Abstract
Adipokines are peptides secreted by adipose tissue that affect whole-body energy metabolism. Their dysregulated production in obesity has implicated them as important mediators in the pathogenesis of obesity-related risk factors for diabetes and cardiovascular disease. PBEF/visfatin/Nampt has recently been described as a novel adipokine with insulin mimetic properties. However, whether it is an authentic adipokine relevant to the metabolic syndrome remains a matter of some debate.
Introduction
Hypertension and dyslipidemia have long been known to be associated with diabetes and cardiovascular disease (CVD); more recently, insulin resistance and obesity have joined them as major risk factors. This association has led some to propose that the excess weight predisposes individuals to diabetes, hypertension, and dyslipidemia, thereby leading to frank CVD. To develop effective therapeutics, a better understanding of the molecular mechanisms underlying these associations is essential and is therefore the subject of much research. One particularly active area of research is the role of adipose tissue-derived factors in obesity-related metabolic and cardiovascular alterations. It is now clear that adipose tissue functions as an endocrine organ, producing a variety of secreted factors such as leptin, adiponectin, tumor necrosis factor-α (TNF-α), and plasminogen activator inhibitor-1 [1]. These so-called adipokines play important roles in regulating whole-body energy homeostasis, and their dysregulated production in obesity can contribute to the cluster of metabolic disorders previously known as the metabolic syndrome. The recent finding that visfatin (a novel adipokine) is preferentially produced by visceral adipose tissue [2•] has further fueled interest in adipokines and renewed attention to the fact that central obesity (due primarily to increased intra-abdominal or visceral fat) may have a greater detrimental effect than overall weight/body mass index (BMI). Nonetheless, whether visfatin is a bona fide adipokine remains a matter of debate [3-5].
Visfatin, also known as pre-B cell colony-enhancing factor (PBEF) 1 homolog and nicotinamide phosphoribosyltransferase (NMPRTase or Nampt), was first identified in 1994 as a lymphokine that acts as a growth and differentiation factor on early B-lineage precursor lymphocytes [6]. Eleven years later, another group of researchers, also using differential display, identified it as an insulinmimetic molecule that is expressed at much higher levels in visceral fat than in subcutaneous fat and renamed it visfatin [2•]. This nomenclature can be potentially misleading because the expression of PBEF/visfatin/Nampt mRNA is not restricted to visceral adipose tissue. Its mRNA is ubiquitously expressed in all healthy tissues examined, with notably highest expression in bone marrow, liver, and muscle [6]. Similarly, PBEF/visfatin/Nampt protein expression has been detected in numerous tissues, and immunohistologic studies suggest that it can be found in both the cytoplasm and nucleus, albeit in a growth phase-dependent manner [7]. PBEF/visfatin/Nampt is also found as a secreted protein and is detectable in the circulation, where its levels may correlate with obesity [2•].
The presence of secreted PBEF/visfatin/Nampt in the circulation has raised three pertinent questions: 1) How does a 52-kDa protein lacking a typical signal peptide sequence come to be in the blood? 2) What is the biological role(s) of PBEF/visfatin/Nampt? 3) Is PBEF/visfatin/Nampt relevant to the metabolic syndrome? Although definitive answers to these questions remain to be fully established, some clues are beginning to trickle into the current literature.
Firstly, although PBEF/visfatin/Nampt lacks a typical signal peptide sequence, there is now increasing evidence to suggest that its presence in the circulation is due to more than the remnants of dying cells [3]. The original study by Samal et al. [6] reported that transfected COS-7 and mouse embryonic fibroblasts secreted PBEF into the culture medium. This in vitro observation has also been reproduced by more recent studies showing regulated secretion of endogenous PBEF/visfatin/Nampt from cultured neutrophils, as well as differentiating and stimulated adipocytes [2•,8-10]. In vivo, circulating plasma levels appear to be regulated by factors such as exercise, hormones, and pharmacologic treatment [9-12]. Intriguingly, PBEF/visfatin/Nampt is also an example of an adipocytokine whose expression displays circadian rhythmicity in mouse visceral adipose tissue and liver [13]. Similarly, PBEF/visfatin/Nampt may be regulated in response to calorie restriction (unpublished data cited in [14]). However, because these latter profiles have only been studied at the RNA level, it is currently unclear whether they are reflected in the circulation. Nonetheless, the evidence for regulated secretion, taken together with the fact that other cytokines like interleukin (IL)-1β, which are also secreted despite the lack of an obvious secretion peptide [15], supports the possibility that PBEF/visfatin/Nampt is indeed a secreted protein.
The Roles of PBEF/Visfatin/Nampt
The biological roles of PBEF initially appear as varied as are the locations in which it can be found. It is clearly vital for life, as visfatin-deficient (visfatin−/−) mice die during early embryogenesis [2•]. Initial studies described it to be involved in both innate and adaptive immune responses. Specifically, in addition to its actions on B-cell colony formation, it is upregulated in activated lymphocytes, and is involved in B cell proliferation and maturation, and in the progression of lupus-like autoimmune diseases [16]. In neutrophils, its expression is also upregulated by a variety of inflammatory stimuli, and here it functions as an inhibitor of apoptosis. This is particularly relevant in septic polymorphonuclear neutrophils that exhibit delayed apoptosis (eg, in patients with sepsis) [8]. Similarly, the upregulated expression of PBEF in infected human fetal membranes has been linked to infection-induced preterm births, and at least in part involves the presence of invading neutrophils [17]. Other immunologic disorders in which an increased expression of PBEF has been reported include acute lung injury [18] and psoriasis [19].
Recently, visfatin has been reported to mediate insulin-mimetic effects on cultured adipocytes, muscle cells, and hepatocytes. Here it stimulates glucose uptake in adipocytes and myotubes and inhibits glucose production by hepatocytes. Surprisingly, this is reported to be mediated through direct binding and activation of insulin receptors, but without altering or competing with insulin binding. Intriguingly, it does not bind insulin-like growth factor-1 receptors [2•]. Fukuhara et al. [2•] demonstrated that visfatin lowers plasma glucose levels in vivo, and mice heterozygous for a targeted null mutation in the PBEF/visfatin/Nampt gene exhibit mild hyperglycemia compared to wild-type littermates. This clearly suggests that PBEF/visfatin/Nampt may be a hormone-like peptide with potential to regulate carbohydrate metabolism.
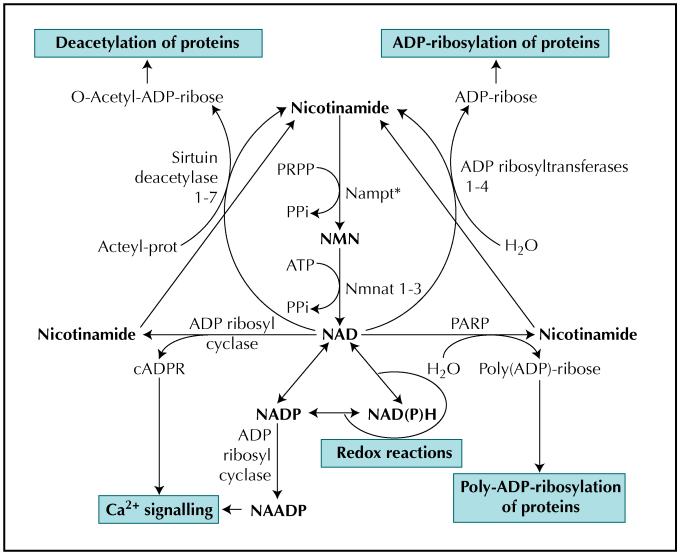
Although lymphokine and hormonal roles of PBEF are linked to the secreted protein, PBEF/visfatin/Nampt is also thought to function intracellularly as a key enzyme in the salvage pathway of nicotinamide adenine dinucleotide (NAD) biosynthesis [20,21•]. PBEF is a nicotinamide phosphoribosyltransferase (NMPRTase or Nampt, Enzyme Commission [EC] 2.4.2.12). It catalyzes the enzymatic condensation of nicotinamide (NAM) with 5-phosphoribosyl-1-pyrophosphate (PRPP) to yield nicotinamide mononucleotide (NMN), an intermediate for the biosynthesis of NAD (Fig. 1). In so doing, it enables the recycling of nicotinamide to NAD, thereby maintaining the NAD pool and preventing NAM leakage out of the cell. This is important in cells undergoing substantial NAD turnover, such as during sustained poly(ADP)-ribosylation activity in tumor cells. In line with this, NMPRTase expression is also upregulated in some cancers [22,23]. Because enzymes that utilize NAD are often compartmentalized within the cell, it is tempting to speculate that the intracellular location of PBEF itself may also reflect enzyme targeting to areas in particular need of NAD regeneration. This may also explain the growth phase-dependent changes in the subcellular localization of PBEF [7].
Figure 1.
The central role of NMPRTase (Nampt*) in the salvage pathway for NAD biosynthesis and related cellular functions. ADP—adenosine diphosphate; ATP—adenosine triphosphate; cADPR—cyclic ADP-ribose; NAADP—nicotinic acid-adenine dinucleotide phosphate; NAD—nicotinamide adenine dinucleotide; NADP—nicotinamide adenine dinucleotide phosphate; NAD(P)H—nicotinamide adenine dinucleotide (phosphate), reduced form; Nampt—nicotinamide phosphoribosyltransferase; NMN—nicotinamide mononucleotide; Nmnat—nicotinamide mononucleotide adenylyltransferase; PARP—poly-ADP-ribose polymerase; PPi—inorganic pyrophosphate; PRPP—5-phosphoribosyl-1-pyrophosphate.
Nonetheless, the possibility that PBEF/visfatin/Nampt can affect NAD metabolism has interesting possibilities for its role(s) in metabolic diseases such as diabetes and CVD. This is true not only because the biochemistry of NAD has well-established roles in redox biochemistry and energy metabolism, but also because nicotinamide cofactors can function as signaling molecules (eg, in Ca2+ signaling) and in a variety of cellular regulatory processes (eg, transcriptional regulation) through their involvement in protein modification reactions as mono- and poly-ADP ribosylation and deacetylation reactions (Fig. 1).
The enzyme activity of NMPRTase has also been subject to pharmacological studies that have yielded a potent and specific chemical inhibitor of NMPRTase, FK866. This is currently in phase II trials for anticancer and anti-angiogenesis therapy. Treatment with FK866 causes the depletion of intracellular NAD levels in tumors, ultimately inducing apoptosis in these cells, while showing little toxicity towards normal cells [24]. FK866 also has potent anti-angiogenic effects in a mouse renal cell carcinoma model [25]. The molecular basis for the specific inhibition of NMPRTase has just been elucidated through determination of the crystal structures of PBEF either alone or in complex with the reaction product, nicotinamide mononucleotide (NMN), or with FK866 [26-28]. Three independent studies all show that PBEF forms homodimers and the active sites lie at the dimer interface. This suggests that dimerization may be an essential requirement for the NMPRTase catalytic activity of PBEF. In addition, Kahn et al. [26] conducted kinetic studies on point mutants to determine the key residues that differ in related enzymes and are responsible for substrate and inhibitor specificity. Wang et al. [27] demonstrated for the first time that Nampt activity may also depend on autophosphorylation.
Unfortunately, because research on the insulin-like action of PBEF/visfatin/Nampt is still in its infancy, no information is currently available regarding the requirement for NMPRTase activity in visfatin’s insulin-mimetic effects or on the effect of FK866 on these properties. Hence, it is far too soon to speculate on the actions of FK866 on features of the metabolic syndrome. Similarly, because the extracellular domains of the insulin receptor have also just been crystallized [29], the molecular interaction between this pair of dimers remains unknown.
PBEF/Visfatin/Nampt and Obesity
Fukuhara et al. [2•] were the first to report that plasma visfatin levels correlated positively with visceral fat area in humans. The plasma levels appear to increase during the development of obesity in both KKAy mice and high-fat diet–fed c57BL/6J mice. The further correlation between increased plasma levels and increased expression in visceral adipose tissue, but not with expression in subcutaneous adipose tissue or liver, presented an interesting paradigm [2•]. This observation has since been reproduced in at least one other study, by Haider et al. [30], who investigated morbidly obese patients with elevated plasma visfatin levels compared to lean controls. Interestingly, this study also observed a significant reduction 6 months after gastric banding and weight loss. However, contradictory reports also exist suggesting that the correlations with obesity may not be so universal. Berndt et al. [31] demonstrated that although visfatin plasma concentration in human subjects correlates positively with the visceral adipose tissue visfatin mRNA expression, BMI, and percent body fat, there is no correlation between circulating visfatin levels and waist-to-hip ratio or the amount (mass) of visceral fat. Furthermore, other recent reports also suggest that although visceral adipose tissue mRNA expression may correlate with BMI, circulating levels of visfatin do not show the same association with BMI. Instead, plasma visfatin is negatively correlated with BMI [32-34]. These observations cast doubt on the notion that visfatin is a secreted adipokine with diagnostic potential in obesity-related disorders.
One study in obese rats stands out because it was unable to find any correlation between visfatin mRNA expression and obesity. However, unlike the above reports, Kloting et al. [35] measured visfatin mRNA in isolated primary adipocytes from subcutaneous and visceral depots of obese WOKW (Wistar Ottawa Karlsburg W) rats. In retrospect, their findings may not be too surprising, because a recent study demonstrated that visfatin protein secretion occurs primarily from macrophages in visceral adipose tissue (ie, the CD14+ population of the stromavascular fraction) and less so from mature adipocytes [36]. Given that the proportion of adipose tissue resident macrophages increases with obesity, this highlights a common problem when interpreting expression data from whole tissues. Conversely, working with isolated primary cells is not without its limitations. It now appears that visfatin production by cultured adipocytes can be significantly stimulated by glucose in the culture media [9]. This new information does influence the interpretation of Kloting’s study, wherein any potential differential expression may have been masked by the culture conditions.
In short, although the mRNA expression of visfatin in visceral adipose tissue may correlate with obesity, the levels of circulating visfatin can exhibit variable associations. However, as is the case for TNF-α, the possibility remains that visfatin expression is primarily upregulated locally in visceral adipose tissue of obese individuals. Here it may be primarily secreted by activated macrophages to act in an autocrine or paracrine manner within this adipose depot. Additionally, it is likely that nonsecreted visfatin is also elevated in visceral adipose tissue from obese subjects, and here its intracellular activity may be important in responding to alterations in metabolic stress. Future studies should help to clarify these issues.
PBEF/Visfatin/Nampt and Type 2 Diabetes Mellitus
Adipokines are thought to play an influential role in the link between obesity and diabetes. However, the link between visfatin levels and diabetes currently remains tenuous, particularly because most studies have focused on the possible correlations between plasma visfatin levels and diabetes. Fukuhara et al. [2•] suggested that plasma levels (and mRNA expression in visceral fat) increased in two rodent models of obesity-related diabetes. This is consistent with a more recent study of visfatin levels in patients with type 2 diabetes mellitus, which reported increasing concentrations of visfatin as being independently and significantly associated with type 2 diabetes even after full adjustment of known biomarkers [37]. However, it contrasts with other reports suggesting visfatin plasma levels are similar in patients with type 2 diabetes, subjects with impaired glucose regulation, and normal glucose-tolerant subjects [34].
Most adipocytokines (eg, TNF-α) that are elevated in obese diabetic models tend to induce insulin resistance, whereas others that correlate negatively with obesity (eg, adiponectin) enhance insulin sensitivity. Clearly, visfatin fits neither profile because it is an insulin-mimetic, whose mRNA expression is enhanced in insulin-resistant visceral adipose tissue. However, as with the studies addressing correlations with type 2 diabetes mellitus, contrasting data are available. Haider et al. [30] found that individual changes of insulin resistance and visfatin levels were significantly associated in morbidly obese patients undergoing weight loss. Conversely, Berndt et al. [31] found no significant correlation between visfatin plasma concentrations and parameters of insulin sensitivity, including fasting insulin and fasting plasma glucose concentrations. Similarly, Pagano et al. [32] also concluded that plasma visfatin levels are not related to insulin resistance in humans. This last study also monitored visfatin levels following free fatty acid infusions that induced a state of insulin resistance. However, neither changes in circulating visfatin nor its adipose tissue mRNA were found after this treatment.
Like Pagano et al. [32], other studies have investigated the role of visfatin in insulin sensitivity through hormonal or pharmacological manipulation of insulin sensitivity. In vitro studies using cultured adipocytes showed that insulin resistance–inducing agents such as growth hormone, TNF-α, IL-6, and isoproterenol downregulated visfatin mRNA, whereas dexamethasone significantly increased PBEF/visfatin/Nampt mRNA [38,39]. This profile is consistent with the insulin-sensitizing role of visfatin, but it contrasts with the cytokine-mediated induction of visfatin seen in other cell types [6]. Furthermore, the finding that troglitazone does not reverse the negative effect of IL-6 on visfatin synthesis [38] is inconsistent and contrasts with the in vivo actions of other thiazolidindiones. For example, treatment of diabetic OLETF (Otsuka Long-Evans Tokushima Fatty) rats with insulin-sensitizing rosiglitazone or fenofibrate treatments elevated the expression of visfatin mRNA in visceral fat depots [40]. Similarly, rosiglitazone treatment increased systemic plasma visfatin concentrations in healthy subjects and in isolated human adipocytes treated with acute and chronic rosiglitazone [10]. This group also shows that while infusion of free fatty acids has little effect on control subjects, this treatment reduces rosiglitazoneinduced elevated visfatin levels [10]. Intriguingly, treatment of newly diagnosed patients with a different thiazolidindione, pioglitazone, has little effect on gene expression or circulating levels of visfatin in either nondiabetic or diabetic individuals, while showing marked improvement in insulin sensitivity [33]. These inconsistencies suggest that visfatin’s actions as a regulator of insulin sensitivity do not follow the conventional profile of an adipokine involved in insulin resistance or type 2 diabetes.
PBEF/Visfatin/Nampt and Hyperglycemia
Another hypothesis currently being addressed is the potential for hyperglycemia to lead to increased visfatin levels in plasma. Intriguingly, Haider et al. [30] have reported glucose infusions can stimulate visfatin secretion, and that this is prevented by co-infusion of insulin or somatostatin. This finding is recapitulated in vitro on isolated human adipocytes [9]. Futhermore, elevated plasma visfatin levels have been reported by the same group, in a study of patients with type 1 diabetes mellitus [11]. Finally, the level of circulating visfatin has also been studied in women with gestational diabetes. However, two independent reports have found significant but opposing correlations when compared to levels in healthy pregnant women [41,42].
PBEF/Visfatin/Nampt, Hyperlipidemia, and Hypertension
To date, no study has directly addressed the relationship between visfatin and either hyperlipidemia or hypertension. However, the insulin-mimetic actions of visfatin also extend to promoting lipogenesis and lipid accumulation in differentiating adipocytes [2•]. This raises the possibility that the autocrine/paracrine effects of visfatin could facilitate the expansion of adipose depots, which may be more biologically relevant in preventing dyslipidemia than its endocrine effects on improving global insulin sensitivity [43].
Additionally, with regard to vascular function, one study worthy of note has found that PBEF expression promotes vascular smooth muscle cell maturation in a NAD+-dependent manner. Knocking down endogenous PBEF/visfatin/Nampt increases apoptosis of vascular smooth muscle cells, and reduces their capacity to mature to a contractile state [44•]. Hence, this study may provide the molecular link by which redox regulation of vascular tone can alter vascular structure in hypertension [45]. So the stage is also now set for future investigations to explore the role of PBEF/visfatin/Nampt in both lipid metabolism and vascular remodeling.
Conclusions
The recent explosion in the knowledge of how adipose tissue-derived factors influence metabolic and cardiovascular disease has revealed the multiple levels at which putative adipokines may affect energy metabolism. It also supports an emerging view that adipose tissue and cells of the immune system are closely related, potentially allowing the coordination of energy metabolism with immunologic responses. In this context, the discovery of PBEF/visfatin/Nampt as a novel adipokine is not without interest. However, although PBEF/visfatin/Nampt clearly has properties of a cytokine, hormone, and enzyme with potential to affect cellular metabolism, the current physiologic evidence suggests that the link between PBEF/visfatin/Nampt expression in visceral adipose tissue and metabolic syndrome is more complicated. Much remains to be addressed before PBEF/visfatin/Nampt can be considered a true adipokine with pathophysiologic relevance to the metabolic syndrome.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 2•.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [Original paper reporting the first identification of visfatin as putative adipokine with insulin mimetic properties.] [DOI] [PubMed] [Google Scholar]
- 3.Hug C, Lodish HF. Medicine. Visfatin: a new adipokine. Science. 2005;307:366–367. doi: 10.1126/science.1106933. [DOI] [PubMed] [Google Scholar]
- 4.Stephens JM, Vidal-Puig AJ. An update on visfatin/pre-B cell colony-enhancing factor, an ubiquitously expressed, illusive cytokine that is regulated in obesity. Curr Opin Lipidol. 2006;17:128–131. doi: 10.1097/01.mol.0000217893.77746.4b. [DOI] [PubMed] [Google Scholar]
- 5.Arner P. Visfatin—a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:28–30. doi: 10.1210/jc.2005-2391. [DOI] [PubMed] [Google Scholar]
- 6.Samal B, Sun Y, Stearns G, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitani T, Okuno S, Fujisawa H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colonyenhancing factor. FEBS Lett. 2003;544:74–78. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 8.Jia SH, Li Y, Parodo J, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haider DG, Schaller G, Kapiotis S, et al. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 10.Haider DG, Mittermayer F, Schaller G, et al. Free fatty acids normalize a rosiglitazone-induced visfatin release. Am J Physiol Endocrinol Metab. 2006;291:E885–890. doi: 10.1152/ajpendo.00109.2006. Epub 2006 May 30. [DOI] [PubMed] [Google Scholar]
- 11.Haider DG, Pleiner J, Francesconi M, et al. Exercise training lowers plasma visfatin concentrations in patients with type 1 diabetes. J Clin Endocrinol Metab. 2006 Aug 8; doi: 10.1210/jc.2006-1013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Frydelund-Larsen L, Akerstrom T, Nielsen S, et al. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise. Am J Physiol Endocrinol Metab. 2006 Jul 25; doi: 10.1152/ajpendo.00113.2006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Ando H, Yanagihara H, Hayashi Y, et al. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Lavu S, Sinclair DA. Nampt/PBEF/visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrei C, Dazzi C, Lotti L, et al. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu LG, Wu M, Hu J, et al. Identification of downstream genes up-regulated by the tumor necrosis factor family member TALL-1. J Leukoc Biol. 2002;72:410–416. [PubMed] [Google Scholar]
- 17.Ognjanovic S, Bao S, Yamamoto SY, et al. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JG. Searching for candidate genes in acute lung injury: SNPs, chips and PBEF. Trans Am Clin Climatol Assoc. 2001;116:205–219. discussion 220. [PMC free article] [PubMed] [Google Scholar]
- 19.Koczan D, Guthke R, Thiesen HJ, et al. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur J Dermatol. 2005;15:251–257. [PubMed] [Google Scholar]
- 20.Rongvaux A, Shea RJ, Mulks MH, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21•.Mattevi A. A close look at NAD biosynthesis. Nat Struct Mol Biol. 2006;13:563–564. doi: 10.1038/nsmb0706-563. [Excellent review of both structure and function of Nampt.] [DOI] [PubMed] [Google Scholar]
- 22.Hufton SE, Moerkerk PT, Brandwijk R, et al. A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett. 1999;463:77–82. doi: 10.1016/s0014-5793(99)01578-1. [DOI] [PubMed] [Google Scholar]
- 23.Van Beijnum JR, Moerkerk PT, Gerbers AJ, et al. Target validation for genomics using peptide-specific phage antibodies: a study of five gene products overexpressed in colorectal cancer. Int J Cancer. 2002;101:118–127. doi: 10.1002/ijc.10584. [DOI] [PubMed] [Google Scholar]
- 24.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 25.Drevs J, Loser R, Rattel B, Esser N. Antiangiogenic potency of FK866/K22.175, a new inhibitor of intracellular NAD biosynthesis, in murine renal cell carcinoma. Anticancer Res. 2003;23:4853–4858. [PubMed] [Google Scholar]
- 26.Khan JA, Tao X, Tong L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat Struct Mol Biol. 2006;13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Zhang X, Bheda P, et al. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- 28.Kim MK, Lee JH, Kim H, et al. Crystal structure of visfatin/pre-b cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866. J Mol Biol. 2006;362:66–77. doi: 10.1016/j.jmb.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 29.McKern NM, Lawrence MC, Streltsov VA, et al. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature. 2006;443:218–221. doi: 10.1038/nature05106. [DOI] [PubMed] [Google Scholar]
- 30.Haider DG, Schindler K, Schaller G, et al. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–1581. doi: 10.1210/jc.2005-2248. [DOI] [PubMed] [Google Scholar]
- 31.Berndt J, Kloting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 32.Pagano C, Pilon C, Olivieri M, et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006;91:3165–3170. doi: 10.1210/jc.2006-0361. [DOI] [PubMed] [Google Scholar]
- 33.Hammarstedt A, Pihlajamaki J, Rotter Sopasakis V, et al. Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J Clin Endocrinol Metab. 2006;91:1181–1184. doi: 10.1210/jc.2005-1395. [DOI] [PubMed] [Google Scholar]
- 34.Jian WX, Luo TH, Gu YY, et al. The visfatin gene is associated with glucose and lipid metabolism in a Chinese population. Diabet Med. 2006;23:967–973. doi: 10.1111/j.1464-5491.2006.01909.x. [DOI] [PubMed] [Google Scholar]
- 35.Kloting N, Kloting I. Visfatin: gene expression in isolated adipocytes and sequence analysis in obese WOKW rats compared with lean control rats. Biochem Biophys Res Commun. 2005;332:1070–1072. doi: 10.1016/j.bbrc.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 36.Curat CA, Wegner V, Sengenes C, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 37.Chen MP, Chung FM, Chang DM, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 38.Kralisch S, Klein J, Lossner U, et al. Interleukin-6 is a negative regulator of visfatin gene expression in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2005;289:E586–590. doi: 10.1152/ajpendo.00090.2005. [DOI] [PubMed] [Google Scholar]
- 39.Kralisch S, Klein J, Lossner U, et al. Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J Endocrinol. 2005;185:R1–8. doi: 10.1677/joe.1.06211. [DOI] [PubMed] [Google Scholar]
- 40.Choi KC, Ryu OH, Lee KW, et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun. 2005;336:747–753. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- 41.Chan TF, Chen YL, Lee CH, et al. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J Soc Gynecol Investig. 2006;13:364–367. doi: 10.1016/j.jsgi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Krzyzanowska K, Krugluger W, Mittermayer F, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 43.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.van der Veer E, Nong Z, O’Neil C, et al. Pre-B-cell colonyenhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [Seminal paper suggesting PBEF action is important for vascular smooth muscle cell biology.] [DOI] [PubMed] [Google Scholar]
- 45.Archer SL. Pre-B-cell colony-enhancing factor regulates vascular smooth muscle maturation through a NAD+-dependent mechanism: recognition of a new mechanism for cell diversity and redox regulation of vascular tone and remodeling. Circ Res. 2005;97:4–7. doi: 10.1161/01.RES.0000174111.52307.64. [DOI] [PubMed] [Google Scholar]