Abstract
This study aimed to determine the possible preventive effects of dexmedetomidine on postoperative intra-abdominal adhesions. Dexmedetomidine is a highly selective and potent α2 adrenergic agonist with sedative, analgesic, anxiolytic, sympatholytic, hemodynamic, and diuretic properties. In recent years, investigations have shown that dexmedetomidine possesses secondary antioxidant and also anti-inflammatory effects. Thirty Wistar albino male rats were randomized and divided into 3 groups of 10 animals each: group 1, sham-operated; group 2, cecal abrasion + peritoneal dissection; group 3, cecal abrasion + peritoneal dissection followed by daily intravenous injection of 10 μg/kg dexmedetomidine for 10 days. The animals were killed on postoperative day 21. Blood and cecal samples were taken for biochemical and histopathologic evaluation. In this study, biochemical and pathologic parameters were significantly better in the cecal abrasion + peritoneal dissection + dexmedetomidine group when compared with the cecal abrasion + peritoneal dissection group. Tissue malondialdehyde, myeloperoxidase, total sulfhydryl, and catalase were found to be significantly different between the cecal abrasion/peritoneal dissection + dexmedetomidine and the cecal abrasion/peritoneal dissection groups. Plasma malondialdehyde and total sulfhydryl values were also statistically different between these groups (P < 0.05). Statistical analyses of mean pathologic scores showed that the histopathologic damage in the cecal abrasion/peritoneal dissection + dexmedetomidine group was significantly less than the damage in the control group (P < 0.05 for all pathologic parameters). The results of this study show that dexmedetomidine had a significant preventive effect on postoperative intra-abdominal adhesions. We concluded that these effects might be due to antioxidant and anti-inflammatory activities.
Key words: Intraabdominal adhesions, Dexmedetomidine, Oxidative stress, Antioxidant, Anti-inflammatory
Intraperitoneal adhesions are fibrous tissue bands inside the peritoneal cavity that occur as a consequence of inflammation or surgical manipulation.1 Adhesions may remain silent or cause pathologic complications. Postsurgical adhesions severely affect the quality of life of millions of people worldwide, causing small-bowel obstruction, difficult reoperations, chronic abdominal and pelvic pain, and female infertility.2 Although not commonly recognized, adhesions develop in up to 94% of patients after abdominal operations.3 The goal of adhesion prevention is to abolish or reduce the incidence, severity, extent, and consequences of adhesions while retaining normal healing and preventing infection.4 Prevention strategies can be grouped into 4 categories: general principles, surgical techniques, mechanical barriers, and chemical agents.5 Over the years, although numerous approaches have been used to prevent adhesions, none of the measures have proved to completely prevent adhesion development.
Dexmedetomidine, which was approved by the US Food and Drug Administration in 1999 for the sedation of patients hospitalized in an intensive care setting, is a potent α2 adrenergic agonist with sedative, analgesic, sympatholytic, hemodynamic, and diuretic properties.6 It has been increasingly used in clinical practice for anxiolysis, analgesia, sedation, and anesthetic sparing.7 Both in vivo and in vitro studies have demonstrated that dexmedetomidine has a protective effect against ischemia-reperfusion (I/R) injury of the heart, kidney, brain, and testis in animal models.8 In preclinical studies on oxidative stress and free radical formation, prophylactic administration of dexmedetomidine in various experimental I/R injury models has been found to protect tissues against the formation of free radicals after reperfusion. Moreover, preclinical studies have shown that dexmedetomidine could decrease systemic inflammation and increase the survival rate following sepsis caused by endotoxins.9 Whole studies have demonstrated that apart from its anesthetic property, dexmedetomidine possesses anti-inflammatory, antioxidant, and antiapoptotic effects.
In the light of all these features of dexmedetomidine, the aim of this study was to investigate the effects of dexmedetomidine on experimental intraperitoneal adhesions and the possible mechanisms of these effects. To the best of our knowledge, the effect of dexmedetomidine on intra-abdominal adhesions has not been previously investigated in the literature.
Materials and Methods
Animals
Thirty Wistar albino male rats, weighing 250 ± 25 g, were allowed to adapt to laboratory conditions for 1 week before experimental use. The animals had free access to water and standard laboratory chow. They were housed under constant temperature (21°C ± 2°C) individually in wire cages under a 12-hour light-dark cycle. Twelve hours before anesthesia, the animals were deprived of food but had free access to water until 2 hours before anesthesia. No enteral or parenteral antibiotics were administered at any time. Rats that died during the experiment were excluded from the study and no new rats were included. The procedures in this experimental study were performed in accordance with the National Guidelines for The Use and Care of Laboratory Animals, and approval for the study was granted by the Animal Ethics Committee of Ankara Education and Research Hospital.
Study groups and operative procedure
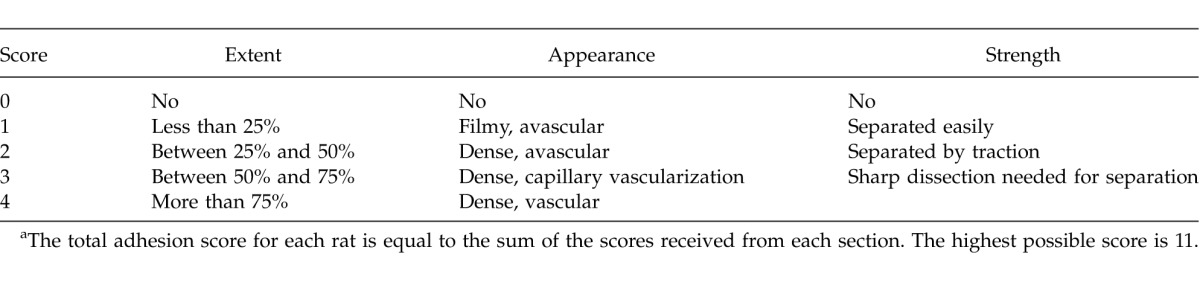
The rats were randomized and divided into 3 groups of 10 animals each. All animals were anesthetized by intramuscular injection of 50 mg/kg ketamine hydrochloride (Ketalar, Parke-Davis, Istanbul, Turkey) and 5 mg/kg xylazine (Rompun, Bayer, Istanbul, Turkey). The abdomen was shaved and prepared with povidone-iodine. Under sterile conditions, a midline laparotomy was performed. The cecum was abraded with sterile gauze until subserosal hemorrhage had developed. A 1 × 1 cm patch of peritoneum located opposite the cecal abrasion was completely dissected. The groups were formed as group 1, sham-operated (laparotomy and cecal mobilization only); group 2 (control), cecal abrasion + peritoneal dissection and no treatment group; and group III, cecal abrasion + peritoneal dissection followed by daily intravenous injection of 10 μg/kg dexmedetomidine for 10 days. The animals were allowed to feed and drink water after the operation. All operations were performed by the same surgeon. The animals were killed on postoperative day 21. The adhesions were classified by a surgeon who was unaware of the groups, according to a scoring system based on the evaluation of the appearance, extent, and strength of the adhesions (Table 1). Blood and tissue (cecum and peritoneum) samples were taken for biochemical and histopathologic evaluation.
Table 1.
Adhesion scoring systema
Evaluation of oxidative stress
The evaluation of oxidative stress parameters was performed in the Biochemistry Department of the Ankara Education and Research Hospital. Tissues were stored at −80°C until the assays. Tissue malondialdehyde (MDA), total sulfhydryl (SH) levels, catalase (CAT), and myeloperoxidase (MPO) enzyme activities were measured. Plasma MDA and total SH levels were also evaluated. MDA levels were calculated by the fluorometric method as described by Wasowicz et al.10 After the reaction of thiobarbituric acid with MDA, the reaction product was extracted in butanol and was measured spectrofluorometrically at wavelengths of 525 nm for excitation and 547 nm for emission. A total of 0 to 5 μmol/L 1,1′,3,3′-tetraethoxypropane solution was used as a standard. For the measurement of tissue MDA levels, 50 μL of homogenate was added and introduced into 10-mL glass tubes containing 1 mL of distilled water. After the addition of 1 mL of the solution containing 29 mmol/L thiobarbituric acid in acetic acid and mixing, the samples were placed in a water bath and heated for 1 hour at 95°C to 100°C. The samples were then cooled, 25 μL of 5 mol/L HCl was added, and the reaction mixture was extracted by agitation for 5 minutes with 3.5 mL n-butanol. After separation of the butanol phase by centrifugation at 1500g for 10 minutes, the fluorescence of the butanol extract was measured with a fluorometer (Hitachi F-2500) at wavelengths of 525 nm for excitation and 547 nm for emission. A total of 0 to 5 μmol/L 1,1′,3,3′-tetraethoxypropane solution was used as a standard. MDA levels were given as micromoles per gram (μmol/g) wet tissue.10
Total SH groups were measured spectrophotometrically using the method of Sedlak and Lindsay.11 Aliquots of 250 μL of the supernatant fraction of the tissue homogenate were mixed in 5-mL test tubes with 750 μL of 0.2 M Tris buffer (pH 8.2), and 50 μL of 0.01 M 5,5′-dithiobis(2-nitrobenzoic acid). The mixture was brought to 5 mL with 3950 μL of absolute methanol. A reagent blank (without sample) and a sample blank [without 5,5′-dithiobis(2-nitrobenzoic acid)] were prepared in a similar manner. The test tubes were stoppered with rubber caps, the color was developed for 15 minutes, and the reaction mixtures were centrifuged at approximately 3000g at room temperature for 15 minutes. The absorbance of supernatant fractions were read in a spectrophotometer at 412 nm.11
MPO activity was assayed spectrophotometrically by determining the decomposition of hydrogen peroxide using o-Dianisidine as the hydrogen donor. Tissue samples of approximately 50 mg were taken, weighed, and homogenized 3 times for 30 seconds at 4°C in 1 mL of ice-cold 0.5% hexadecyltrimethylammonium bromide in 50 mmol/L phosphate buffer (pH 6). The homogenate was subjected to 3 freeze-thaw cycles and centrifuged for 15 minutes at 40,000g. MPO activity was determined by the addition of 0.1 mL of the supernatant to 2.9 mL of 50 mmol/L phosphate buffer containing 0.167 mg/mL o-Dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm during a 5-minute period was measured at 25°C. The data were expressed as the change in absorbance per minute per gram wet weight.12
CAT activity was assayed using the method of Yasmineh and Theologides.13 The absorbance change of hydrogen peroxide, which was degraded by CAT activity, was monitored at 240 nm. CAT activity was calculated by using change of absorbance per minute, molar absorptivity coefficient of hydrogen peroxide, and dilution factor. Results were expressed as units per milligram (U/mg) protein.
Histopathologic examination
The histopathologic analyses were performed in the Pathology Department of the Harran University Faculty of Veterinary Medicine. For light microscopy analyses, the samples obtained from the cecum and peritoneum were fixed in 10% neutral-buffered formalin solution for 2 days. The tissues were washed in running water and were dehydrated with increasing concentrations of ethanol (50%, 75%, 96%, and 100%). After dehydration, the specimens were put into xylene to obtain transparency and were then infiltrated with and embedded in paraffin. The embedded tissues were cut into 5-μm–thick sections using a Leica RM 2125 RT microtome and stained with hematoxylin-eosin. Histopathologic examinations were performed with a light microscope (Olympus BX51TF) by a pathologist blinded to the study design. Inflammatory activities and fibrosis were graded semiquantitatively (0, no inflammation or fibrosis; 1, rare; 2, mild; 3, prominent; 4, severe).
Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS) version 15.0 for Windows (SPSS Inc, Chicago, Illinois). All variables were normally distributed about the mean. Data were presented as mean ± SD. Differences between the groups were evaluated by one-way analysis of variance or Kruskal-Wallis variance analysis, whichever was appropriate. When the P values from the variance analysis were statistically significant, the Tukey honestly significant difference or Mann-Whitney U multiple comparison test was used to determine which group was different from the others. A value of P < 0.05 was considered to be statistically significant.
Results
General
Four rats from the control group died during the study. These rats were excluded from the study and no new rats were included. The remaining rats were killed on postoperative day 21.
Adhesion scores
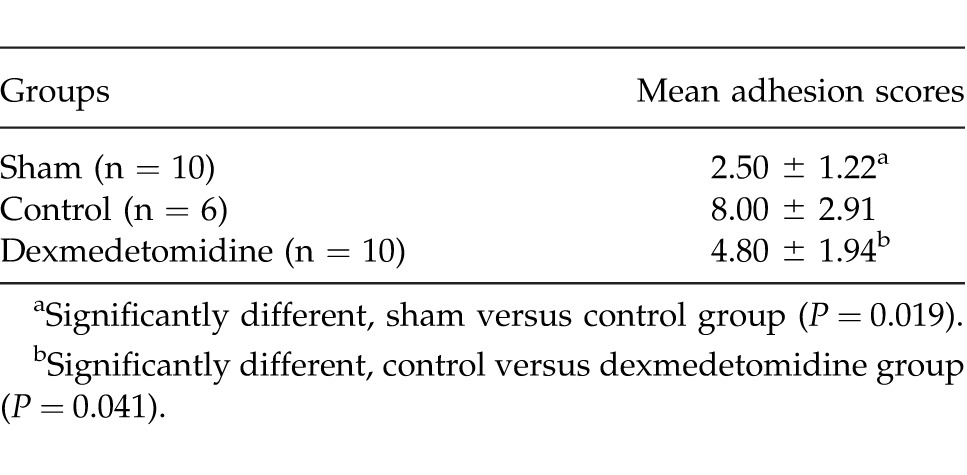
The mean adhesion scores are summarized in Table 2. There was a significant difference between the control group and the dexmedetomidine group (P = 0.041). The difference between the sham and dexmedetomidine groups was not statistically significant (P > 0.05). In other words, dexmedetomidine prevented adhesion formation significantly.
Table 2.
The mean adhesion scores of the groups
Oxidative stress
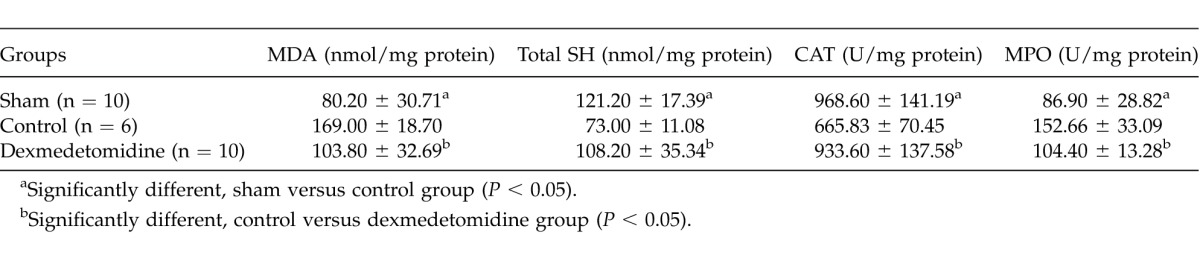
Tissue MDA, total SH levels, CAT, and MPO enzyme activities are summarized in Table 3. According to the MDA levels, there was a significant difference between the control and other groups (P = 0.001 for sham group, P = 0.003 for dexmedetomidine group). The difference between the sham and dexmedetomidine groups was not significant (P > 0.05). Total SH levels were significantly different between the control and other groups (P = 0.001 for the sham group and 0.011 for the dexmedetomidine group). When CAT and MPO activities were compared, the differences between the control and other groups were significant (P = 0.002 for CAT, 0.003 for MPO between control and sham groups; P = 0.001 for CAT, 0.002 for MPO between control and dexmedetomidine groups). No significant difference was found between the sham and dexmedetomidine groups according to tissue CAT and MPO activities and total SH levels (P > 0.05 for all parameters).
Table 3.
Mean tissue oxidative stress parameters of the groups
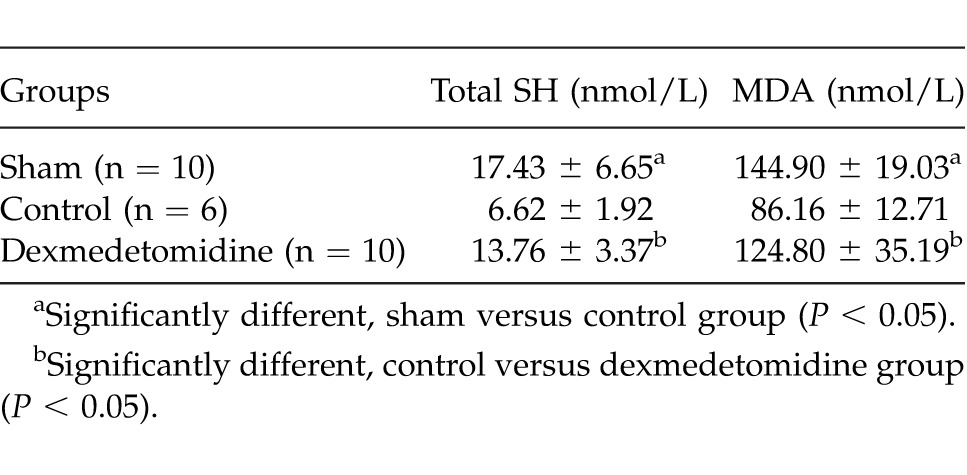
The mean plasma MDA and total SH levels of groups are given in Table 4. There was a significant difference between control and other groups according to MDA and total SH plasma levels (P = 0.001 and P = 0.03 for MDA; P = 0.001 and P = 0.001 for total SH, respectively, for sham and dexmedetomidine groups). There was no statistically significant difference between sham and dexmedetomidine groups (P > 0.05).
Table 4.
Mean plasma oxidative stress parameters of the groups
Histopathologic results
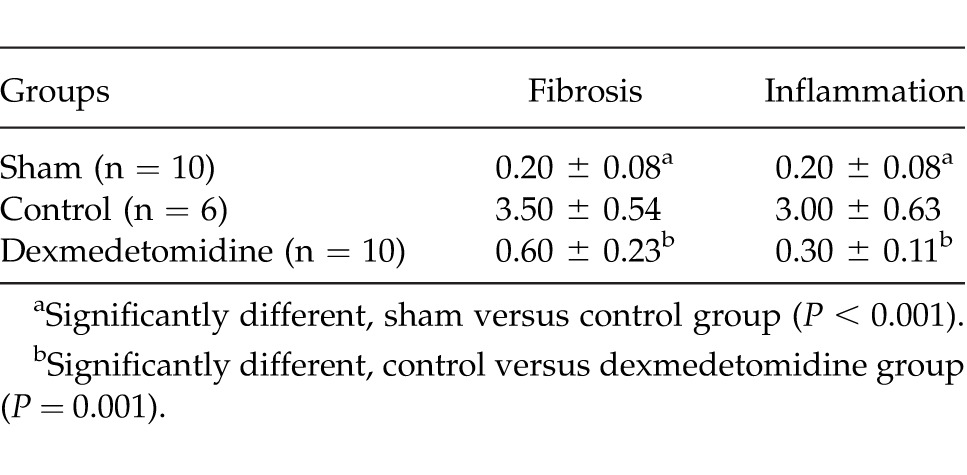
The histologic findings of the groups are represented in Figs. 1, 2, and 3. The mean pathologic scores are summarized in Table 5. The difference between the control and dexmedetomidine groups was statistically significant (P = 0.001 for both inflammation and fibrosis). There was no significant difference between the sham and dexmedetomidine groups for inflammation and fibrosis scores (P > 0.05). The pathologic scores and findings were in accordance with the adhesion scores.
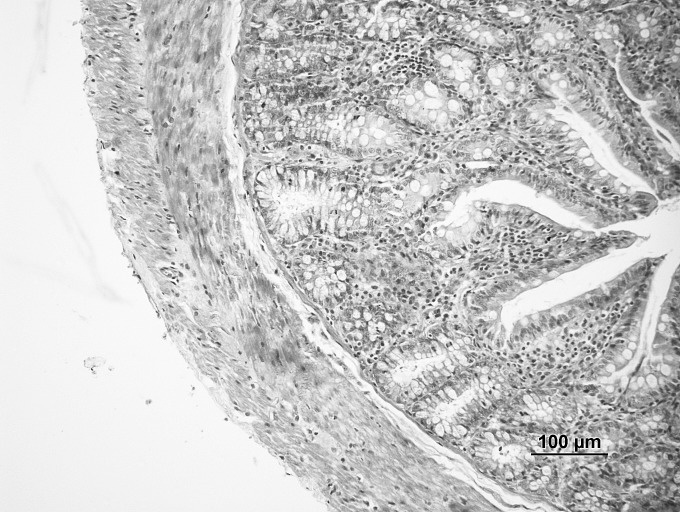
Fig. 1.
Sham group: normal architecture (hematoxylin-eosin, ×20).
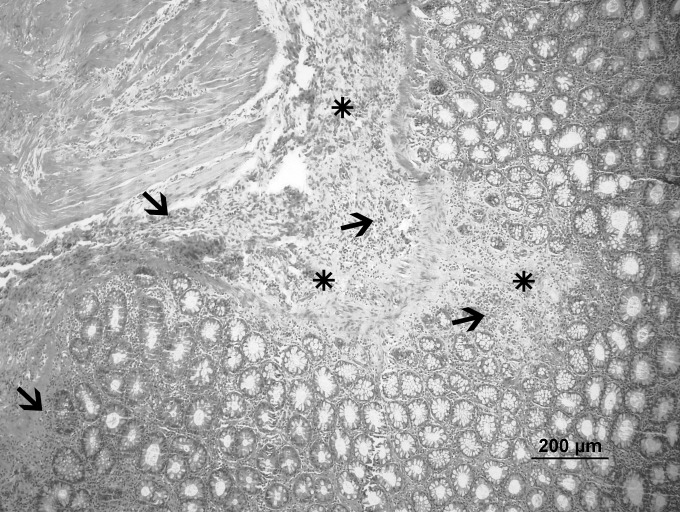
Fig. 2.
Control group: Severe fibrosis (*) and inflammation (arrow) (hematoxylin-eosin, ×10).
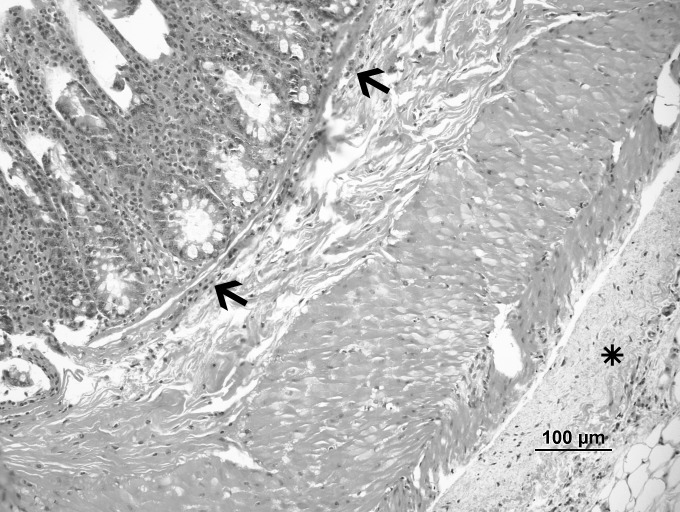
Fig. 3.
Dexmedetomidine group: mild fibrosis (*) and inflammation (arrow) (hematoxylin-eosin, ×20).
Table 5.
The mean pathologic scores of the groups
Discussion
It is already well known that peritoneal adhesions can be found in up to 93% of patients undergoing intra-abdominal surgery, although most of these adhesions, fortunately, are asymptomatic. Nevertheless, adhesion formation still presents a major cause of postoperative complications in abdominal and gynecologic surgeries, such as chronic abdominal or pelvic pain, infertility, and intestinal obstructions. Moreover, existing peritoneal adhesions lead to elevated rates of reoperation, longer operation time, and an increased risk of intraoperative complications.14
The peritoneum is the largest serous membrane in the body. With a surface of 2 m2 it is equivalent to that of the skin, and it covers the visceral organs (visceral peritoneum) and lines the abdominal cavity (parietal peritoneum).15 Peritoneal surface is highly susceptible to trauma. Minimal mobilization or damage to the peritoneum can result in denudation of peritoneal surfaces, which can trigger the formation of adhesions.16
Peritoneal adhesion formation is the result of a complex interactive cascade involving cellular and humoral factors, the exact mechanisms of which are still poorly understood. The cellular factors are mesothelial cells, several types of inflammatory cells, and fibroblasts. The interplay of these cells and the structural organization are regulated by a number of cytokines, growth factors, and signaling molecules.14 Initial local ischemia and the resulting inflammatory reaction in the damaged tissue both play key roles in peritoneal adhesion formation. The inflammation is triggered by inflammatory mediators released by mesothelial cells from the edges of the traumatized area and the dispersion of fibrin onto the affected surface. Both elements cause the migration of inflammatory cells into the traumatized tissue.1
Ischemia has been proposed as the most important insult that leads to adhesion development.17 Hypoxia induces the conversion of fibroblasts with a normal peritoneal phenotype into fibroblasts with an adhesion phenotype; such fibroblasts have a lower fibrinolytic activity and have a significant increase in basal mRNA levels for several cytokines, coagulatory factors, and crucial proteolytic enzymes that play a role in the extracellular matrix remodeling process of healing.16 The fibrin deposits represent an adhesive surface that would be degraded by the fibrinolytic properties of mesothelial cells under physiologic conditions or in cases of limited lesions. However, extensive tissue damage, local ischemia, and the absence of adequate fibrinolytic activity of the mesothelium after peritoneal trauma lead to an imbalance between fibrinolytic and procoagulatory factors, favoring the formation of fibrin clots.1,14 Under normal conditions, this fibrinous exudate serves as a platform for appropriate healing to progress, but under certain pathologic circumstances the deposited fibrin can instead serve as a bridge between unrelated neighboring tissues.18 These fibrin bands are transformed into granulation tissue by the ingrowth of capillaries and fibroblasts and are subsequently converted into permanent, collagenous, highly organized tissue containing nerve fibers and vessels.1
The results of many studies have shown that reactive oxygen species and inflammatory reactions play an important role in adhesion formation. Raa et al19 demonstrated that reactive oxygen species had a key role in the complex pathophysiology of postoperative adhesion formation. Roy et al20 demonstrated the colonization of inflammatory cells and their derivative reactive oxygen species in human peritoneal tissue. Oxidative stress is a process of tissue injury caused by the effect of free radicals. Reactive intermediates produced under conditions of oxidative stress cause the oxidation of polyunsaturated fatty acid in the membrane lipid bilayers, leading eventually to the formation of aldehydes.21 Oxidative stress produces reactive oxygen species and induces uncontrolled lipid peroxidation. The products of oxidative stress, such as MDA, have been found in the blood of patients. These products are extremely cytotoxic and damage cell membranes and intracellular macromolecules.22
Over the years, numerous agents have been investigated for the prevention of intra-abdominal adhesions. Their roles are to activate fibrinolysis, hamper coagulation, diminish the inflammatory response, inhibit collagen synthesis, or create a barrier between adjacent wound surfaces. However, none of them have been found to completely prevent adhesion development.23–25
In the current study, an evaluation was made of the possible adhesion-preventive effects of dexmedetomidine, which is an α2 adrenergic agonist. It acts by binding to G protein–coupled α2 adrenergic receptors, which are found in the central, peripheral, and autonomic nervous systems and also in various vital organs and blood vessels throughout the body. In the intensive care setting, it has been effectively used in postoperative analgesia and sedation of high-risk and complex surgical patients, as well as during transition from other conventional sedatives. The activation of postsynaptic α2 receptors leads to sympatholysis and results in hypotension and bradycardia, thus helping to attenuate the stress response. Dexmedetomidine also offers good perioperative hemodynamic stability and an intraoperative anesthetic-sparing effect. Therefore, it is used as anesthetic adjuvant during surgery.26,27
The attenuation of noradrenaline release in the circulation by dexmedetomidine may prevent the potentially destructive effects of excess metabolism caused by noradrenaline, by means of prohibiting increased free oxygen radical production.28 Tüfek et al29 showed that, when given before the induction of ischemia, dexmedetomidine was a protective agent against oxidative alterations in hepatic I/R injury on the liver and remote organs. Previous studies have demonstrated that dexmedetomidine may lessen systemic inflammation and increase survival rates in sepsis and endotoxin-induced shock in rats.7 In a study by Sun et al,30 it was reported that the protective effects of dexmedetomidine on I/R-induced lung inflammation, capillary barrier dysfunction, tissue edema, and injury were similar to those of the steroid dexamethasone. Studies on rats have supported that it could decrease systemic inflammation and increase the survival rate following sepsis caused by endotoxins.31,32
In the present study it was found that dexmedetomidine had a significant adhesion-preventive effect. There was a statistically significant difference between the control group and the dexmedetomidine group (P = 0.041). The difference between the sham and dexmedetomidine groups was not statistically significant (P > 0.05). To evaluate the underlying mechanism of this adhesion-preventive effect of dexmedetomidine, tissue levels of MDA, MPO, CAT, and total SH, as well as the blood levels of MDA and total SH, were determined. The anti-inflammatory effect of dexmedetomidine was also evaluated by histopathologic examination.
MDA is the end product of lipid peroxidation and is widely used as a marker of oxidative activity.33 In a study by Kurt et al,34 the inhibition of acute I/R damage by dexmedetomidine in rat ovarian tissue was investigated, and microscopic findings, such as very severe edema, very severe vascular congestion, hemorrhage and leukocytic infiltrates, were found to be present in the ovarian tissue with elevated MDA; these histologic observations indicated that the increase in MDA was related to the intensity of the tissue injury. Gideroglu et al35 also reported that ischemic insult resulted in high MDA levels in an inferior epigastric artery skin flap as a flap I/R injury model. Studies of various tissues have shown that dexmedetomidine prevented an increase in MDA levels, thus resulting in a simultaneous decrease in lipid peroxidation.6,9,28,34 In the present study, MDA levels were measured to evaluate lipid peroxidation and tissue damage. The tissue and plasma MDA levels were higher in the control group than in the sham and dexmedetomidine groups. These results show that dexmedetomidine reduced tissue injury and lipid peroxidation.
MPO, which is a member of the heme peroxidase-cyclooxygenase superfamily, is used as an enzyme marker for the degree of neutrophil infiltration. Many reports have shown that activated neutrophils are able to produce oxygen metabolites or protease, and these neutrophil-derived cytotoxic agents cause endothelial cell injury and result in tissue damage. Overreaction of neutrophils may be responsible for organ failure in various pathologic conditions.36 MPO is abundant in the granules of human inflammatory cells, such as activated neutrophils, macrophages, and monocytes.37 It was consistently proven that dexmedetomidine decreased tissue MPO in studies carried out by Uysal et al28 and Kiliç et al.38 In the current study, MPO activity was used to evaluate the degree of neutrophil infiltration. Tissue MPO activity was high in the control group when compared with the sham and dexmedetomidine groups. In other words, the treatment with dexmedetomidine reduced the tissue MPO activity, and this process might be considered as less tissue injury and less neutrophil infiltration.
Aerobic organisms defend themselves against reactive oxygen and nitrogen species through enzymatic and nonenzymatic detoxification mechanisms. The enzymatic detoxification mechanisms involve antioxidant enzymes (superoxide dismutases, CATs, and peroxidases), low–molecular weight antioxidants [vitamin E, vitamin C, glutathione (GSH), ubiquinone, beta-carotene, etc], and adaptive mechanisms leading to antioxidant gene expression. Enzymatic antioxidants reduce or completely eliminate the hazardous effects of reactive oxygen species. CAT reduces H2O2 to O2 and H2O. CAT is more important when H2O2 levels are low. At higher concentrations of H2O2, GSH peroxidase enzyme, which is dependent on reduced GSH, is responsible for detoxifying H2O2. Finally, these enzymes catalyze the conversion of reactive oxygen species into less reactive species.39,40 In this study, CAT levels were low in the control group and high in the dexmedetomidine group. This result proves that dexmedetomidine increases the CAT level.
GSH is a cysteine-containing tripeptide that is abundant in most eukaryotic cells. GSH helps to maintain cellular sulfhydryl residues in a reduced state. GSH also reacts with free radicals, generating glutathionyl. GSH is involved in DNA synthesis, the repair of injured DNA portions, metabolic functions, inactivation of toxic substances, and the prevention of possible damage caused by free radicals.41 Although the physiologic significance of protein glutathiolation has not been fully assessed, it is currently believed that the addition of GSH to protein sulfhydryls prevents excessive oxidation, and thereby preserves protein integrity and function under conditions of oxidative stress. GSH and total SH levels have been found to be lower in injured tissues compared with normal tissues. GSH has been measured to assess the defense mechanism against the hazardous effects of reactive oxygen species, and to roughly estimate the degree of injured tissues. To date, published papers have supported that dexmedetomidine increases the GSH level.34,42 Similarly, in the current study plasma and tissue total SH levels were low in the control group and high in the dexmedetomidine group.
As previously mentioned, oxidative stress plays an important role in postoperative intra-abdominal adhesion formation. In the current study, it was shown that dexmedetomidine had significant antioxidant capacity. Thus, it can be concluded that the antioxidant effects of dexmedetomidine might have a role in adhesion prevention.
It is clearly known that inflammation and fibrosis are the main factors for adhesion formation.1,14 The mean fibrosis and inflammation scores detected in the current study are summarized in Table 3. The difference between the control and dexmedetomidine groups was statistically significant (P = 0.001 for both inflammation and fibrosis). There was no significant difference between the sham and dexmedetomidine groups for inflammation and fibrosis scores (P > 0.05). The pathologic scores and findings were in accordance with the adhesion scores. These results demonstrated that treatment with dexmedetomidine significantly decreased the inflammation and fibrosis. This might be another mechanism to explain the adhesion-preventive effect of dexmedetomidine.
In conclusion, the results of this study clearly demonstrate that dexmedetomidine possessed a significant adhesion-preventive effect in rats. Dexmedetomidine might be used for adhesion prevention in humans. It can be concluded that these effects of dexmedetomidine might be due to antioxidant and anti-inflammatory activities, but further studies are needed to evaluate the exact mechanism of the antiadhesive effect of dexmedetomidine.
References
- 1.Brochhausen C, Schmitt VH, Rajab TK, Plunck CN, Kramer B, Wallwiever M, et al. Intraperitoneal adhesions–an ongoing challenge between biomedical engineering and the life sciences. J Biomed Mater Res A. 2011;98(1):143–156. doi: 10.1002/jbm.a.33083. [DOI] [PubMed] [Google Scholar]
- 2.Attard JP, MacLean AR. Adhesive small bowel obstruction: epidemiology, biology and prevention. Can J Surg. 2007;50(4):291–300. [PMC free article] [PubMed] [Google Scholar]
- 3.Becker JM, Dayton MT, Fazio VW, Beck DE, Stryker SJ, Wexner SD, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183(4):297–306. [PubMed] [Google Scholar]
- 4.Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, van Goor H. Recent clinical developments in pathophysiology, epidemiology, diagnosis and treatment of intra-abdominal adhesions. Scand J Gastroenterol Suppl. 2000;232:52–59. [PubMed] [Google Scholar]
- 5.Schnüriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: a review of the literature. Am J Surg. 2011;201(1):111–121. doi: 10.1016/j.amjsurg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Arslan M, Metin Comu F, Kucuk A, Ozturk L, Yaylak F. Dexmedetomidine protects against lipid peroxidation and erythrocyte deformability alterations in experimental hepatic ischemia reperfusion injury. Libyan J Med. 2012;7:18185. doi: 10.3402/ljm.v7i0.18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D. Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand. 2011;55(10):1272–1278. doi: 10.1111/j.1399-6576.2011.02526.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao X, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012;116(5):1035–1046. doi: 10.1097/ALN.0b013e3182503964. [DOI] [PubMed] [Google Scholar]
- 9.Cekic B, Besir A, Yulug E, Geze S, Alkanat M. Protective effects of dexmedetomidine in pneumoperitoneum-related ischaemia–reperfusion injury in rat ovarian tissue. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):343–346. doi: 10.1016/j.ejogrb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substance in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39(12):2522–2526. [PubMed] [Google Scholar]
- 11.Sedlak J, Lindsay RH. Estimation of total, protein bound, and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 12.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 13.Yasmineh WG, Theologides A. Catalase as a roving scavenger of hydrogen peroxide: a hypothesis. J Lab Clin Med. 1993;122(1):110–114. [PubMed] [Google Scholar]
- 14.Brochhausen C, Schmitt VH, Planck CNE, Rajab TK, Hallemann D, Tapprich C, et al. Current strategies and future perspectives for intraperitoneal adhesion prevention. J Gastrointest Surg. 2012;16(6):1256–1274. doi: 10.1007/s11605-011-1819-9. [DOI] [PubMed] [Google Scholar]
- 15.Van der Wal JBC, Jeekel J. Biology of the peritoneum in normal homeostasis and after surgical trauma. Colorectal Dis. 2007;9(suppl 2):9–13. doi: 10.1111/j.1463-1318.2007.01345.x. [DOI] [PubMed] [Google Scholar]
- 16.Hellebrekers BW, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98(11):1503–1516. doi: 10.1002/bjs.7657. [DOI] [PubMed] [Google Scholar]
- 17.Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: the adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004;11(3):307–314. doi: 10.1016/s1074-3804(05)60041-2. [DOI] [PubMed] [Google Scholar]
- 18.Boland GM, Weigel RJ. Formation and prevention of postoperative abdominal adhesions. J Surg Res. 2006;132(1):3–12. doi: 10.1016/j.jss.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Raa S, van den Tol MP, Sluiter W, Hofland LJ, van Eijck CH, Jeekel H. The role of neutrophils and oxygen free radicals in postoperative adhesions. J Surg Res. 2006;136(1):45–52. doi: 10.1016/j.jss.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Roy S, Clark CJ, Mohebali K. Reactive oxygen species and EGR-1 gene expression in surgical postoperative peritoneal adhesions. World J Surg. 2004;28(3):316–320. doi: 10.1007/s00268-003-7403-z. [DOI] [PubMed] [Google Scholar]
- 21.Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59(4):459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 23.Reijnen MMPJ, Bleichrodt RP, Goor HV. Pathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronan. Br J Surg. 2003;90(5):533–541. doi: 10.1002/bjs.4141. [DOI] [PubMed] [Google Scholar]
- 24.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165(1):91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17(41):4545–4553. doi: 10.3748/wjg.v17.i41.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afsani N. Clinical application of dexmedetomidine. S Afr J Anaesthesiol Analg. 2010;16:50–56. [Google Scholar]
- 27.Arcangeli A, D'Alo C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10(8):687–695. doi: 10.2174/138945009788982423. [DOI] [PubMed] [Google Scholar]
- 28.Uysal HY, Cuzdan SS, Kayıran O, Başar H, Fidancı V, Afyoncu E, et al. Preventive effect of dexmedetomidine in ischemia-reperfusion ınjury. J Craniofac Surg. 2012;23(5):1287–1291. doi: 10.1097/SCS.0b013e3182519f24. [DOI] [PubMed] [Google Scholar]
- 29.Tüfek A, Tokgöz O, Aliosmanoglu I, Alabalık U, Evliyaoglu O, Çiftçi T, et al. The protective effects of dexmedetomidine on the liver and remote organs against hepatic ischemia reperfusion injury in rats. Int J Surg. 2013;11(1):96–100. doi: 10.1016/j.ijsu.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Yang D, Li S, Xu Z, Wang X, Bai C. Effects of curcumin or dexamethasone on lung ischaemia-reperfusion injury in rats. Eur Respir J. 2009;33(2):398–404. doi: 10.1183/09031936.00142407. [DOI] [PubMed] [Google Scholar]
- 31.Qiao H, Sanders RD, Ma D, Wu X, Maze M. Sedation improves early outcome in severely septic Sprague Dawley rats. Crit Care. 2009;13(4):R136. doi: 10.1186/cc8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322–1326. doi: 10.1097/01.ccm.0000128579.84228.2a. [DOI] [PubMed] [Google Scholar]
- 33.Cighetti G, Duca I, Bortone I, Sala S, Nava I, Fiorelli G, et al. Oxidative status and malondialdehyde in beta-thalassemia patients. Eur J Clin Invest. 2002;32(suppl 1):55–60. doi: 10.1046/j.1365-2362.2002.0320s1055.x. [DOI] [PubMed] [Google Scholar]
- 34.Kurt A, Ingec M, Isaoglu U, Yılmaz M, Cetin N, Calık M, et al. An investigation about the inhibition of acute ischemia/reperfusion damage by dexmedetomidine in rat ovarian tissue. Gynecol Endocrinol. 2013;29(3):222–225. doi: 10.3109/09513590.2012.665104. [DOI] [PubMed] [Google Scholar]
- 35.Gideroglu K, Yilmaz F, Aksoy F, Bugdayci G, Saglam I, Yimaz F. Montelukast protects axial pattern rat skin flaps against ischemia/reperfusion injury. J Surg Res. 2009;157(2):181–186. doi: 10.1016/j.jss.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu Y, Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, et al. Enhanced endothelial cell injury by activated neutrophils in patients with obstructive jaundice. J Hepatol. 1997;27(5):803–809. doi: 10.1016/s0168-8278(97)80316-9. [DOI] [PubMed] [Google Scholar]
- 37.Ho E, Galougahi KK, Liu CC, Bhindi R. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1(1):483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiliç K, Hanci V, Selek S, Sözmen M, Kiliç N, Citil M, et al. The effects of dexmedetomidine on mesenteric arterial occlusion-associated gut ischemia and reperfusion-induced gut and kidney injury in rabbits. J Surg Res. 2012;178(1):223–232. doi: 10.1016/j.jss.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 39.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1(1):244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin T, Begec Z, Toprak HI, Polat A, Vardi N, Yücel A, et al. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J Surg Res. 2013;183(1):385–390. doi: 10.1016/j.jss.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 41.Chavan SSL, Saxena V, Pillai S, Sontakke A, Ingole D. Reduced glutathione: importance of specimen collection. Indian J Clin Biochem. 2005;20(1):150–152. doi: 10.1007/BF02893062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong X, Xing Q, Li Y, Han X, Sun L. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186(1):240–245. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]