL/6 mice were prepared, the cells were washed, and the washed cells were cultured in RPMI-1640 containing 10% heat-inactivated fetal bovine serum, 2 mmol/L l-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin (all from Life Technologies, Grand Island, New York), 10 ng/mL recombinant mouse granulocyte-macrophage colony–stimulating factor (R&D Systems, Minneapolis, Minnesota), and 10 ng/mL recombinant mouse interleukin-4 (R&D Systems). On day 2, suspended cells were discarded. Fresh RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum and cytokines was added to the adherent cells. On days 4 and 6, half of the culture medium was replaced with fresh RPMI-1640 containing 10% heat-inactivated fetal bovine serum and cytokines. On day 7, nonadherent and loosely adherent cells (i.e., immature dendritic cells) were collected. The mouse prostate cancer cell line RM-1, syngeneic to C57BL/6 mice, was purchased from Shanghai Cell Institute (Shanghai City, China). For preparation of tumor cell lysate, RM-1 tumor cells were suspended in phosphate-buffered saline and subjected 4 times to rapid freeze-thaw exposures. In order to induce dendritic cell maturation, immature dendritic cells were incubated with RM-1 tumor cell lysate at the ratio of 3 tumor cells lysate to 1 dendritic cell in RPMI-1640. After incubation for 8 hours, dendritic cells were further matured in the presence of 10 ng/mL recombinant mouse tumor necrosis factor-α (R&D Systems) and 10 ng/mL recombinant mouse interferon-γ (R&D Systems). After 16 hours of incubation, RM-1 tumor lysate–pulsed dendritic cells (i.e., mature dendritic cells) were harvested and used as dendritic cell–based vaccine in our study. Dendritic cells were confirmed by phenotype analysis.
Phenotype analysis of dendritic cells
Immature and mature dendritic cells were harvested, stained with fluorescein isothiocyanate–labeled anti-CD11c (dendritic cell marker) monoclonal antibody (BD PharMingen, San Diego, California), and phycoerythrin-labeled anti-CD83 (maturation marker of dendritic cells) monoclonal antibody (BD PharMingen), and then analyzed by flow cytometry for expressions of CD11c and CD83 on the cell surface.
Analysis of CD27 expression on T cells
C57BL/6 mice either were not treated or were immunized subcutaneously with RM-1 tumor lysate–pulsed dendritic cells (1 × 106). Some of the mice were killed at 24-hour intervals and spleens were harvested. T cells were separated from splenocytes by using the T-cell isolation kit according to the manufacturer's protocol (Miltenyi Biotec, Auburn, California). Purified T cells were stained with fluorescein isothiocyanate–labeled anti-CD3 (T-cell marker) monoclonal antibody (BD PharMingen) and phycoerythrin-labeled anti-CD27 monoclonal antibody (BD PharMingen), and then analyzed by flow cytometry for CD27 expression.
Tumor challenge and treatment
C57BL/6 mice were injected subcutaneously in the right flank with 2 × 105 RM-1 tumor cells on day 0. After 4 days, tumor-bearing mice were randomly divided into 4 groups. Control group received no treatment. Mice in the dendritic cell–treated group were immunized subcutaneously in the left flank with RM-1 tumor lysate–pulsed dendritic cells (1 × 106) on days 4 and 11.23 In the antibody-treated group, anti-CD27 monoclonal antibody (100 μg; BD PharMingen) was given intraperitoneally on days 4 and 11. The combination therapy group underwent both subcutaneous administration of RM-1 tumor lysate–pulsed dendritic cells (1 × 106) on days 4 and 11, and intraperitoneal injection of anti-CD27 monoclonal antibody (100 μg) on days 7 and 14. The maximal perpendicular diameters of tumor were measured in a blind, randomly numbered fashion using a vernier caliper twice a week, and tumor size was recorded as tumor area (mm2). Mice were killed on day 21.
T-cell proliferation assay
Twenty-one days after tumor implantation, control or differently treated mice were killed and spleens were harvested. T cells were separated from splenocytes by using a T-cell isolation kit (Miltenyi Biotec). Purified T cells were plated at 1 × 105 cells per well in 96-well round-bottom plates and stimulated with RM-1 tumor lysate–pulsed dendritic cells (1 × 104 per well). After stimulation for 4 days, 1 μCi of [3H]thymidine (PerkinElmer, Waltham, Massachusetts) was added to each well for an additional 16 hours. Cells were harvested on glass microfiber filters using a cell harvester. T-cell proliferation was assessed by measuring incorporated [3H]thymidine with a liquid scintillation counter. Results were expressed as counts per minute.
Cytotoxicity assay
CD8+ T-cell–mediated cytotoxicity was determined using a standard 51Cr-release assay. In brief, RM-1 tumor cells were used as target cells. In order to label the target cell with 51Cr, RM-1 tumor cells were incubated with Na251CrO4 (PerkinElmer) for 60 minutes at 37°C and washed 3 times prior to use. Twenty-one days after tumor challenge, spleens were harvested from control or differently treated mice. CD8+ T cells were separated from splenocytes by using the CD8+ T-cell isolation kit (Miltenyi Biotec) and were stimulated in vitro with RM-1 tumor lysate–pulsed dendritic cells at a 10:1 ratio. After 5 days, the primed CD8+ T cells (effector cells) were harvested, washed twice, and cocultured in 96-well round-bottom plates with 51Cr-labeled RM-1 tumor cells (target cells) at an effector-to-target cell ratio of 100:1. After a 4-hour incubation at 37°C, supernatants were harvested and 51Cr release was assessed using a gamma counter. The percentage of target cell–specific lysis was determined as: [(experimental 51Cr release – spontaneous 51Cr release) / (maximal 51Cr release – spontaneous 51Cr release)] × 100.24 The amount of spontaneous 51Cr release was obtained by incubating target cells in medium alone. The amount of maximal 51Cr release was determined by incubation of target cells in 1% Triton X-100 (Sigma-Aldrich, St Louis, Missouri).
Assessment of interferon-γ production
Spleens were collected from control or differently treated mice 21 days after tumor challenge. CD4+ T cells were separated from splenocytes by using a CD4+ T-cell isolation kit (Miltenyi Biotec) and were cocultured with RM-1 tumor lysate–pulsed dendritic cells at a 10:1 ratio for 24 hours. Supernatants were harvested, and interferon-γ level was measured by an enzyme-linked immunosorbent assay kit (BD PharMingen).
Statistical analysis
Data were expressed as mean ± SD. Data among groups were compared by one-way analysis of variance, followed by the Student-Newman-Keuls test. P < 0.05 was considered statistically significant. GraphPad Prism software (GraphPad Software Inc, San Diego, California) was used for data analysis.
Results
Evaluation of dendritic cell surface marker expression
As shown in Fig. 1, cultured cells highly expressed CD11c (dendritic cell marker), suggesting that these cells were dendritic cells. RM-1 tumor lysate–pulsed dendritic cells (i.e., mature dendritic cells) expressed higher levels of CD83 (maturation marker of dendritic cells) in comparison with unpulsed dendritic cells (i.e., immature dendritic cells).
Fig. 1.
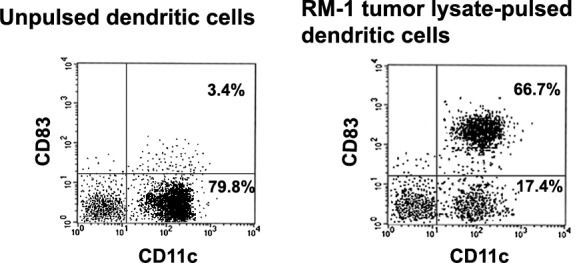
Flow cytometric analysis of immature and mature dendritic cells. The dendritic cells were cultured as described in “Materials and Methods.” Immature and mature dendritic cells were harvested, stained with fluorescein isothiocyanate–labeled anti-CD11c (dendritic cell marker) monoclonal antibody and phycoerythrin-labeled anti-CD83 (maturation marker of dendritic cells) monoclonal antibody, and then analyzed by flow cytometry. Expression rates of CD11c and CD83 in unpulsed dendritic cells (i.e., immature dendritic cells) and RM-1 tumor lysate–pulsed dendritic cells (i.e., mature dendritic cells) were, respectively, 83.2% (i.e., 3.4% + 79.8%) and 3.4% for CD11c, and 84.1% (i.e., 66.7% + 17.4%) and 66.7% for CD83, respectively.
Tumor lysate–pulsed dendritic cell vaccination up-regulates CD27 expression on T cells
As shown in Fig. 2, T cells in untreated mice displayed low expression of CD27. However, after mice were immunized with RM-1 tumor lysate–pulsed dendritic cells, T cells increased the expression of CD27.
Fig. 2.
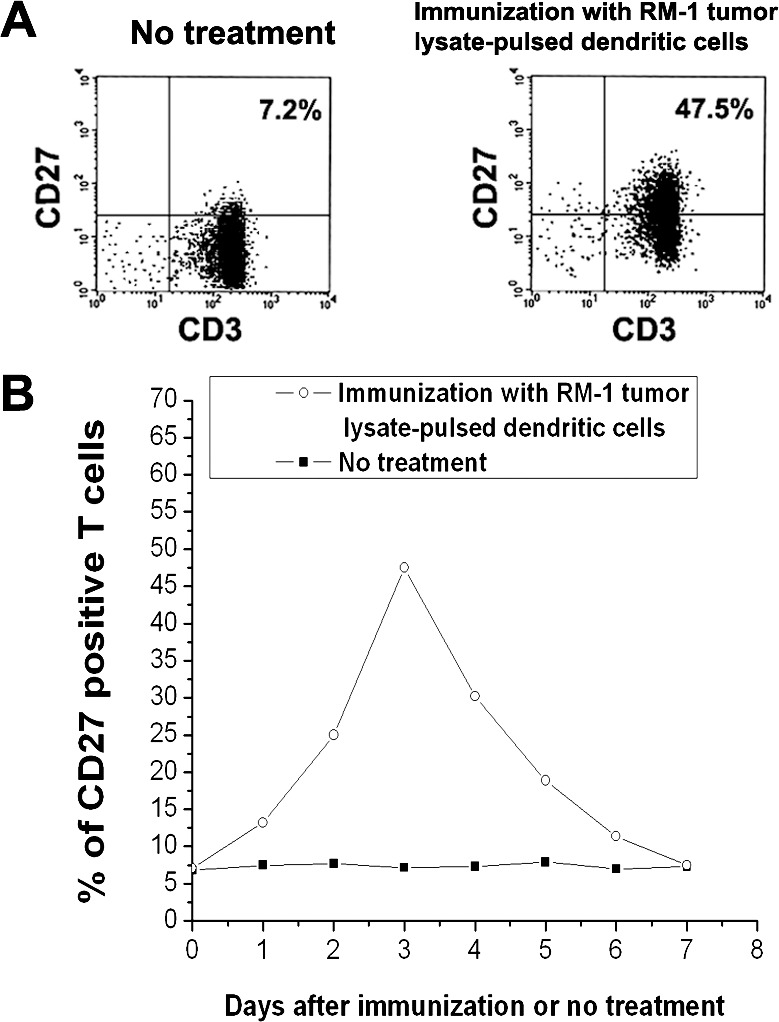
Up-regulation of CD27 expression on T cells induced by RM-1 tumor lysate–pulsed dendritic cells. C57BL/6 mice either were not treated or were immunized subcutaneously with RM-1 tumor lysate–pulsed dendritic cells. Some mice were killed at 24-hour intervals and spleens were harvested. T cells were isolated from splenocytes and stained with fluorescein isothiocyanate–labeled anti-CD3 (T-cell marker) monoclonal antibody and phycoerythrin-labeled anti-CD27 monoclonal antibody. CD27 expression on T cells was analyzed by flow cytometry. (A) CD27 expression on T cells 3 days after immunization or no treatment. Approximately 7.2% of T cells expressed CD27 in untreated mice. However, 47.5% of T cells expressed CD27 3 days after immunization of mice with RM-1 tumor lysate–pulsed dendritic cells. Data were representative of three independent experiments. (B) Kinetics of CD27 expression on T cells. CD27 expression reached peak level on T cells 3 days after immunization of mice with RM-1 tumor lysate–pulsed dendritic cells.
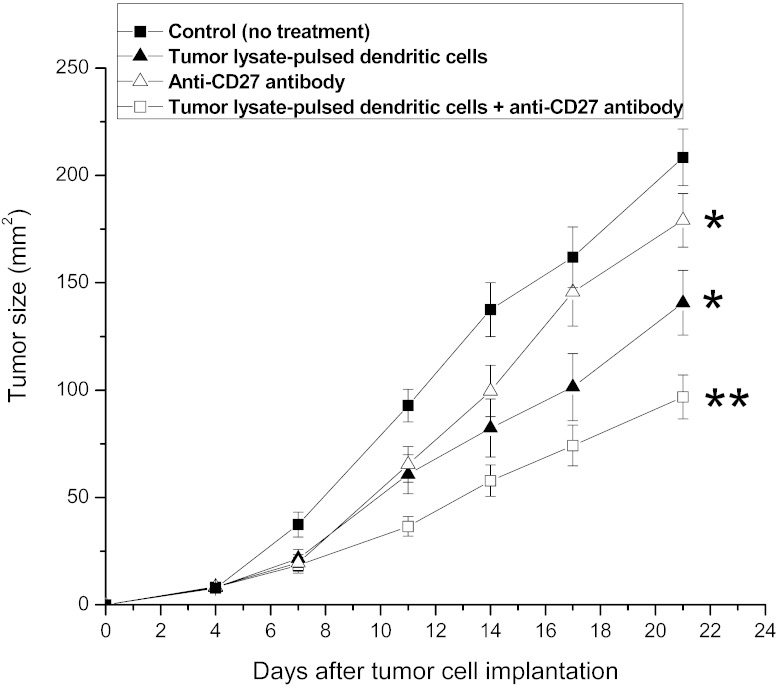
Combination therapy augments the antitumor efficacy
As shown in Fig. 3, mice treated with tumor lysate–pulsed dendritic cells or anti-CD27 antibody alone had significantly reduced tumor size compared with control (untreated) mice (P < 0.05). However, the combination of tumor lysate–pulsed dendritic cells with anti-CD27 antibody resulted in the greatest reduction of tumor size (P < 0.05), showing a synergistic anticancer effect of the combined therapy.
Fig. 3.
Antitumor effect of tumor lysate–pulsed dendritic cells and anti-CD27 antibody. C57BL/6 mice were inoculated subcutaneously with 2 × 105 RM-1 tumor cells. Four days later, tumor-bearing mice were randomly divided into 4 groups. Each group contained 10 mice. Control group received no treatment. Mice in the dendritic cell–treated group were immunized subcutaneously with tumor lysate–pulsed dendritic cells on days 4 and 11. In the antibody-treated group, anti-CD27 antibody was given intraperitoneally on days 4 and 11. Combination therapy group underwent both subcutaneous administration of tumor lysate–pulsed dendritic cells on days 4 and 11, and intraperitoneal injection of anti-CD27 antibody on days 7 and 14. The maximal perpendicular diameters of tumors were measured twice a week, and tumor size was recorded as tumor area (mm2). Mice were killed 21 days after tumor cell implantation. *P < 0.05 compared with control group; **P < 0.05 compared with all other groups.
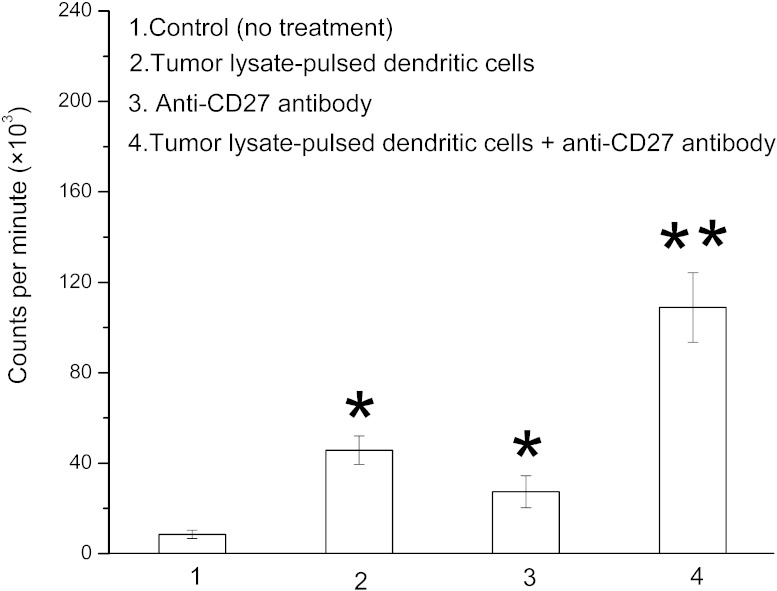
Combination therapy enhances T-cell proliferation
As shown in Fig. 4, therapy with tumor lysate–pulsed dendritic cells or anti-CD27 antibody significantly increased T-cell proliferation (P < 0.05), with the highest increase in the combination treatment (P < 0.05).
Fig. 4.
Evaluation of T-cell proliferation in tumor lysate–pulsed dendritic cells + anti-CD27 antibody–treated mice. RM-1 tumor–bearing mice (10 per group) were not treated or were treated with tumor lysate–pulsed dendritic cells, anti-CD27 antibody, or tumor lysate–pulsed dendritic cells plus anti-CD27 antibody. T cells separated from the splenocytes of untreated or differently treated mice were stimulated in vitro with RM-1 tumor lysate–pulsed dendritic cells for 4 days. These cells were pulsed with [3H]thymidine for an additional 16 hours. T-cell proliferation was assessed by measuring incorporated [3H]thymidine. Results were expressed as counts per minute. *P < 0.05 compared with control group; **P < 0.05 compared with all other groups.
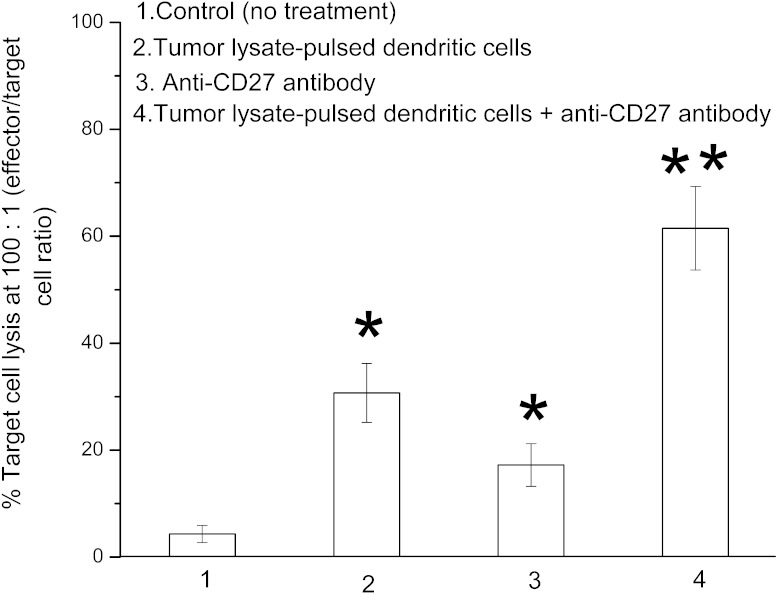
Combination therapy potentiates cytotoxic T-lymphocyte activity
As shown in Fig. 5, tumor lysate–pulsed dendritic cells or anti-CD27 antibody treatment significantly improved CD8+ T-cell activity compared with the control (untreated) group (P < 0.05). However, the combination-treated mice exhibited a much stronger CD8+ T-cell activity than the tumor lysate–pulsed dendritic cell mice or the anti-CD27 antibody–only mice (P < 0.05).
Fig. 5.
Enhancement of cytotoxic T-lymphocyte activity by treatment with tumor lysate–pulsed dendritic cells and anti-CD27 antibody. CD8+ T cells separated from the splenocytes of RM-1 tumor–bearing mice (10 per group)—which either were not treated or were treated with tumor lysate–pulsed dendritic cells, anti-CD27 antibody, or a combination of tumor lysate–pulsed dendritic cells with anti-CD27 antibody—were stimulated in vitro with RM-1 tumor lysate–pulsed dendritic cells for 5 days. The primed CD8+ T cells (effector cells) were harvested and cocultured with 51Cr-labeled RM-1 tumor cells (target cells) at an effector-to-target cell ratio of 100:1 for 4 hours. Cytotoxic T-lymphocyte activity against RM-1 tumor cells was determined by the 51Cr release assay. Results were shown as the percentage of target cell lysis. *P < 0.05 compared with control group; **P < 0.05 compared with all other groups.
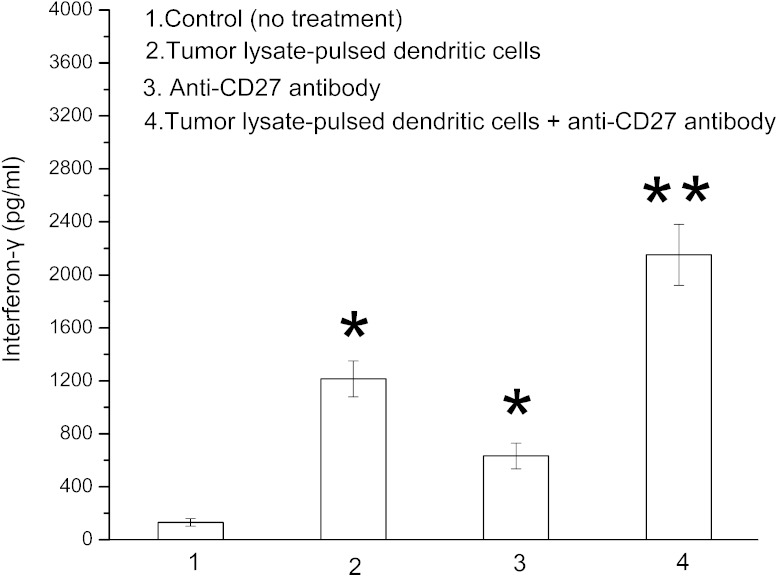
Combination therapy improves interferon-γ level
The interferon-γ level in tumor lysate–pulsed dendritic cell mice or anti-CD27 antibody–only mice was significantly increased in comparison with control (untreated) mice (P < 0.05; Fig. 6). The combination treatment with tumor lysate–pulsed dendritic cells and anti-CD27 antibody caused a much higher interferon-γ level than either monotherapy (P < 0.05; Fig. 6).
Fig. 6.
Effect of tumor lysate–pulsed dendritic cells and anti-CD27 antibody on interferon-γ production. RM-1 tumor–bearing mice (10 per group) either were not treated or were treated with tumor lysate–pulsed dendritic cells, anti-CD27 antibody, or tumor lysate–pulsed dendritic cells plus anti-CD27 antibody. CD4+ T cells separated from the splenocytes of untreated or differently treated mice were stimulated in vitro with RM-1 tumor lysate–pulsed dendritic cells for 24 hours. Supernatants were harvested, and interferon-γ level was measured by enzyme-linked immunosorbent assay kit. *P < 0.05 compared with control group; *P < 0.05 compared with all other groups.
Discussion
Dendritic cell–based vaccine has been applied clinically for the treatment of metastatic castration-resistant prostate cancer.9 However, the overall clinical benefit of this vaccine remains moderate. A resting T cell expresses a small amount of CD27, which can be greatly enhanced upon T-cell activation.16 Ligation of CD27 by anti-CD27 monoclonal antibody provides costimulatory signals for T-cell proliferation and activation.17 It further enhances T-cell survival and effector function.17 Previous studies have demonstrated that anti-CD27 antibody can exert a potent antitumor effect.18–21 For the reason mentioned in the introduction, we hypothesized that anti-CD27 antibody may enhance the antitumor effect mediated by dendritic cell–based vaccine. Our study showed that CD27 expression on T cells was up-regulated after mice were immunized with tumor lysate–pulsed dendritic cells (i.e., dendritic cell–based vaccine; Fig. 2), suggesting that tumor lysate–pulsed dendritic cells activated T cells and induced up-regulation of CD27 expression on T cells. The result provided a scientific rationale for subsequent administration of anti-CD27 antibody. In addition, the present study found that the combined treatment with tumor lysate–pulsed dendritic cells and anti-CD27 antibody significantly reduced tumor growth compared with monotherapy with tumor lysate–pulsed dendritic cells or anti-CD27 antibody (Fig. 3). These data demonstrate that administration of anti-CD27 antibody augments the antitumor effect of tumor lysate–pulsed dendritic cells.
The antitumor immunologic responses can be monitored by different approaches, such as T-cell proliferation assay, cytotoxicity test, and enzyme-linked immunosorbent assay.25 In our study, a stronger T-cell proliferation was observed in mice that received combination therapy with tumor lysate-pulsed dendritic cells and anti-CD27 antibody than those treated with tumor lysate–pulsed dendritic cells or anti-CD27 antibody alone (Fig. 4). These results suggest that combined therapy enhances T-cell proliferation. Moreover, mice undergoing combination therapy showed a higher cytotoxic T-lymphocyte activity compared with mice immunized with tumor lysate-pulsed dendritic cells or anti-CD27 antibody alone (Fig. 5), implying that combination therapy improves CD8+ T-cell activity to destroy tumor cells. Cytokine levels were determined by an enzyme-linked immunosorbent assay. The cytokine interferon-γ levels were higher in mice receiving combination treatment than in mice treated with tumor lysate-pulsed dendritic cells or anti-CD27 antibody alone (Fig. 6), suggesting that combination treatment increases CD4+ T cell activity to release interferon-γ. The interferon-γ can provide help for CD8+ T cell activation.13 After considering all these results, we think that combined treatment strengthens antitumor efficacy by improving T-cell proliferation and activity.
It is well known that tumor cells can produce various immunosuppressive factors, including vascular endothelial growth factor, transforming growth factor-β, and interleukin-10.26 These immunosuppressive factors can suppress the maturation of dendritic cells in vivo as well as their ability to present tumor antigens and to activate T cells, thereby diminishing antitumor immunity.27 These findings provide the rationale for the use of in vitro–generated dendritic cells. A population of immature dendritic cells can be generated in vitro by differentiation of bone marrow cells by the addition of granulocyte-macrophage colony–stimulating factor and interleukin-4 for 5 to 7 days.28 The immature dendritic cells can be pulsed with tumor antigen and further differentiated into mature dendritic cells by the addition of maturation stimulus, such as tumor necrosis factor-α, interferon-γ, prostaglandin E2, or lipopolysaccharide.28 In the present study, generated cells expressed high levels of CD11c, which is a characteristic of dendritic cells (Fig. 1). The result suggests that the generated cells are dendritic cells. In most clinical studies, immature or mature dendritic cells have been used. However, studies comparing the immunogenic potential of immature versus mature dendritic cells show that maturation is essential for the induction of full antitumor immunity.14 Furthermore, recent studies have reported that the use of mature dendritic cells can produce a better clinical outcome.9 Therefore, mature dendritic cells (i.e., RM-1 tumor lysate–pulsed dendritic cells) were used in our study. The mature dendritic cells express high levels of CD83 (maturation marker of dendritic cells; Fig. 1).
Results from numerous studies have indicated that dendritic cells pulsed with tumor antigens, such as tumor lysate, defined tumor-associated peptides, apoptotic or necrotic tumor cells, or tumor RNA, are capable of eliciting specific antitumor T-cell responses.29 In the current experiment, tumor lysate was used as tumor antigen. The use of tumor lysate has two important advantages. Tumor lysate contains the entire repertoire of tumor-associated antigens. It provides the ability to sensitize T cells to the entire spectrum of tumor-associated antigens, thereby reducing the possibility of tumor escape from immune recognition. Furthermore, the use of tumor lysate circumvents the need for the identification of tumor-associated antigens.
Our data showed that CD27 expression on T cells increased after mice were immunized with tumor lysate–pulsed dendritic cells, and it generally peaked on day 3 (Fig. 2B). Subsequently, a gradual overall reduction in CD27 expression was observed (Fig. 2B). For this reason, in the combined treatment group anti-CD27 antibody was injected 3 days after therapy along with tumor lysate–pulsed dendritic cells. Some reports have indicated that anti-CD27 antibody at a dose of 100 μg is effective in the treatment of mouse tumors, such as lymphoma and melanoma.17,18 Therefore, this dose was chosen in our mouse prostate cancer model.
Conclusions
The findings of our study present the first evidence that a dendritic cell–based vaccine in combination with anti-CD27 antibody can strengthen antitumor efficacy in prostate cancer–bearing mice by improving T-cell proliferation and activity. Therefore, it is reasonable to suggest that combined treatment may have clinical applicability in patients with prostate cancer. For this purpose, further clinical studies will be required.
Acknowledgments
This work was supported by grants from the Medical Science and Technology Planning Project of Zhejiang Province (2014KYA051), the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talent, and the Undergraduate Scientific and Technological Innovation Project (i.e., Xinmiao Talent Project) of Zhejiang Province (2014R434009), China. Si-Ming Wei, Jin-Xuan Fei, and Feng Tao contributed equally to this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.2012;62(1):10–29. [Google Scholar]
- 3.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20(15):3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 4.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 5.Stamey TA, Yemoto CM, McNeal JE, Sigal BM, Johnstone IM. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163(4):1155–1160. doi: 10.1016/s0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 6.Wesley JD, Whitmore J, Trager J, Sheikh N. An overview of sipuleucel-T: autologous cellular immunotherapy for prostate cancer. Hum Vaccin Immunother. 2012;8(4):520–527. doi: 10.4161/hv.18769. [DOI] [PubMed] [Google Scholar]
- 7.Daniels-Wells TR, Helguera G, Leuchter RK, Quintero R, Kozman M, Rodríguez JA, et al. A novel IgE antibody targeting the prostate-specific antigen as a potential prostate cancer therapy. BMC Cancer. 2013;13:195. doi: 10.1186/1471-2407-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jachetti E, Mazzoleni S, Grioni M, Ricupito A, Brambillasca C, Generoso L, et al. Prostate cancer stem cells are targets of both innate and adaptive immunity and elicit tumor-specific immune responses. Oncoimmunology. 2013;2(5):e24520. doi: 10.4161/onci.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Sæter T, Vlatkovic L, Servoll E, Waaler G, Axcrona U, et al. Dendritic and lymphocytic cell infiltration in prostate carcinoma. Histol Histopathol. 2013;28(12):1621–1628. doi: 10.14670/HH-28.1621. [DOI] [PubMed] [Google Scholar]
- 10.Draube A, Klein-González N, Mattheus S, Brillant C, Hellmich M, Engert A, et al. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS One. 2011;6(4):e18801. doi: 10.1371/journal.pone.0018801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantia-Smaldone GM, Chu CS. A review of dendritic cell therapy for cancer: progress and challenges. BioDrugs. 2013;27(5):453–468. doi: 10.1007/s40259-013-0030-9. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal N, Padmanabh S, Vogelzang NJ. Development of novel immune interventions for prostate cancer. Clin Genitourin Cancer. 2012;10(2):84–92. doi: 10.1016/j.clgc.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Xiao L, Joo KI, Lim M, Wang P. Dendritic cell-directed vaccination with a lentivector encoding PSCA for prostate cancer in mice. PLoS One. 2012;7(11):e48866. doi: 10.1371/journal.pone.0048866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafer-Weaver KA, Watkins SK, Anderson MJ, Draper LJ, Malyguine A, Alvord WG, et al. Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. Cancer Res. 2009;69(15):6256–6264. doi: 10.1158/0008-5472.CAN-08-4516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Kim JH, Lee Y, Bae YS, Kim WS, Kim K, Im HY, et al. Phase I/II study of immunotherapy using autologous tumor lysate-pulsed dendritic cells in patients with metastatic renal cell carcinoma. Clin Immunol. 2007;125(3):257–267. doi: 10.1016/j.clim.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Bozdogan G, Dogu F, Güloglu D, Yuksek M, Aytekin C, Ikinciogullari A. CD27 expression on lymphocyte and sCD27 levels in children with asthma. Allergol Immunopathol (Madr) 2010;38(6):327–332. doi: 10.1016/j.aller.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Taraban VY, Rowley TF, Kerr JP, Willoughby JE, Johnson PM, Al-Shamkhani A, et al. CD27 costimulation contributes substantially to the expansion of functional memory CD8(+) T cells after peptide immunization. Eur J Immunol. 2013;43(12):3314–3323. doi: 10.1002/eji.201343579. [DOI] [PubMed] [Google Scholar]
- 18.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33(8):769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakanishi T, Yagita H. Anti-tumor effects of depleting and non-depleting anti-CD27 monoclonal antibodies in immune-competent mice. Biochem Biophys Res Commun. 2010;393(4):829–835. doi: 10.1016/j.bbrc.2010.02.092. [DOI] [PubMed] [Google Scholar]
- 20.He LZ, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191(8):4174–4183. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]
- 21.French RR, Taraban VY, Crowther GR, Rowley TF, Gray JC, Johnson PW, et al. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109(11):4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 22.Vitale LA, He LZ, Thomas LJ, Widger J, Weidlick J, Crocker A, et al. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res. 2012;18(14):3812–3821. doi: 10.1158/1078-0432.CCR-11-3308. [DOI] [PubMed] [Google Scholar]
- 23.Lim DS, Kim JH, Lee DS, Yoon CH, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother. 2007;56(11):1817–1829. doi: 10.1007/s00262-007-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirtskhalaishvili G, Shurin GV, Gambotto A, Esche C, Wahl M, Yurkovetsky ZR, et al. Transduction of dendritic cells with Bcl-xL increases their resistance to prostate cancer-induced apoptosis and antitumor effect in mice. J Immunol. 2000;165(4):1956–1964. doi: 10.4049/jimmunol.165.4.1956. [DOI] [PubMed] [Google Scholar]
- 25.Williams BJ, Bhatia S, Adams LK, Boling S, Carroll JL, Li XL, et al. Dendritic cell based PSMA immunotherapy for prostate cancer using a CD40-targeted adenovirus vector. PLoS One. 2012;7(10):e46981. doi: 10.1371/journal.pone.0046981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thara E, Dorff TB, Averia-Suboc M, Luther M, Reed ME, Pinski JK, et al. Immune response to sipuleucel-T in prostate cancer. Cancers (Basel) 2012;4(2):420–441. doi: 10.3390/cancers4040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang SY, Thara E, Quinn DI, Dorff TB. Blood cells and their use in active immunotherapy of prostate cancer. Hum Vaccin Immunother. 2012;8(4):528–533. doi: 10.4161/hv.19188. [DOI] [PubMed] [Google Scholar]
- 28.Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Y, Wang C, Wei W, Shen C, Deng X, Chen L, et al. Dendritic cells pulsed with leukemia cell-derived exosomes more efficiently induce antileukemic immunities. PLoS One. 2014;9(3):e91463. doi: 10.1371/journal.pone.0091463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1(7):1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]