Abstract
A 53-year-old man with multiple liver metastasis of esophageal cancer underwent four courses of chemotherapy. After four courses of chemotherapy, positron emission tomography showed progressive disease. Because it was difficult to control the cancer only by chemotherapy, we performed proton beam therapy (PBT) combined with chemotherapy. The irradiated parts were the primary tumor, liver metastases (S2/S4/S6), and mediastinal lymph nodes. The primary tumor including the mediastinal lymph nodes and the S2/S4/S6 metastases received proton beam irradiation at a total dose of 68.2 Gy in 31 fractions and 66.0 Gy in 30 fractions, respectively, according to tumor location. This resulted in a complete response as shown by positron emission tomography. In our experience, PBT exerted a curative effect on liver metastases of esophageal cancer. It is thought that PBT may be effective in the treatment of esophageal cancer. This is the first report about PBT for liver metastases of esophageal cancer.
Key words: proton beam therapy, esophageal cancer, liver metastasis
Esophageal cancer is one of the most difficult malignancies to cure because of its early metastasis to lymph nodes and distant organs. Radiotherapy was developed during the last decade, and particle radiation therapy has come into use for the treatment of cancer in clinical practice. Proton beam therapy (PBT) is effective because protons have excellent dose localization according to the Bragg peak compared with photons, and are biologically equivalent to conventional X-ray for cancer.1 Indications for PBT include ocular melanoma, head and neck cancer, lung cancer, esophageal cancer, and others. However, there are currently no reports of PBT for liver metastases of esophageal cancer despite reports on the utility of PBT for hepatocellular carcinoma and other cancers in clinical practice. In this manuscript, we present a case in which PBT was effective for multiple liver metastases of esophageal cancer.
Case Report
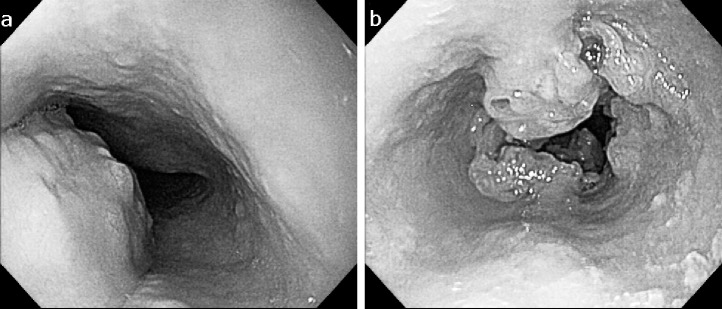
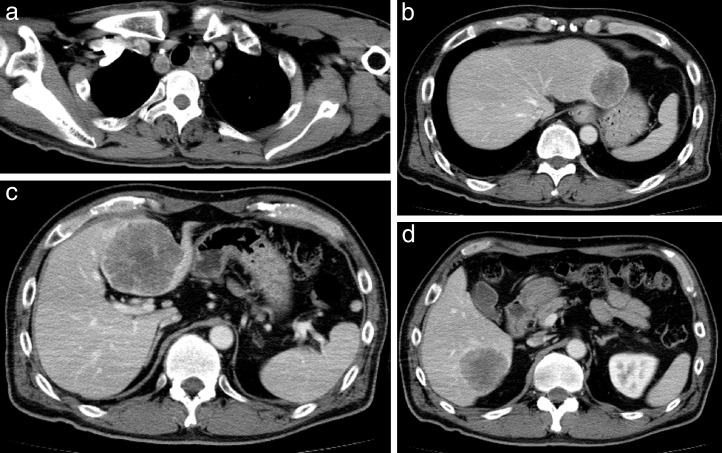
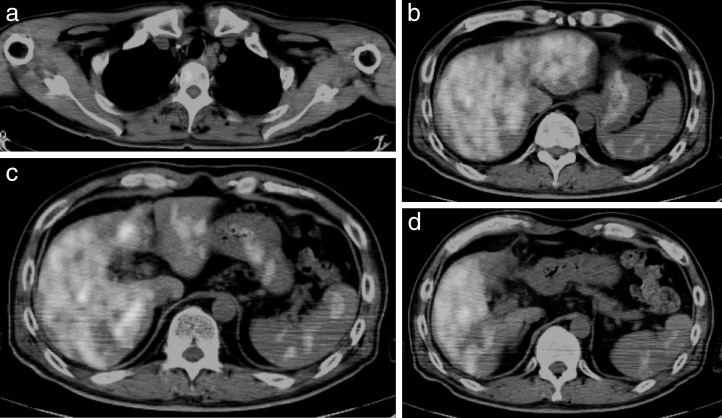
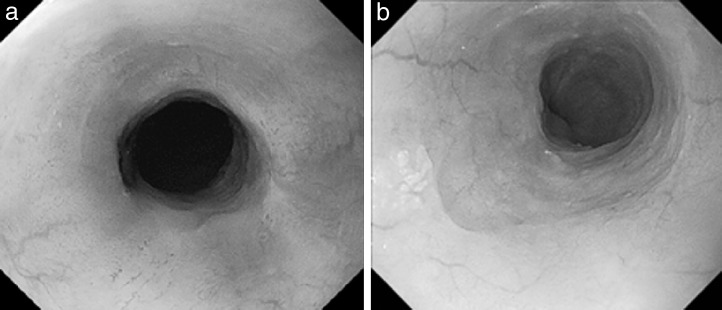
A 53-year-old man with no history of health disorders presented to another clinic with complaints of heartburn and choking. He underwent esophagogastroduodenoscopy, and cancer of the esophagogastric (EG) junction and upper thoracic esophagus was discovered (Fig. 1A and 1B). In addition, multiple liver metastases and mediastinal lymph node metastases were detected by computed tomography (CT). There were three liver metastases (45.5 mm in S2, 67.5 mm in S4, and 48.3 mm in S6) and one mediastinal lymph node metastasis (17.3 mm in the upper mediastinum; Fig. 2A–2D). He was then referred to our hospital.
Fig. 1.
The first esophagogastroduodenoscopy before PBT. (A) A type 1 primary tumor is located in the upper thoracic esophagus. (B) Another type 3 primary tumor is located in the lower thoracic esophagus.
Fig. 2.
CT before proton beam therapy. CT shows three liver metastases: (a) S2, 45.5 mm; (b) S4, 67.5 mm; and (c) S6, 48.3 mm. (d) Lymph node metastasis in the upper mediastinum is also present (17.3 mm).
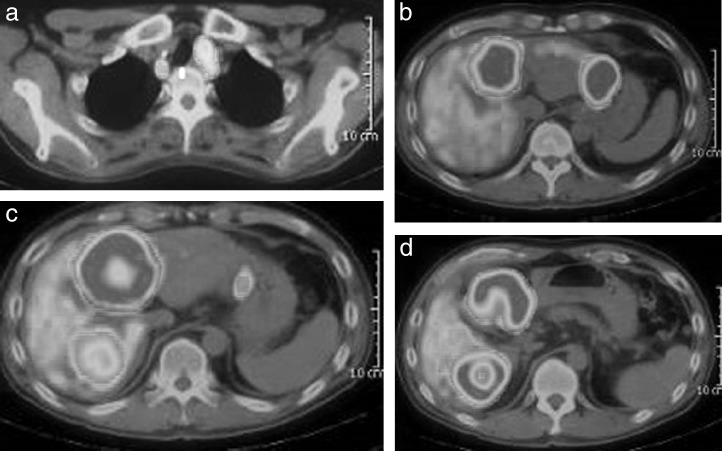
Routine laboratory investigation results, including tumor marker levels, were normal with the exception of an increased lactate dehydrogenase level in November 2011. Positron emission tomography (PET) demonstrated accumulation of fluorodeoxyglucose (FDG) in the liver (S2/S4/S6) and primary cancers at the EG junction, upper thoracic esophagus, and left supraclavicular lymph node (Fig. 3A–3D). Based on these results, we diagnosed lower thoracic esophagus T3N1M1 (liver) Stage IV and upper thoracic esophagus T3 cancer (UICC/TNM Classification of Malignant Tumors, 7th edition) and planned chemotherapy.
Fig. 3.
PET before proton beam therapy. Accumulations of fluorodeoxyglucose are demonstrated in the upper thoracic esophagus, including: (a) a lymph node; (b) S2; (c) S4; and (d) S6 of the liver.
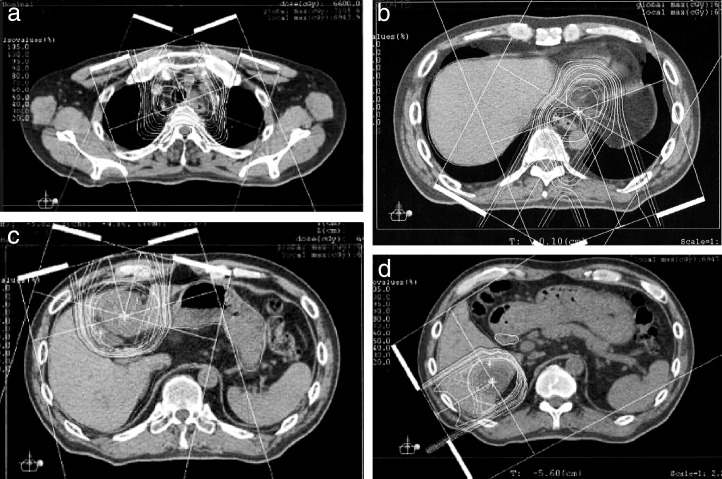
DCF (docetaxel: 70 mg/m2; cisplatin: 70 mg/m2; 5-FU: 700 mg/m2) was started in February 2012. We used the World Health Organization (WHO) Response Evaluation Criteria in Solid Tumors (RECIST) to estimate the value of chemotherapy. Progressive disease remained after four courses; thus, we applied PBT combined with chemotherapy. PBT was carried out in the Southern Tohoku Proton Therapy Center. Proton beams were delivered through two oblique ports. First, the primary tumor and left supraclavicular lymph node and S6 liver metastases were treated with 68.2 Gy in 31 fractions and 66.0 Gy in 30 fractions, respectively. Next, DNF (docetaxel: 100 mg/m2; nedaplatin: 160 mg/m2; 5-FU: 1000 mg/m2) was administered. Finally, the S2/S4 liver metastases were treated with 66.0 Gy in 30 fractions (Fig. 4A–4D). Approximately 2 months after the administration of PBT combined with chemotherapy, PET revealed the disappearance of FDG accumulation in each liver metastasis site, the primary tumor, and the mediastinal lymph nodes (Fig. 5A–5D). Because CT and PET showed that all target lesions (S2, S4, S6, and upper mediastinal lymph node) were completely abolished and esophagogastroduodenoscopy showed that the nontarget lesions (primary tumors in the upper thoracic esophagus and EG junction) were obliterated, the outcome of the esophageal cancer was graded as a complete response (CR) according to RECIST (Figs. 5A–5D, 6A, and 6B). There were no acute or late toxicities associated with treatment. After PBT, the patient received adjuvant chemotherapy in our hospital and the CR has been maintained for 10 months.
Fig. 4.
CT for proton beam therapy planning. (a) The primary tumor in the upper thoracic esophagus, including the left supraclavicular lymph node, was treated with 68.2 Gy in 31 fractions. Three liver metastases, S2 (b), S4 (c), and S6 (d) were treated with 66.0 Gy in 30 fractions.
Fig. 5.
PET after proton beam therapy. Proton beam therapy resulted in the disappearance of fluorodeoxyglucose in all targets: the primary tumor located in the upper thoracic esophagus, including: (a) a lymph node; (b) S2; (c) S4; and (d) S6 of the liver.
Fig. 6.
Esophagogastroduodenoscopy after proton beam therapy. The primary tumors located in the (a) upper and (b) lower thoracic esophagus have disappeared, and no malignancy is present in biopsy specimens.
Discussion
It is well known that chemoradiotherapy results in significant improvement in the prognosis of esophageal cancer. Recently, other than conventional irradiation with photons, PBT has attracted attention and is widely expected to deliver a higher dose to the target while decreasing the side effects.1
The present patient was treated with PBT combined with chemotherapy. Although conventional irradiation cannot provide a target with an adequate dose because of the high incidence of adverse events, protons can be spread in a sufficient dose. To our knowledge, there are no studies on the treatment of liver metastases of esophageal cancer by PBT, although it has come to be used for metastases of the lung and liver at some facilities. Although we have experienced only one case of a solitary liver metastasis from esophageal cancer treated by conventional radiation,2 this is the first report of radiotherapy for multiple liver metastases of esophageal cancer and PBT for liver metastases of esophageal cancer.
Among primary liver tumors, PBT for hepatocellular carcinoma showed good outcomes in which 5-year local progression-free rates were over 85%.3 In addition, a prospective phase II trial of high-dose PBT for patients with hepatocellular carcinoma targeting those with cirrhosis showed good results; the median progression-free survival time for the entire group of patients was 36 months, and the 3-year progression-free survival rate was 60%.4 We thought that these results were established due to the ability to deliver a high dose to the target. These studies show the therapeutic superiority of PBT over alternative treatment options for ocular melanoma, neck and head cancer, lung cancer, esophageal cancer, prostate cancer, and others.
Some studies have reported that PBT decreased adverse events.3,4 In particular, there are fewer reports of adverse events such as liver dysfunction and gastrointestinal tract disturbances above grade 3 compared with conventional radiotherapy.1,3–5 Furthermore, in a phase II trial, acute toxicity during PBT was minimal and included only grade 1 erythema and mild fatigue. No acute toxicities disrupted the PBT. After treatment, there were also no radiotherapy-induced toxicities above grade 3; there were only grade 2 gastrointestinal adverse events comprising ulceration and bleeding.4
The most common treatment strategy for inoperable esophageal cancer is currently palliative chemotherapy or best supportive care, which is administered according to the patient's stage of progression or general condition. It is difficult to establish a clinical chemoradiotherapy regimen for patients with metastases of esophageal cancer because of the high rate of adverse events and low curability. In the present case, we achieved CR in all target-innovated PBT combined with chemotherapy without acute critical troubles. We believe this case will have a considerable impact on therapeutic strategies. PBT is still included in highly advanced medical technology, and many patients in Japan cannot undergo PBT for economic reasons. It is hoped that these problems are resolved, multicenter trials are carried out, and indications are expanded.
Acknowledgments
The authors declare that they have no conflict of interest.
References
- 1.Hata M, Tokuuye K, Sugahara S, Tohno E, Nakayama H, Fukumitsu N, et al. Proton beam therapy for aged patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69(3):805–812. doi: 10.1016/j.ijrobp.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda Y, Niimi M, Kan S, Shatari T, Takami H, Kodaira S, et al. Conformal radiation therapy for liver metastasis of esophageal carcinoma. Hepatogastroenterology. 2003;50(50):532–534. [PubMed] [Google Scholar]
- 3.Chiba T, Tokuuye K, Matsuzaki Y, Sugahara S, Chuganji Y, Kagei K, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162patients. Clin Cancer Res. 2005;11(10):3799–3805. doi: 10.1158/1078-0432.CCR-04-1350. [DOI] [PubMed] [Google Scholar]
- 4.Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117(13):3053–3059. doi: 10.1002/cncr.25809. [DOI] [PubMed] [Google Scholar]
- 5.Mizumoto M, Okumura T, Hashimoto T, Fukuda K, Oshiro Y, Fukumitsu N, et al. Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(3):e529–e535. doi: 10.1016/j.ijrobp.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 6.Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. PhaseIIstudy of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23(9):1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 8.Hata M, Tokuuye K, Sugahara S, Fukumitsu N, Hashimoto T, Ohnishi K, et al. Proton beam therapy for hepatocellular carcinoma patients with severe cirrhosis. Strahlenther Onkol. 2006;182(12):713–720. doi: 10.1007/s00066-006-1564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oshiro Y, Mizumoto M, Okumura T, Hashimoto T, Fukumitsu N, Ohkawa A, et al. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2012;7(2):370–375. doi: 10.1097/JTO.0b013e31823c485f. [DOI] [PubMed] [Google Scholar]