Abstract
A 57-year-old woman without any past medical history underwent abdominoperineal resection for rectal cancer in our department. On postoperative day 15, the patient complained of sudden abdominal pain, and high fever was noted in addition to the appearance of erythema around the stoma. The diagnosis of phlegmon was made, and antibiotic infusion was started. However, a few days later, the patient developed hypovolemic shock with hypoalbuminemia and hemoconcentration. Fasciotomy was performed to exclude the necrotizing fasciitis, though all cultures were negative. Upon exclusion of the differential diagnoses, idiopathic systemic capillary leak syndrome (ISCLS) was diagnosed. She was successfully treated with massive fluid infusion under ventilation and continuous hemodiafiltration. Here, we report the first case of ISCLS that occurred during the postoperative period of colorectal surgery.
Key words: Idiopathic systemic capillary leak syndrome (ISCLS), Surgery, Abdominoperineal resection
Idiopathic systemic capillary leak syndrome (ISCLS) is a rare disease characterized by severe hyperpermeability of unknown cause, followed by hemoconcentration and hypoalbuminemia. During such episodes, approximately 70% of the plasma acutely shifts from the intravascular area into the interstitial space causing severe hypovolemic shock. The first case of ISCLS was described by Clarkson et al in 1960. Since then approximately 150 cases of ISCLS have been reported worldwide. To the best of our knowledge, this is the first report of a case of ISCLS that occurred during the postoperative period of colorectal surgery.
Case Report
A 57-year-old woman without any past medical history was admitted to our hospital in May 2011. The patient was found with rectal cancer (tumor, node, metastasis stage: T3 N1b M0) 3 months before the admission, for which she underwent neoadjuvant chemoradiotherapy (CRT) on an outpatient basis. The CRT protocol was a combination of radiation therapy (10 megavoltage X-ray linac, total 50.4 Gy/28 F, whole pelvis) and chemotherapy (oral intake of fluorouracil/leucovorin on the day of radiation). The adverse effects of the CRT included moderate diarrhea and loss of appetite.
Abdominoperineal resection (APR) with regional lymph node dissection was performed for the rectal cancer without any complications (operation time, 438 min; blood loss, 1060 mL). A prophylactic antibiotic (cefmetazole) was given for 3 days after the surgery and the patient's postsurgical progress was favorable.
On postoperative day 15, the patient complained of sudden abdominal pain and had a high fever (38.8°C) in addition to the appearance of erythema around the stoma. Laboratory findings revealed a white blood cell (WBC) count of 13,000/μL and C-reactive protein (CRP) level of 0.73 mg/dL. Phlegmon caused by the surgical procedure was suspected, and empirical antibiotic therapy was started. However, the patient's fever persisted and the erythema around the stoma rapidly expanded. A computed tomographic (CT) scan revealed edematous subcutaneous tissue at the left lower quadrant of the abdomen (Fig. 1). Laboratory test results showed a highly increased inflammatory status (WBC count 42,000/μL, CRP level 25.19 mg/dL) and severe hemoconcentration (hematocrit level 49.4%) with hypoalbuminemia (albumin level 1.5 g/dL) on the fourth day after onset (Fig. 2). Creatine kinase (CK) level was also increased, up to 1632 U/L. On the fifth day after onset, the patient developed hypovolemic shock and required large colloid and crystalloid infusions, and catecholamine to stabilize the blood pressure. Even at that time, all cultures were negative; however, we still could not exclude the possibility of septic shock caused by necrotizing fasciitis. Accordingly, we decided to perform a fasciotomy. We made an incision around the red skin and then separated the subcutaneous tissue to split the fascia of the external oblique muscle, which was considered the most inflamed on CT examination. The fascia of the external oblique muscle looked ischemic and had a dark red color, but did not show any microbiological infection with serous discharge. All cultures, including that of the discharge sample and resected fascia obtained during the surgery, were negative (Fig. 3).
Fig. 1.
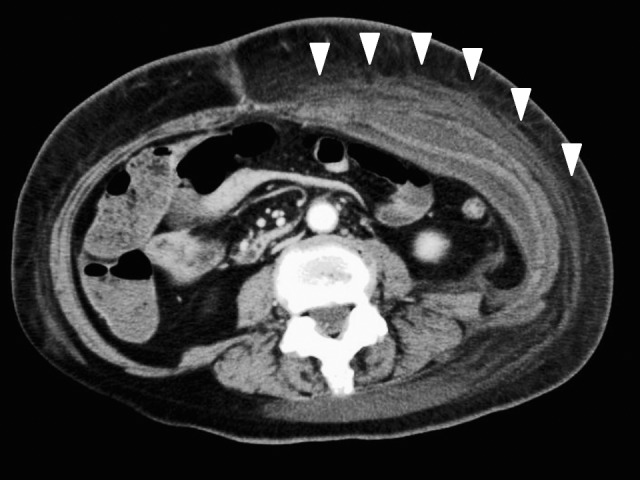
A CT scan detected edematous subcutaneous tissue at the left lower quadrant of the abdomen (arrow head).
Fig. 2.
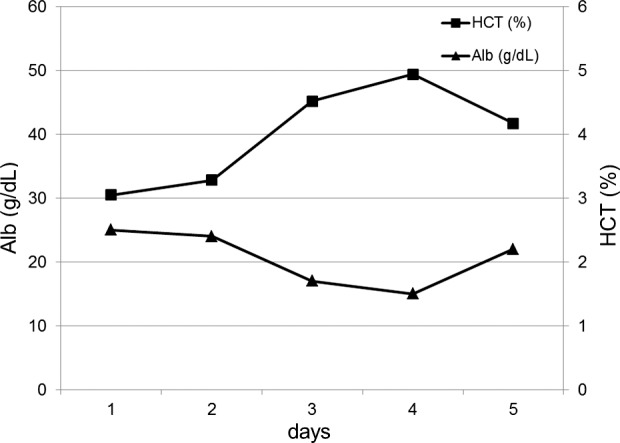
Transitional changes in the serum albumin level and hematocrit level after the onset of ISCLS.
Fig. 3.
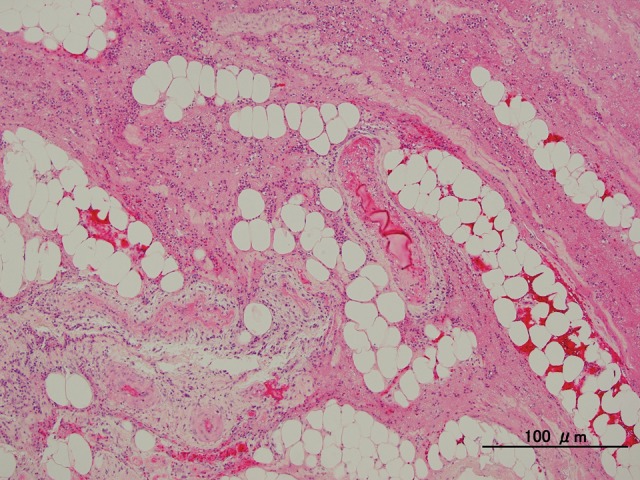
Pathological findings of resected specimen detected degenerated fascia of the abdominal wall with neutrophil infiltration. Any pathogenic microbe was not detected. Original magnification ×100; bars, 100 μm.
The patient showed severe hyperpermeability and inflammatory status despite the negative cultures. Considering the symptoms and laboratory findings, other diseases that cause noninfectious hypovolemic shock—massive edema with hemoconcentration and hypoalbuminemia—were excluded, and the patient's condition was diagnosed as ISCLS.
The patient had large amounts of discharge from the incision site and pleural effusion, and gained 30 kg of body weight. Although massive fluid and inotropic agents were infused, it was difficult to control the patient's hemodynamics. Subsequently oliguria was developed; thus we treated the patient under mechanical ventilation and continuous hemodiafiltration (CHDF). Theophylline (750 mg/day), tulobuterol (2 mg/day), and steroid (prednisolone 20 mg/day), which were reported to have some effect on a hyperpermeable state, were administered.
The blood pressure was stabilized on day 13 and the urine volume increased gradually; ultimately, the patient was extubated, and the CHDF was discontinued on day 26 (Fig. 4).
Fig. 4.
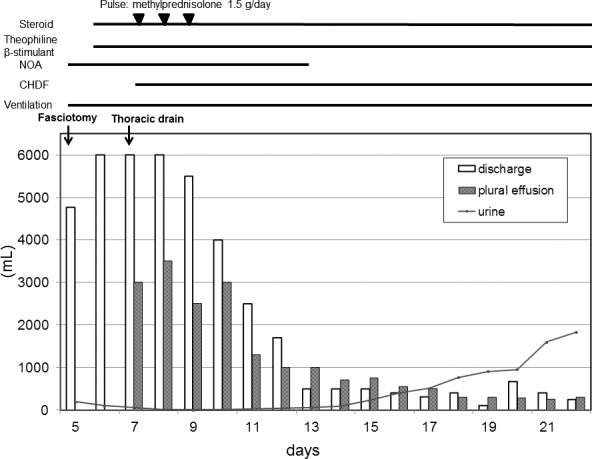
Clinical course and the treatment after the onset of ISCLS.
The patient was discharged 10 months after the abdominoperineal resection and has now lived a healthy life without recurrent attacks for 2 years.
Discussion
SCLS is characterized by severe hyperpermeability and accompanied by hemoconcentration and hypoalbuminemia, which sometimes attacks recurrently. Approximately 150 cases of SCLS have been reported since Clarkson published the first case1; however, the cause of the hyperpermeability remains unclear. Kapoor et al reported that monoclonal gammopathy, predominantly IgG-κ, were detected in 76% of cases, suggesting that these paraproteins are involved in the pathogenesis of hyperpermeability.2 Paraproteins were not detected in our case.
Interleukin-2, tumor necrosis factor, granulocyte colony-stimulating factor (G-CSF), and gemcitabine have been reported to cause secondary SCLS.3–6 Nakagawa et al reported high serum levels of G-CSF in a case of ISCLS.7 Vascular endothelial growth factor (VEGF) expression has also been reported to be a possible mediator of SCLS, although Lesterhuis et al reported that anti-VEGF therapy was not clinically effective on the therapy of ISCLS.8 In this case, G-CSF and VEGF levels were both high (79.5, and 252 pg/mL, respectively), suggesting some relationship to the pathogenesis of ISCLS.
The increase in capillary permeability causes leakage of plasma into the interstitial space, leading to a hypovolemic state. A CT scan of our patient detected edematous subcutaneous tissue. Compartment syndrome can also occur due to massive fluid leakage into the muscle. In our patient, the elevated serum CK level indicated rhabdomyolysis caused by compartment syndrome.
To diagnose ISCLS, exclusion of a differential diagnosis showing hyperpermeability is needed. Sepsis is the most common condition that causes hyperpermeability after colorectal surgery. In the present case, we consistently suspected involvement of the biological agent, but all the cultures tested negative. Necrotizing fasciitis, a severe complication after APR, mimics the clinical course of ISCLS, making it difficult to diagnose ISCLS. Other differential diagnoses did not fulfill the clinical and laboratory findings in the present patient; thus, ISCLS was ultimately diagnosed.
ISCLS is divided into two phases, namely the leaking and the refilling phases, and treatment depends on the phase.
During the leaking phase, maintaining the patient's intravenous volume is the most important goal. Here, we infused as much as 12,000 mL of fluid daily and used an inotropic agent to stabilize the patient's blood pressure. We also used agents that have been reported to have roles in reversing the hyperpermeable state of the vasculature. Steroid administration is considered effective because cytokine-mediated endothelial damage has been implicated.9 Theophylline and β2 agonists are reported to have a prophylactic effect on ISCLS consisting of anti-inflammatory and immunomodulatory effects, but the effect during the acute stage is controversial.10 Lambert et al reported that high doses of intravenous immunoglobulin reversed SCLS, although its exact mechanism of action is still unknown.11
In the refilling phase, the infused fluid during the leaking phase acutely returns into the vasculature, causing pulmonary congestion or heart failure; therefore, it is essential to control the hemodynamics using diuretics and sometimes with mechanical ventilation or hemodiafiltration.
The prognosis of ISCLS is not clear, but estimated 5-year survival rate is reported to be 70 to 76%.2,12,13 Shock and pulmonary congestion are the main causes of the death, so early diagnosis and intensive treatment are most important to improve the outcome.14
Conclusion
This is the first case report of ISCLS that occurred during the perioperative period of colorectal cancer surgery. We were able to diagnose ISCLS relatively early in its clinical course and treated the patient successfully by infusing massive volumes of fluids under mechanical ventilation and CHDF. After colorectal surgery, it is difficult to differentiate ISCLS from sepsis, particularly necrotizing fasciitis, but early diagnosis and treatment are crucial. Therefore, increased awareness of this rare but critical syndrome is needed.
References
- 1.Clarkson B, Thompson D, Horwith M, Luckey EH. Cyclical edema and shock due to increased capillary permeability. Am J Med. 1960;29:193–216. doi: 10.1016/0002-9343(60)90018-8. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor P, Greipp PT, Schaefer EW, Mandrekar SJ, Kamal AH, Gonzalez-Paz NC, et al. Idiopathic systemic capillary leak syndrome (Clarkson's disease): the Mayo clinic experience. Mayo Clin Proc. 2010;85(10):905–912. doi: 10.4065/mcp.2010.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenstein M, Ettinghausen SE, Rosenberg SA. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137(5):1735–1742. [PubMed] [Google Scholar]
- 4.Dowden AM, Rullo OJ, Aziz N, Fasano MB, Chatila T, Ballas ZK. Idiopathic systemic capillary leak syndrome: novel therapy for acute attacks. J Allergy Clin Immunol. 2009;124(5):1111–1113. doi: 10.1016/j.jaci.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Rechner I, Brito-Babapulle F, Fielden J. Systemic capillary leak syndrome after granulocyte colony-stimulating factor (G-CSF) Hematol J. 2003;4(1):54–56. doi: 10.1038/sj.thj.6200211. [DOI] [PubMed] [Google Scholar]
- 6.Baron D, Mayo A, Kluger Y. Gemcitabine-induced chronic systemic capillary leak syndrome: a life-threatening disease. Clin Oncol (R Coll Radiol) 2006;18(1):90–91. doi: 10.1016/j.clon.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa N, Ota H, Tanabe Y, Kabara M, Matsuki M, Chinda J, et al. A case of idiopathic systemic capillary leak syndrome with high serum levels of G-CSF on exacerbation. Intern Med. 2011;50(6):597–600. doi: 10.2169/internalmedicine.50.4857. [DOI] [PubMed] [Google Scholar]
- 8.Lesterhuis WJ, Rennings AJ, Leenders WP, Nooteboom A, Punt CJ, Sweep FC, et al. Vascular endothelial growth factor in systemic capillary leak syndrome. Am J Med. 2009;122(6):e5–e7. doi: 10.1016/j.amjmed.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Amoura Z, Papo T, Ninet J, Hatron PY, Guillaumie J, Piette AM, et al. Systemic capillary leak syndrome: report on 13 patients with special focus on course and treatment. Am J Med. 1997;103(6):514–519. doi: 10.1016/s0002-9343(97)00272-6. [DOI] [PubMed] [Google Scholar]
- 10.Droder RM, Kyle RA, Greipp PR. Control of systemic capillary leak syndrome with aminophylline and terbutaline. Am J Med. 1992;92(5):523–526. doi: 10.1016/0002-9343(92)90749-2. [DOI] [PubMed] [Google Scholar]
- 11.Lambert M, Launay D, Hachulla E, Morell-Dubois S, Soland V, Queyrel V, et al. High-dose intravenous immunoglobulins dramatically reverse systemic capillary leak syndrome. Crit Care Med. 2008;36(7):2184–2187. doi: 10.1097/CCM.0b013e31817d7c71. [DOI] [PubMed] [Google Scholar]
- 12.Dhir V, Arya V, Malav IC, Suryanarayanan BS, Gupta R, Dey AB. Idiopathic systemic capillary leak syndrome (SCLS): case report and systematic review of cases reported in the last 16 years. Intern Med. 2007;46(12):899–904. doi: 10.2169/internalmedicine.46.6129. [DOI] [PubMed] [Google Scholar]
- 13.Gousseff M, Arnaud L, Lambert M, Hot A, Hamidou M, Duhaut P, et al. The systemic capillary leak syndrome: a case series of 28 patients from a European registry. Ann Intern Med. 2011;154(7):464–471. doi: 10.7326/0003-4819-154-7-201104050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Lissoni P, Barni S, Cattaneo G, Archili C, Crispino S, Tancini G, et al. Activation of the complement system during immunotherapy of cancer with interleukin-2: a possible explanation of the capillary leak syndrome. Int J Biol Markers. 1990;5(4):195–197. doi: 10.1177/172460089000500405. [DOI] [PubMed] [Google Scholar]


