Abstract
Cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) is a radical but effective treatment option for select peritoneal malignancies. We sought to determine our early experience with this method for peritoneal carcinomatosis secondary to mucinous adenocarcinomas of appendiceal origin. As such, we performed a retrospective clinical study of 30 consecutive patients undergoing CRS with planned HIPEC at the Princess Alexandra Hospital, between June 2009 to December 2012, with mucinous adenocarcinomas of the appendix. CRS was performed in 30 patients, 13 received HIPEC intraoperatively and 17 received early postoperative intra-peritoneal chemotherapy (EPIC) in addition. Mean age was 52.3 years and median hospital stay was 26 days (range 12–190 days). Peritoneal cancer index scores were 0–10 in 6.7% of patients, 11–20 in 20% of patients and >20 in 73.3% of patients. Complete cytoreduction was achieved overall in 21 patients. In total, 106 complications were observed in 28 patients. Ten were grade 3-A, five were grade 3-B and one grade-5 secondary to a fatal PE on day 97. In patients who received HIPEC, there was no difference in disease-free survival (P = 0.098) or overall survival (P = 0.645) between those who received EPIC versus those who did not. This study demonstrates that satisfactory outcomes with regards to morbidity and survival can be achieved with CRS and HIPEC, at a single-centre institution with growing expertise in the technique. Our results are comparable with outcomes previously described in the international literature.
Key words: Pseudomyxoma peritonei, Cytoreductive surgery, Heated intraperitoneal chemotherapy, Early postoperative intraperitoneal chemotherapy
The combination of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) is an effective treatment option for select peritoneal malignancies.1,2 The management centers on improving survival for a historically palliative disease. CRS aims to achieve complete tumor clearance, requiring extensive peritoneal and visceral resection. Once optimal cytoreduction is complete, HIPEC is employed intraoperatively and is selectively followed by early postoperative intraperitoneal chemotherapy (EPIC). Direct delivery into the peritoneal cavity facilitates higher regional dosing and, because of decreased systemic exposure,3 is achieved with fewer toxicities, although the role for EPIC following HIPEC remains debatable.4
Such an aggressive treatment comes with significant potential morbidity; thus, appropriate patient selection is vital.5 A considerable learning curve exists.6 However, with increasing experience, an institution can significantly reduce its morbidity and mortality.7
Our aim was to evaluate the outcomes early in our experience with CRS and HIPEC at the Princess Alexandra Hospital, specifically for mucinous adenocarcinoma of the appendix, and compare these outcomes with those reported in the literature globally for primary end points such as morbidity, mortality, and survival. We further sought to determine the role of HIPEC with or without EPIC because the literature remains ambivalent on this topic to date.
Patients and Methods
A retrospective analysis of medical records was performed on all consecutive patients with peritoneal carcinomatosis of appendiceal origin who underwent CRS between June 2009 and December 2012. Selection for surgery was on a case-by-case basis in a multidisciplinary team setting, led by the two senior surgeons. All appendiceal malignancies encountered were classified in accordance with the World Health Organization classification for tumors of the digestive system.8 These were further classified into either low grade or high grade depending on the level of cytoarchitectural atypia. Suitability for resection was evaluated based on available tumor histopathology, radiology, and extent of disease evident at diagnostic laparoscopy, objectively classified using the peritoneal cancer index (PCI). The medical oncology team assessed suitability for HIPEC and/or EPIC. Patients with extraperitoneal metastatic disease were excluded from treatment unless simultaneous resection of metastases was considered feasible. Ethics approval was obtained for the study through the local ethics committee (Metro South Human Research Ethics Committee: HREC/13/QPAH/63).
Cytoreductive surgery
The goal of CRS is to resect all visible peritoneal disease, and the procedure is composed of a series of resections dependent on disease dissemination. Abdominal resections were followed in accordance with Sugarbaker.9 This approach often employed splenectomy, cholecystectomy, and stripping of the omental bursa peritoneum. Other visceral resections variably included ascending and descending colon, uterus, ovaries, stomach, gallbladder, transverse colon, and small bowel (depending on serosal involvement). Such aggressive multiorgan resection was only indicated if complete cytoreduction was achievable.
The PCI is a score calculated after determining the extent of disease in 13 defined regions of the abdomen and pelvis, giving a possible score of between 1 and a maximum of 39. Intraoperative calculation of the PCI was determined as a marker of presurgical disease burden.10 In 3 of the early patients included in the study, where an intraoperative calculation of PCI was not performed, the treating surgeon calculated a score retrospectively after evaluation of preoperative imaging, histopathology results, and the operative report.
The degree of tumor clearance, or “completeness of cytoreduction,” was determined intraoperatively using the CC score.10 CC-0 indicates no visible residual disease is present. CC-1 describes remaining tumor nodules of <2.5 mm. CC-2 describes residual tumor nodules between 2.5 mm and 2.5 cm, and CC-3 describes residual tumor >2.5 cm. Both CC-0 and CC-1 are considered to be complete cytoreduction in mucinous appendiceal adenocarcinoma, because any residual tumor <2.5 mm can be theoretically effectively eradicated through the use of HIPEC.11 Incomplete clearance indicates gross macroscopic residual disease, where attempts at debulking will contribute to greater morbidity, without long-term benefit.3
Heated intraperitoneal chemotherapy
Intraperitoneal chemotherapy aims to complement surgical resection by eradicating free tumor cells or persistent nodules <2.5 mm in size. Increased dose intensity is facilitated by intraperitoneal administration, which limits systemic absorption because of the peritoneal-plasma barrier, thus minimizing toxicity while maximizing efficacy.3 We used the “open coliseum” technique for intraperitoneal administration,12 which permits an even spread of the chemotherapeutic agent throughout the abdominal cavity.
Mitomycin C was infused at 35 mg/m2 body surface area after CRS, with a capped dose of 140 mg. Mean infusion temperature was 39.5°C for 90 minutes. Necessary anastomoses were completed after cessation of HIPEC. Intra-abdominal drains remained in a dependent position to facilitate ongoing drainage and permitted EPIC in select patients. For EPIC, 5-fluorouracil was administered at 500 mg/m2 body surface area and was left in the abdominal cavity to dwell for up to 23 hours. EPIC was repeated on days 2 to 6 in those patients who could tolerate it.
Clinical variables
Patient clinical data were collected retrospectively from medical records and a chemotherapy database. Preoperative demographic indicators examined included age, sex, Eastern Cooperative Oncology Group (ECOG) status (a measure of premorbid functional status), disease duration), tumor markers [carcinoembryonic antigen (CEA) and cancer antigen 19.9 (CA-19.9)] and histopathologic grade. Intraoperative indicators included operation duration, intraoperative transfusion requirements, procedures performed intraoperatively, PCI score, and CC score. Postoperative complications were reported and graded using the Clavien and Dindo classification.13 In patients who had complete cytoreduction, disease-free survival (DFS) was recorded as time from date of surgery to date of recurrence, death, or last date of follow-up. Overall survival (OS) was recorded as time from date of surgery to date of death or date of last follow-up. Patient data on survival and recurrence were acquired through clinical follow-up and evaluation of the death registry.
Statistics
Where appropriate, patient clinical variables were summarized with a count (n) and percentage, mean (±SD), or median (range). Univariate analysis was performed to evaluate the relationship of relevant clinical variables with morbidity (hospital stay, highest complication, total complications), mortality, DFS, and OS. For continuous variables, negative binomial regression analysis was used. For categoric variables, logistic regression analysis and ordinal regression analysis were performed as appropriate. DFS and OS were expressed as Kaplan-Meier curves for selected variables. Mantel-Cox regression and χ2 analysis were performed to evaluate the level of significance for each variable where appropriate. Statistical significance was defined as P < 0.05. Data were analyzed using SPSS for Windows v.17 (SPSS, Chicago, Illinois).
Results
A total of 30 patients received CRS and HIPEC at the Princess Alexandra Hospital between June 2009 and December 2012 (Table 1). The mean age was 53.3 years (12.87 years) for 16 men and 14 women. Median disease duration preoperatively was 5 months (1–75 months) and the ECOG status was ≤1 in 93% of patients (Table 1).
Table 1.
Patient characteristicsa
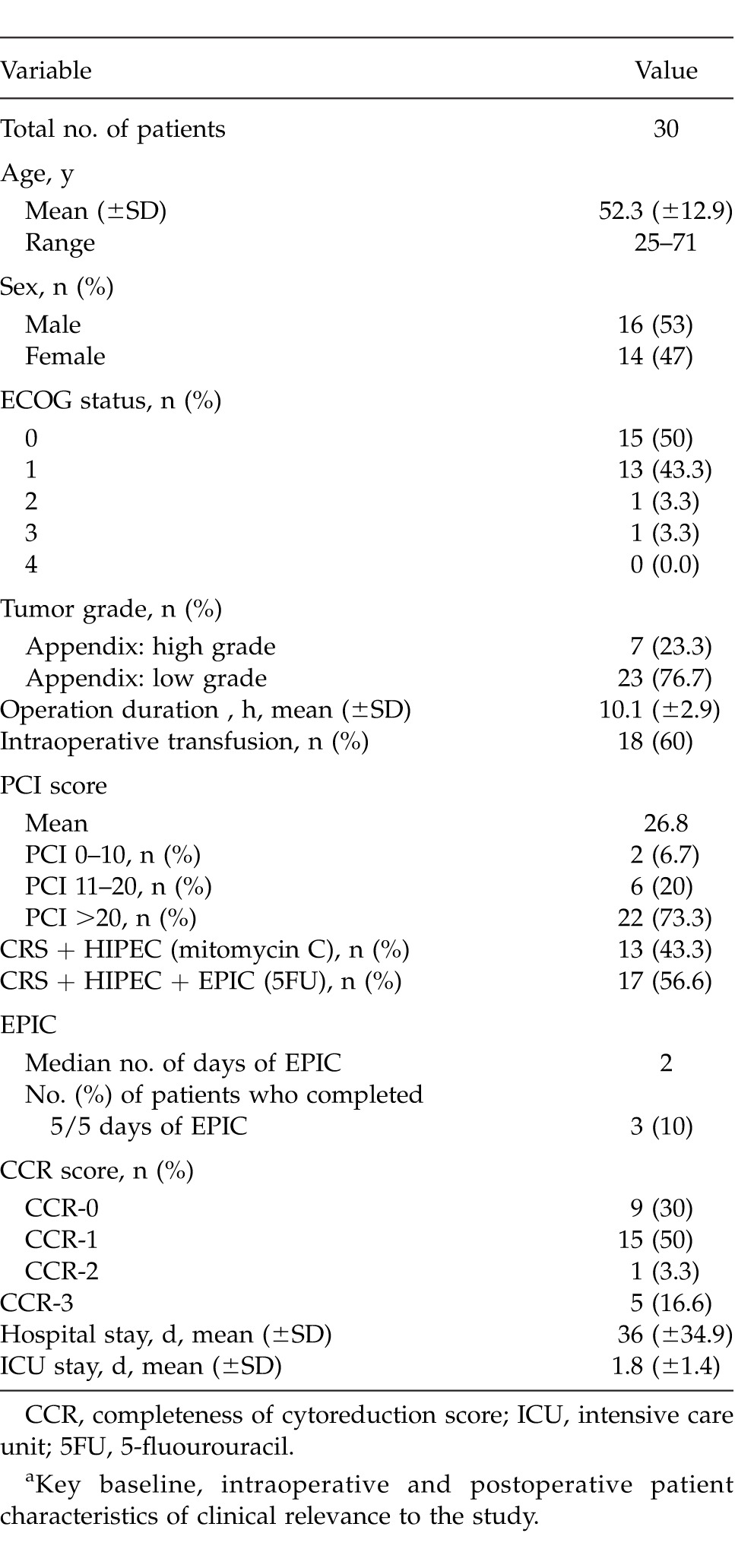
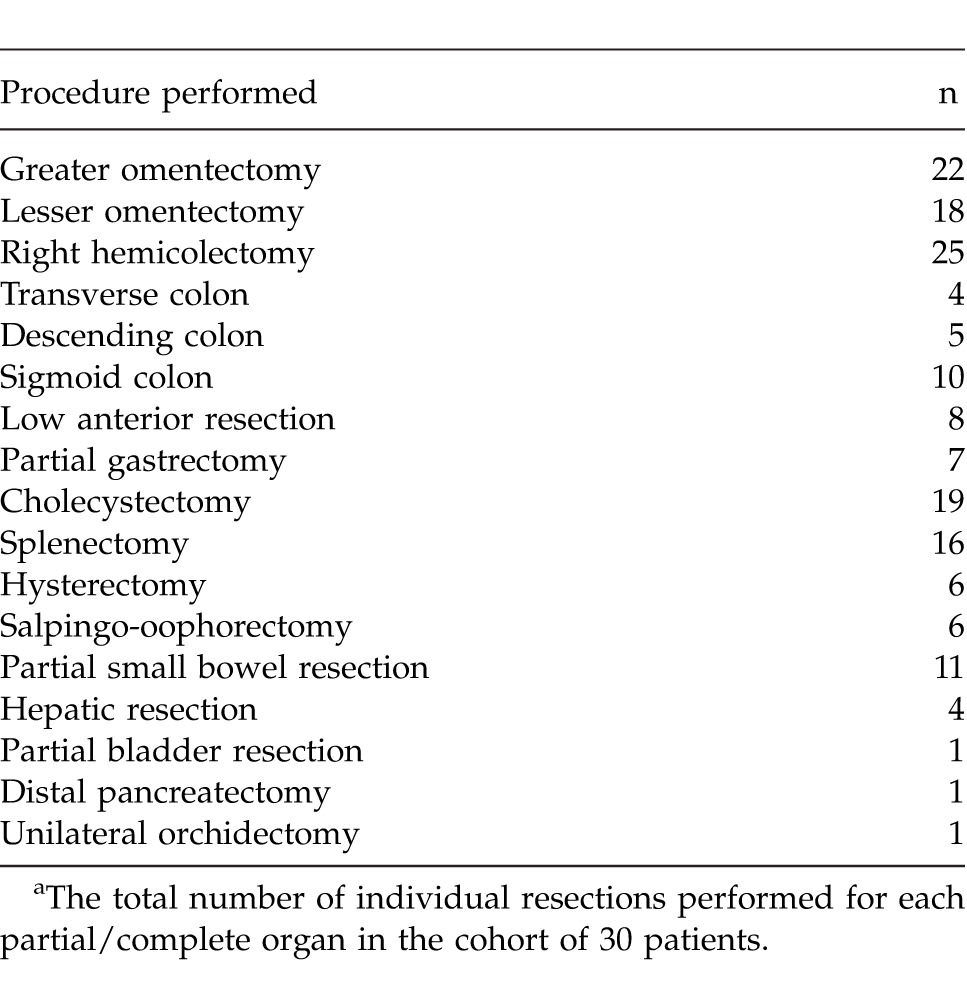
High-grade adenocarcinoma of the appendix accounted for tumor origin in 7 patients (23.3%), with low-grade adenocarcinoma in 23 patients. In patients who had preoperative measurement of tumor markers CEA (n = 26) and CA-19.9 (n = 30), elevations were observed in 15 patients (57.7%) and 8 patients (26.7%), respectively. One patient was initially treated as having ovarian cancer and received 6 cycles of carboplatin/paclitaxel prior to CRS. The PCI was 0 to 10 in 2 patients (6.7%), 11 to 20 in 6 patients (20%), and >20 in 22 patients (73.3%). Details of the surgical resections performed can be found in Table 2. Adequate completeness of cytoreduction (CC score 0–1) was achieved in 21 patients (70%).
Table 2.
Total visceral resections performeda
A total of 17 patients (56.7%) received EPIC following CRS and HIPEC, with a median administration of 2 days (1–5 days). Reasons why EPIC was either not given or not completed include incomplete cytoreduction (23%), surgical complications/illness (50%), patient refusal (15.4%), and incomplete documentation (11.5%).
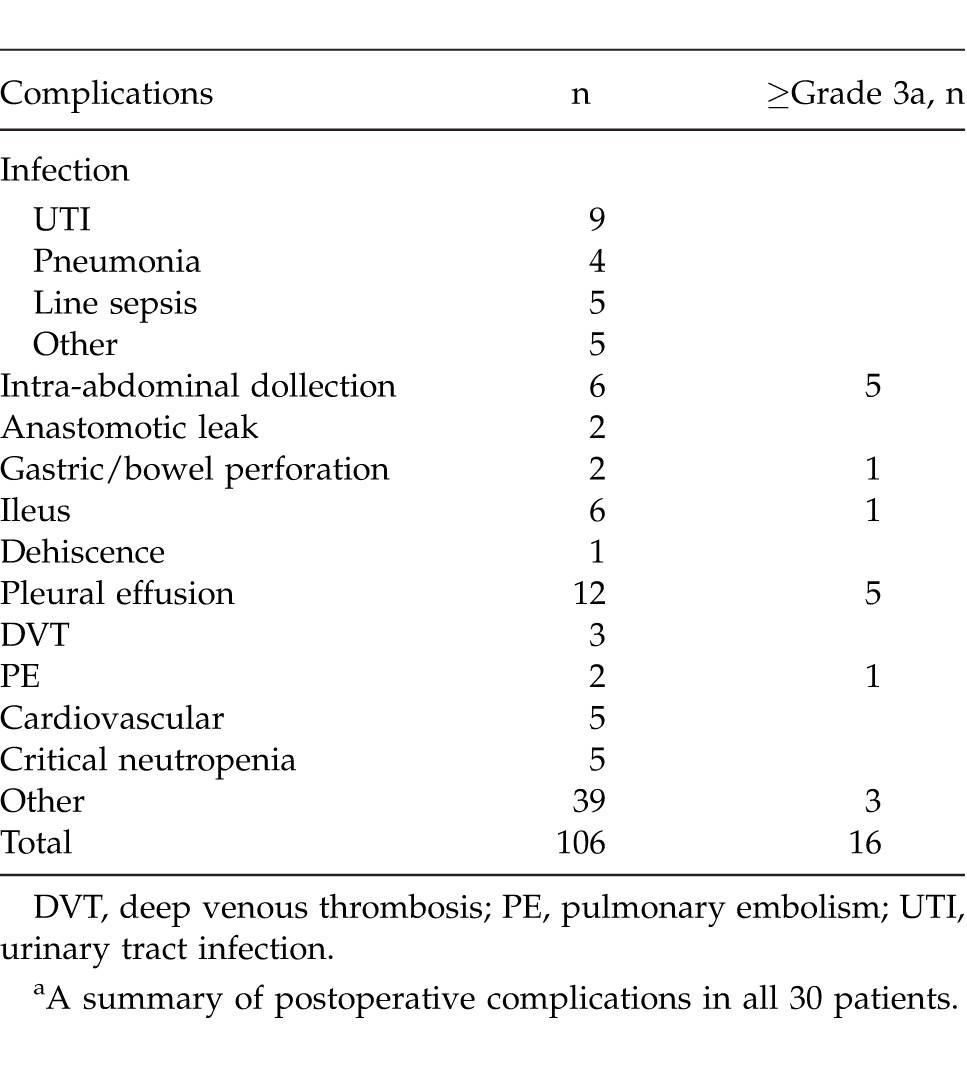
There were a total of 106 complications (Clavien and Dindo classification) observed in 28 patients (Table 3). Of these, 41 complications were grade 1, 49 were grade 2, 10 were grade 3A, 5 were grade 3B, and 1 was grade 5. The 10 grade 3A complications included 3 intra-abdominal collections, 4 pleural effusions, a pneumothorax, an upper gastrointestinal bleed, and a prolonged gastroparesis (both requiring an endoscopic assessment). The 5 grade 3B complications included 2 intra-abdominal collections, a loculated pleural effusion requiring posterolateral thoracotomy for drainage, a vesical leak, and a gastric perforation. A single grade 5 complication was an in-hospital death due to PE that occurred on postoperative day 97.
Table 3.
Postoperative complications
A total of 30 patients were evaluated, with a median follow-up time of 222 days (40–1268 days). At the latest time of review, there was no identifiable disease recurrence for patients in our series with complete cytoreduction. A total of 3 deaths (10%) were recorded, with a median time to death of 126 days (97–576 days).
Morbidity and mortality analysis
Hospital stay
Univariate analysis showed that age was associated positively with prolonged hospital stay (standardized beta, 1.33; P = 0.022). No other variables were associated with hospital stay to a level of significance.
Highest complication
No variables were associated significantly with higher complications on univariate analysis.
Total complications
Univariate analysis showed that age was also associated with total complications (standardized beta, 0.08; P = 0.02), and a nonsignificant trend toward higher total complications was observed between the use of CRS + HIPEC + EPIC compared with CRS + HIPEC (P = 0.154).
Mortality
No variables were associated significantly with mortality on univariate analysis.
Survival analysis
Disease-free survival
Specifically, there was no statistically significant difference between HIPEC + EPIC and HIPEC alone in terms of DFS (P = 0.098), nor were there differences of significance noted for other variables, such as age, tumor grade, completeness of cytoreduction, and PCI.
Overall survival
In those patients who received HIPEC (n = 13), there was no difference in OS between those who received EPIC and those who did not (P = 0.645; Fig. 1). Other key variables evaluated (age, completeness of cytoreduction, PCI, tumor grade) did not reveal any significant relationship with OS.
Fig. 1.
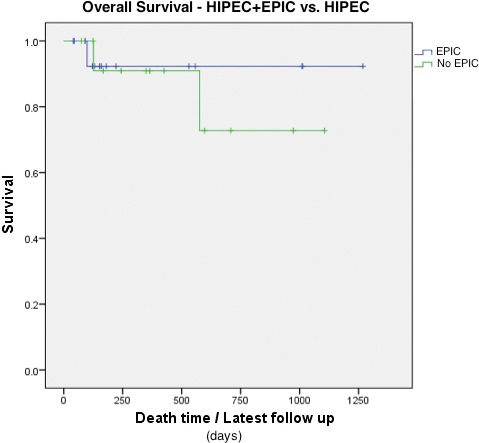
Overall survival (OS) for no EPIC versus EPIC. Kaplan-Meier curve comparing the relationship between those patients who did not receive EPIC (green line) to those who did (blue line) for OS.
Discussion
CRS alongside HIPEC is a radical but effective treatment option for select peritoneal carcinomatoses,2,11,14,15 and is considered by some to be the standard of care for mucinous peritoneal carcinomatosis of appendiceal origin.1 Because distant parenchymal metastases are rare in low-grade mucinous adenocarcinoma,1 an aggressive locoregional approach using both CRS and HIPEC is an ideal approach.16 Importantly, the efficacy of this combined modality treatment in peritoneal carcinomatosis appears to be heavily influenced by factors such as the histopathologic grade and premorbid status, as well as the completeness of cytoreduction.3 Furthermore, improvements in morbidity and survival associated with this operator-dependent approach have a direct relationship to the experience of the surgical team.7
Low-grade mucinous appendiceal adenocarcinoma with peritoneal carcinomatosis appears to have a unique pathophysiology. Locoregional spread of well-differentiated mucinous adenocarcinoma is typically in a minimally invasive fashion along the peritoneal surface, with lymph node invasion and parenchymal visceral metastases rarely observed.1 Visceral peristalsis provides a mechanism for resistance against invasion by cancerous deposits, a phenomenon that likely has a role in the lack of invasion observed in the stomach and colon.17 This process occurs in contrast to areas of relative stasis or fluid reservoirs within the peritoneal cavity, where the seeding of malignancy tends to predominate. These unique aspects to the distribution of appendiceal mucinous adenocarcinoma make it particularly amenable to successful management with complete surgical cytoreduction and HIPEC.16
As our understanding of this disease process has expanded, the combination of CRS and HIPEC has largely superseded palliative surgical debulking for isolated peritoneal metastases associated with mucinous adenocarcinoma of the appendix.2 As a treatment option, surgical debulking only provides temporary control of the disease process, and although this permits symptom control in a variable proportion of patients, the disease always reemerges, demanding further surgical treatment, with concomitant morbidity.18,19 Our disease control and survival experience with CRS and HIPEC are comparable with those of other studies.20–23 A long-term study performed by Youssef et al21 reported 5-year and 10-year survival rates of 87% and 74%, respectively, in patients with complete cytoreduction. Completeness of cytoreduction appears to be a key prognostic measure for this malignancy,2 and this was illustrated in our series, with no identifiable disease recurrence at latest follow-up in those with complete surgical clearance.
The potential efficiency of tumor clearance through the use of EPIC remains debatable.4,24 Although limited by the statistical power of our cohort, this study suggests that EPIC was not clearly associated with improved survival when used as an adjunct treatment measure after HIPEC (Fig. 1). Furthermore, the use of EPIC alongside CRS and HIPEC has been shown to account for an increase in overall morbidity in the literature.4 With poor patient tolerance, an extensive cost burden to the institution, and no long-term established evidence for a survival benefit, routinely adding EPIC to HIPEC may be difficult to justify. Further research is essential to establish the role of EPIC in patients with pseudomyxoma peritonei.
Reviewing the morbidity of our experience with CRS and HIPEC, we observed 16 severe complications (≥grade 3A) in 11 patients, which is comparable with internationally described cohorts.4,25–27 Gastrointestinal complications and infections appeared to dominate total complications, as is concordant with the existing literature (Table 3).16,26–28 These findings are unsurprising, given the extent of abdominal surgery performed alongside the use of perioperative intraperitoneal chemotherapy. Potential clinical variables of prognostic significance identified elsewhere25, 28 (use of blood products, operation duration, and PCI) were not predictive in our series. However, increasing age was significantly associated with increased hospital stay and total complications (P < 0.05). We had no postoperative mortality in this series, which is consistent with the size of our study in comparison with that reported elsewhere26–28 as well as the almost uniformly favorable performance status of our patients preoperatively (ECOG status ≤1 in 93.3%). With the incorporation of CRS and HIPEC into the armamentarium for the treatment of peritoneal carcinomatosis, we are in accord with the literature on the importance of carefully selecting patients for this still relatively morbid procedure.16
Limitations
Our study has several limitations. Because of our early experience with this management option, our case numbers were restricted. As such, the sample size of our study was not large enough to permit multivariate analysis. Of particular significance is the retrospective nature of our data collection, which is prone to chronologic, recall, and transfer bias. Our measurement of follow-up was restricted to outpatient notes and resulting investigations as well as identification through the death registry. Furthermore, patients managed early in this series were not evaluated preoperatively with the PCI score, and this may have contributed to the failure of this marker to accurately predict outcome. The combination of these weaknesses limits, to some degree, the extrapolation of our results to widespread clinical practice.
Conclusion
The optimal combination of treatment modalities remains an evolving topic in the management of peritoneal carcinomatosis of appendiceal origin. As evidenced by this study and others in the literature,2,16 the combination of CRS and HIPEC has an acceptable rate of morbidity and mortality when taking into consideration the potential for prolonged DFS and cure. Although EPIC may have theoretical advantages as an adjunctive treatment measure, it is unclear whether this modality offers a better prognosis at the expense of increased complications and patient intolerance.4 In conclusion, this study demonstrates that a single center can achieve satisfactory outcomes, with acceptable morbidity, in CRS and HIPEC for patients with peritoneal carcinomatosis secondary to mucinous adenocarcinoma of the appendix.
Acknowledgments
The authors would like to thank Dr Nicole Fairweather, Dr Pal Sivalingam, Mr Mark Landy, Ms Catherine Jowett, Ms Kere Klein and Mr Samuel Bunting for their assistance. This study was presented in part at the Annual Scientific Conference for the Royal Australian College of Surgeons in Auckland, May 6–10, 2013, and at the Annual Scientific Meeting for the Medical Oncology Group of Australia Inc in Melbourne, July 31–August 2, 2013.
References
- 1.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7(1):69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 2.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 3.Brücher BLDM, Piso P, Verwaal V, Esquivel J, Derraco M, Yonemura Y, et al. Peritoneal carcinomatosis: cytoreductive surgery and HIPEC–overview and basics. Cancer Invest. 2012;30(3):209–224. doi: 10.3109/07357907.2012.654871. [DOI] [PubMed] [Google Scholar]
- 4.McConnell YJ, Mack LA, Francis WP, Ho T, Temple WJ. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol. 2013;107(6):591–596. doi: 10.1002/jso.23276. [DOI] [PubMed] [Google Scholar]
- 5.Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14(2):484–492. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 6.Moradi BN, Esquivel J. Learning curve in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2009;100(4):293–296. doi: 10.1002/jso.21326. [DOI] [PubMed] [Google Scholar]
- 7.Kusamura S, Baratti D, Virzì S, Bonomi S, Iusco DR, Grassi A, et al. Learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies: analysis of two centres. J Surg Oncol. 2013;107(4):312–319. doi: 10.1002/jso.23231. [DOI] [PubMed] [Google Scholar]
- 8.Bosman FT, Carneiro F, Hruban RH, Theise ND, Bosman FT, Carneiro F. et al. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: World Health Organization;; 2010. [Google Scholar]
- 9.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement: Society of Surgical Oncology. Ann Surg Oncol. 2007;14(1):128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 11.Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol. 2012;13(8):e362–e369. doi: 10.1016/S1470-2045(12)70210-3. [DOI] [PubMed] [Google Scholar]
- 12.Glehen O, Cotte E, Kusamura S, Deraco M, Baratti D, Passot G, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242–246. doi: 10.1002/jso.21061. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu XJ, Yuan P, Li ZY, Bu ZD, Zhang LH, Wu AW, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol. 2013;34(1):463–469. doi: 10.1007/s13277-012-0571-4. [DOI] [PubMed] [Google Scholar]
- 15.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 16.Levine EA, Stewart JH, 4th, Perry S, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218(4):573–585. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79–100. doi: 10.1007/978-1-4613-1247-5_6. [DOI] [PubMed] [Google Scholar]
- 18.Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, et al. Pseudomyxoma peritonei: long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219(2):112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241(2):300–308. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27(3):239–243. doi: 10.1053/ejso.2000.1038. [DOI] [PubMed] [Google Scholar]
- 21.Youssef H, Newman C, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin. Dis Colon Rectum. 2011;54(3):293–299. doi: 10.1007/DCR.0b013e318202f026. [DOI] [PubMed] [Google Scholar]
- 22.Vaira M, Cioppa T. DE Marco G, Bing C, D'Amico S, D'Alessandro M et al. Management of pseudomyxoma peritonei by cytoreduction+HIPEC (hyperthermic intraperitoneal chemotherapy): results analysis of a twelve-year experience. In Vivo. 2009;23(4):639–644. [PubMed] [Google Scholar]
- 23.Rout S, Renehan AG, Parkinson MF, Saunders MP, Fulford PE, Wilson MS, et al. Treatments and outcomes of peritoneal surface tumors through a centralized national service (United Kingdom) Dis Colon Rectum. 2009;52(10):1705–1714. doi: 10.1007/DCR.0b013e3181b5504e. [DOI] [PubMed] [Google Scholar]
- 24.Chua TC, Liauw W, Zhao J, Morris DL. Comparative analysis of perioperative intraperitoneal chemotherapy regimen in appendiceal and colorectal peritoneal carcinomatosis. Int J Clin Oncol. 2013;18(3):439–446. doi: 10.1007/s10147-012-0397-5. [DOI] [PubMed] [Google Scholar]
- 25.Chua TC, Liauw W, Saxena A, Al-Mohaimeed K, Fransi S, Zhao J, et al. Evolution of locoregional treatment for peritoneal carcinomatosis: single-center experience of 308 procedures of cytoreductive surgery and perioperative intraperitoneal chemotherapy. Am J Surg. 2011;201(2):149–156. doi: 10.1016/j.amjsurg.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Yang XJ, Li Y. al-shammaa Hassan AH, Yang GL, Liu SY, Lu YL et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: results of 21 cases. Ann Surg Oncol. 2009;16(2):345–351. doi: 10.1245/s10434-008-0226-2. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt U, Dahlke MH, Klempnauer J, Schlitt HJ, Piso P. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31(1):53–58. doi: 10.1016/j.ejso.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Hadi R, Saunders V, Utkina O, Clingan P, Kam P, Links M, et al. Review of patients with peritoneal malignancy treated with peritonectomy and heated intraperitoneal chemotherapy. ANZ J Surg. 2006;76(3):156–161. doi: 10.1111/j.1445-2197.2006.03579.x. [DOI] [PubMed] [Google Scholar]


