Abstract
Purpose of review
Mitochondrial uncoupling proteins uncouple oxidative phosphorylation. The physiological role ascribed to this process is thermoregulation. The metabolic consequence of mitochondrial respiration uncoupled from ATP production is increased substrate oxidation and metabolic rate. The recent discovery of uncoupling protein 1 (UCP1) positive mitochondria in human adipose tissue has rekindled interest in the role of UCP1 in energy balance and metabolic health.
Recent findings
Recently, there have been numerous reports of functional brown adipose tissue in humans. Further, data from cell and murine studies suggest that beige adipocytes can be induced within white adipose tissue. The presence of brown/beige adipocytes with mitochondria expressing UCP1 negatively correlates with adiposity. Further, activation of these adipocytes alters energy balance and substrate metabolism. However, in humans, brown fat content varies significantly. Further, although beige adipocytes can be induced in white adipose tissue of rodents, whether this is also true in humans remains unclear.
Summary
The presence of UCP1-positive mitochondria in human adipose tissue represents an exciting therapeutic target for treating obesity and its metabolic complications. Understanding the mechanisms governing brown fat activation will be crucial if the therapeutic potential of UCP1 is to be realized.
Keywords: adipose tissue, brown fat, mitochondria, uncoupling protein 1
INTRODUCTION
Obesity results from discordance in energy intake and energy expenditure. Thus, obesity should be treatable by reducing energy intake and/or augmenting energy expenditure. However, despite a clear understanding of the thermodynamics of weight balance, a lack of adherence to diet and exercise prescription means that these strategies often fail to positively impact obesity in humans. Indeed, the prevalence of obesity has risen in recent decades to epidemic proportions, posing a significant clinical and economic burden in both the developed and developing world.
Therapeutic strategies, which alter energy balance without reducing caloric intake or increasing physical activity, represent a means of treating obesity and in particular, the metabolic abnormalities that accompany obesity. Although 2,4-dinitrophenol (DNP) proved efficacious with regards to augmenting energy expenditure in humans, potentially lethal side-effects curtailed its use [1]. Currently, no therapeutic agents, which can alter energy expenditure in humans, are licensed for use in the treatment of obesity.
The recent observation that a significant proportion of humans have depots of brown adipose tissue (BAT) [2-7] represents a genuine opportunity for researchers interested in strategies that increase resting energy expenditure. Activation of mitochondrial uncoupling protein 1 (UCP1) in BAT results in increased substrate oxidation to sustain high levels of leak respiration (mitochondrial respiration not linked to ATP production). Further, recent evidence suggests that some white adipose tissue (WAT) depots in humans contain pockets of UCP1-positive adipocytes [8], often termed beige adipose tissue (BeAT) or BRITE (brown-in-white) fat. Moreover, a significant body of work in cells and rodents suggests that BeAT is inducible in WAT depots [9,10,11■■]. This is intriguing given that humans have far larger WAT depots (kilograms) compared with BAT depots (grams).
THE DIFFERENT SHADES OF FAT
There are numerous WAT depots within the human body, which play differing metabolic and endocrine roles. However, with regards to metabolic rate, these WAT depots are thought to play a minimal role. Indeed, the principle role of WAT appears to be energy storage; only a small number of mitochondria are present to provide ATP for local cellular needs. The identification of symmetrical 2-deoxy-2-(18F)fluoro-D-glucose uptake in the subclavicular region of humans [2-5], particularly following mild cold exposure [2], prompted a paradigm shift in current scientific thinking regarding adipose tissue mitochondrial function. These studies confirmed that adult humans have functional BAT with UCP1-containing mitochondria. Subsequently, humans have at least two distinct depots of adipose tissue, BAT and WAT. With that said, there is significant debate as to whether the supraclavicular BAT seen in humans is truly bona-fide BAT like that seen in animals, or more like the inducible BeAT seen in rodents, as respiratory capacities of human WAT and BAT are not as strikingly different as those of rodents [11■■,12].
With regards to rodents, the picture appears to be clearer. In addition to abundant visceral and subcutaneous WAT depots, distinct BAT depots can be found in the intrascapular region. Furthermore, inducible BeAT can be found in WAT depots, particularly inguinal subcutaneous fat, meaning that adipose tissue in rodents can be classified into three distinct groups according to their oxidative capacity. In what can be considered as analogous to white, intermediate and red skeletal muscle fibers, BAT, BeAT and WAT represent three adipose tissue types with high, intermediate and low oxidative capacity. Similar to that of skeletal muscle, oxidative capacity is governed by vascularization, mitochondrial density (size and number) and intrinsic function (Table 1). However, the distinguishing feature of BAT and BeAT-influencing oxidative capacity is the abundance of UCP1.
Table 1.
Adipocyte characteristics
| WAT | BeAT | BAT | |
|---|---|---|---|
| Cell size | XXX | XX | X |
| Lipid droplet size | XXX | XX | X |
| Lipid droplet number | X | XX | XXX |
| Mitochondrial density | X | XX | XXX |
| UCP1 expression | X | XX | XXX |
| IMM proton conductance | X | XX | XXX |
| Proportion of body mass | 10–50% | ? | 0–0.2% |
BeAT, beige adipose tissue; IMM, inner mitochondrial membrane; UCP1, uncoupling protein 1.
MITOCHONDRIA DICTATE METABOLIC RATE
What we now know as mitochondria were first identified in the cytoplasm of striated muscle by the Swiss anatomist Albert von Kölliker in the mid 1800s. These organelles were studied more comprehensively by the German anatomist Richard Altmann in the late 19th century. In his seminal work The Elementary Organism (1890), Altmann described small subcellular granules similar in size to bacteria. Altmann postulated that these ‘Bioblasts’ represented basic units of cellular life that were somewhat autonomous from the cell. A few years after the publication of The Elementary Organism, the term mitochondria, meaning stringy granule, was coined to the German microbiologist Carl Benda.
In the first half of the 20th century, work focused on characterizing the role of mitochondria in energy metabolism. This culminated in the chemiosmostic theory of oxidative phosphorylation [13], for which Peter Mitchel was awarded the Nobel prize in chemistry in 1978. Oxidative phosphorylation couples electron transfer to oxygen consumption (respiration) with proton excursion across the inner mitochondrial membrane (phosphorylation). This pinpoints the mitochondrion as the site of molecular oxygen consumption in mammals. Indeed, it has been estimated that approximately 90% of whole body oxygen consumption in mammals occur within the mitochondrion [14], where cytochrome C oxidase reduces molecular oxygen to water. Subsequently, the mitochondrion is the cellular organelle that dictates metabolic rate in mammals.
OXIDATIVE PHOSPHORYLATION
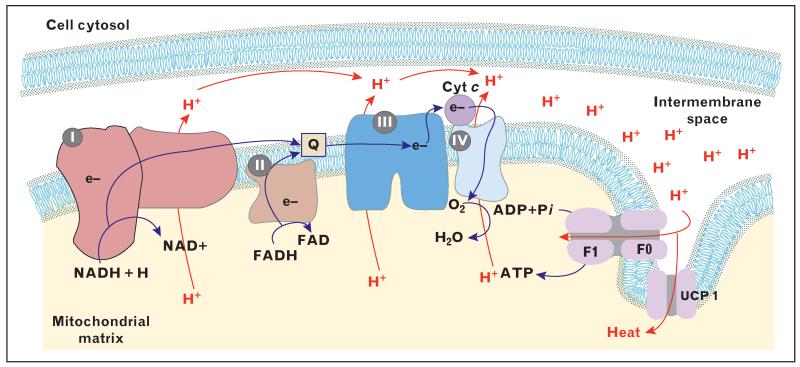
Mitochondria harness redox potential generated from the oxidation of substrates by transferring electrons through a series of membrane-bound respiratory complexes and electron carriers (Fig. 1). Electrons from NADH and FADH2 are shuttled to ubiquinone via complex I (NADH oxidase) and complex II (succinate dehydrogenase), respectively. Reduced ubiquinone subsequently transfers these electrons to complex III (cytochrome bc oxidoreductase) and then to complex IV (cytochrome C oxidase) via the electron carrier cytochrome C. At complex IV, electrons are transferred to molecular oxygen, forming water. As such, the mitochondrial electron transfer chain controls respiration (oxygen consumption), as oxygen is the terminal electron acceptor of the chain (Fig. 1).
FIGURE 1.
Schematic representation of the components of the electron transport chain. ADP, adenosine diphosphate; ATP, adenosine triphosphate; Cyt c, cytochrome C; e, electrons; F1F0, ATP synthase; FAD, oxidized flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide; H, protons; H2O, water; I, NADH oxidase; II, succinate dehydrogenase; III, cytochrome bc oxidoreductase; IV, cytochrome C oxidase; NAD, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; O2, molecular oxygen; Pi, inorganic phosphate; Q, Ubiquinone; UCP1, uncoupling protein 1.
The process of mitochondrial electron transfer drives proton excursion across the inner mitochondrial matrix (Fig. 1). Specifically, complexes I, III and IV pump matrix protons into the space enclosed by the inner and outer mitochondrial membranes. The accumulation of protons within the membrane space results in the development of proton motive force. Protons re-enter the mitochondrial matrix by chemiosmosis. Proton movement through the oligomycin-sensitive FO unit of ATP synthase results in a conformational change in the F1 unit of the enzyme complex, catalyzing the phosphorylation of ADP.
MITOCHONDRIAL COUPLING CONTROL: THE PHYSIOLOGY OF UNCOUPLING PROTEIN 1-POSITIVE MITOCHONDRIA
Not all mitochondrial respiration is linked to ATP production in vivo. Indeed, it has been estimated that around 80% of mitochondrial oxygen consumption is coupled to ATP production in mammals [14]; the remaining 20% of mitochondrial respiration is uncoupled from phosphorylation. This discordance is explained by proton leaks, where protons within the enclosed mitochondrial membrane space can re-enter the mitochondrial matrix independently of ATP synthase. As such, leak respiration (the transfer of electrons to oxygen that is not accompanied with mitochondrial ATP production) represents a significant portion of whole body oxygen consumption. Thus, the coupling of oxidative phosphorylation in vivo represents a key regulator of whole body metabolic rate.
The local ATP demands of a tissue dictate the number and functionality of mitochondria that reside within that particular tissue. For example, adipocytes require ATP for cellular housekeeping functions only (protein turnover etc). Therefore, adipocytes have few mitochondria per unit of tissue. Contrastingly, myocytes require ATP for both cellular housekeeping and for muscular contraction. As such, myocytes are densely populated with mitochondria. Further mitochondrial coupling control is also governed by ATP turnover, where tissues such as cardiac muscle are particularly sensitive to ADP.
A key feature of BAT and BeAT, which sets them apart from other tissues, is the disassociation of their mitochondrial density and functionality from ATP production. This difference in bioenergetics is mediated by UCP1. UCP1, originally named thermogenin, has the ability to almost completely dissipate mitochondrial membrane potential [15], meaning the proton gradient of the mitochondrial membranes is lost as heat. Although numerous other homologues of UCP1 exist in other tissues, such as muscle and liver, it is thought that these UCPs play a much smaller role in inner mitochondrial membrane proton conductance. Indeed, it is has been postulated that these other UCPs permit a small degree of proton conductance to maintain electron transfer to oxygen and prevent free radical production.
UCP1 does not appear to be intrinsically active in BAT mitochondria [16■], where purine nucleotides (presumably ADP and ATP in vivo) inhibit UCP1 [12]. As such, there is little residual proton conductance. However, upon activation by fatty acids, UCP1-containing mitochondria respire at a marked rate, but do not produce any measurable membrane potential [11■■,12,16■]. Further, chemically uncoupling activated BAT mitochondria with the ionophore carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP) only marginally increases respiration above that of leak respiration (respiration with substrates alone and no phosphate acceptor) [11■■,17,18]. This suggests that electron transfer in BAT is almost completely uninhibited when UCP1 is activated, meaning that fuel demand will be elevated above that of a well coupled mitochondria. For example, leak respiration is generally low in muscle mitochondria, and respiration is only elevated when both substrates and ADP are provided. As such, electron flow and thus substrate oxidation in muscle are dependent on ATP needs, where phosphorylation rather than electron transfer is the rate-limiting step. This is not the case in BAT mitochondria when UCP1 is activated, where mitochondrial respiration and thus fuel oxidation are not curtailed in such a manner. Indeed, only GDP appears to reduce mitochondrial respiration and restore mitochondrial membrane potential in BAT mitochondria [11■■,12,16■], underscoring the fact that activated UCP1 is responsible for proton conductance in BAT mitochondria.
Intriguingly, it has recently been shown that mice acclimated to 4 °C develop functional UCP1 in inguinal WAT [11■■]. Isolated mitochondria from this WAT depot exhibit marked increases in leak respiration in response to fatty acids, pyruvate, or glycerol-3-phosphate. Further, this leak respiration can largely be blocked by GDP. Again, what is quiet striking is that in mice acclimated to 30 °C, uncoupling both BAT and inguinal WAT mitochondria with FCCP results in a marked increase in respiration above that of leak respiration with substrates alone, suggesting that mitochondrial leak respiration (i.e. thermogenesis) in these animals is well within the maximal capacity of the mitochondria to transfer electrons to oxygen. However, the addition of FCCP to mitochondria of 4 °C acclimated mice, where UCP1 has been inhibited by GDP, results in mitochondrial respiratory rates almost identical to those when mitochondrial were provided with substrate alone (in the absence of GDP) [11■■]. This again highlights the unusual functional characteristics of mitochondria with activated UCP1, where electron transfer and fuel oxidation precede at or near the maximal capacity of the mitochondria. This is something not seen in mitochondria in other tissues, where respiration is limited by proton accumulation within the mitochondrial membrane.
THE ROLE OF BROWN ADIPOSE TISSUE/BEIGE ADIPOSE TISSUE IN HUMAN ENERGY EXPENDITURE AND SUBSTRATE METABOLISM
Since the discovery of BAT in humans, there has been a significant research effort to ascertain whether recruitment of UCP1-positive mitochondria has metabolic consequences for humans. Early studies, showing that glucose uptake in supraclavicular regions was increased following cold exposure, suggested that pockets of BAT in humans were indeed thermogenic [3]. Further, the presence of BAT in humans is inversely correlated with BMI, suggestive of BAT being protective against obesity [4]. Indeed, BAT activity following mild cold exposure is lower in obese individuals compared with lean individuals, where BMI and adiposity are negatively correlated with BAT activity, and resting metabolic rate is positively correlated with BAT activity [2]. Collectively, these observations suggest that in humans, BAT activation plays a role in energy expenditure.
The chronic activation of BAT by cold exposure has been shown to augment energy expenditure in humans [19]. This was achieved by exposing individuals to mild (17 °C) cold exposure for 2 h/day for a total of 6 weeks. Further, increased BAT activity was accompanied by reduced adiposity [19]. Moreover, others have shown that 10 days of mild cold exposure (6 h/day) resulted in an increase in the activity of BAT, which was accompanied by increased nonshivering thermogenesis [20■]. Interestingly, these researchers showed that this cold exposure protocol did not alter subcutaneous WAT function.
Little data are available concerning the role of BAT in fuel metabolism in humans. Orava et al. [21] showed that acute cold exposure resulted in a 12-fold increase in glucose uptake by BAT, suggesting that BAT plays a role in plasma glucose clearance. However, these investigators did not show differences in whole-body insulin-stimulated glucose disposal between individuals with detectable BAT (BAT+) and nondetectable BAT (BAT−), questioning the ability of BAT to modulate whole-body glucose metabolism. More recently, Quellet et al. [22] showed that acute cold exposure resulted in a 1.8-fold increase in energy expenditure. However, even though BAT activation resulted in increased oxidation of intracellular fatty acids, plasma substrate oxidation by BAT was minimal, casting doubt over the role of BAT in whole-body metabolism in humans.
We have recently studied the role of BAT activation in whole-body insulin sensitivity in humans. Using glucose clamps and stable isotope methodologies, we found that prolonged (5–8 h) cold exposure increases whole body glucose disposal and insulin sensitivity in humans [23■]. These data suggest that the activation of BAT can acutely alter whole-body energy expenditure, glucose homeostasis and insulin sensitivity in humans, supporting the notion that BAT may function as an antidiabetic tissue.
RECRUITING BROWN ADIPOSE TISSUE/BEIGE ADIPOSE TISSUE IN HUMANS
Several studies have shown that acute mild cold exposure activates existing BAT depots in humans. Further, chronic (6 weeks) exposure to mild cold stimulus (2 h/day) results in a significant increase in BAT activity in humans [19]. This increase in BAT activity was associated with reduced adiposity, suggesting that recruitment of BAT is possible in humans and that this has favorable metabolic consequences. However, BAT depots in humans are small, amounting to no more than a few hundred grams. In contrast, humans have vast depots of WAT, which suggest that if WAT could acquire a BAT-like phenotype in humans, huge alterations in energy expenditure and fuel metabolism could be achieved.
Although the adrenergic response to mild cold exposure is sufficient to acutely activate pre-existing BAT in humans, prolonged mild cold exposure for 10 days does not recruit BeAT in the subcutaneous WAT of humans (i.e. browning of WAT) [20■]. One potential issue is that mild cold exposure, regardless of its duration, may be an insufficient stress to cause browning of WAT. For example, browning in animals is achieved by acclimation to 4 °C [11■■], whereas mild cold exposure in humans usually comprises a few hours at ~16-20°C. van der Lans et al. [20■] demonstrated that mild cold exposure resulted in a ~1.5-fold increase in circulating norepinephrine levels. Whether these transient increases in catecholamines, which activate BAT, can cause browning of WAT is doubtful. Interestingly, Frontini et al. [8] reported that in a cohort of patients affected by pheochromocytoma, adrenal tumors that result in chronic catecholamine release, around 50% of patients had UCP1-positive adipocytes in omental fat depots. Unfortunately, mitochondrial function assays were not performed, so it cannot be concluded whether the oxidative capacity or more specifically the thermogenic capacity of this adipose tissue was different to that of normal WAT.
Despite a wealth of data from cells and rodents, and the study by Frontini et al. in omental adipocytes, evidence of browning of human subcutaneous WAT is currently lacking. We have recently shown in children with massive burns that subcutaneous WAT develops a BAT-like phenotype. In particular, the presence of UCP1 mRNA and protein is accompanied by increased uncoupled mitochondrial respiration [24]. Furthermore, this was accompanied by hypermetabolism (elevated resting metabolic rate) in these burn victims. These data are the first to demonstrate browning of human subcutaneous WAT. Burn patients are unique in that chronic 10-fold increases in circulating norepinephrine levels are observed for months post injury, which is accompanied by persistent increases in metabolic rate [25]. We saw the appearance of subcutaneous BeAT at approximately 2 weeks post injury, suggesting that the persistent adrenergic response to burns is sufficient to cause browning of WAT. Subsequently, our data suggest that if the stimulus (adrenergic stress) is intense and persistent for over 2 weeks, classical WAT depots of humans can adopt a BAT phenotype.
The metabolic consequences of browning of subcutaneous WAT in unburned individuals remain to be seen. However, if a similar degree of browning could be induced in healthy or indeed obese individuals, it is likely that this would have a significant impact on energy expenditure and substrate metabolism. For example, BeAT mitochondria of mice acclimated to 4°C have a leak respiratory capacity (thermogenic capacity) around 20% of that of BAT [11■■]. However, when considering that humans have at best a few hundred grams of BAT but tens of kilograms of WAT, suggests the conversion of WAT to a BeAT phenotype will likely have a more profound impact on energy balance. Even a more conservative change in WAT thermogenic capacity could be of significance to energy expenditure and metabolic health in humans. By way of example, our data suggest that in response to burn injury, WAT tissue mitochondrial thermogenesis increases 2-fold to 3-fold. As WAT represents ~6.2% of resting energy expenditure in humans, or ~125 kcal/day in a 70-kg male with 20% body fat [17], a 2-fold to 3-fold increase in WAT thermogenesis computes to a 125-250 kcal/day increase in energy expenditure. If this could be achieved in humans, then this would likely impact adiposity and metabolism.
CONCLUSION
Adipose tissue mitochondria were long considered to be quiescent cellular organelles that played a minor role in whole-body metabolism. Since the discovery of functional BAT in humans, the spotlight has been put on adipose tissue mitochondria and the potential therapeutic impact of UCP1 expression. Activation of BAT and its UCP1-positive mitochondria augments energy expenditure and improves circulating substrate metabolism in humans. However, the quantity of BAT varies significantly in humans, and is never more than a few hundred grams. The possibility of BeAT induction in the far larger WAT depots of humans holds much promise. However, for the therapeutic potential of BeAT to be realized, the events leading to the induction of UCP1 mitochondria need to be better understood.
KEY POINTS.
UCP1-positive mitochondria can increase energy in humans when activated.
Browning of classical white adipose tissue in humans is possible after prolonged adrenergic stress.
The recruitment/activation of UCP1-positive mitochondria represents a means of treating obesity and its metabolic complications.
Effective strategies that recruit/activate UCP1-positive mitochondria are needed before the therapeutic potential of uncoupled mitochondria can be realized.
Acknowledgements
This study is supported by grants from the National Institutes of Health (P50-GM60338, R01-GM05668, Clinical and Translational Science Award (UL1TR000071), grants from the Shriners Hospitals for Children (85310, 84090), the John Sealy Memorial Endowment Fund for Biomedical Research (66992), ADA (1-14-TS-35) and the Sealy Center on Aging at UTMB. C.P. is supported by a National Institute of Disability and Rehabilitation Research and Department of Education Postdoctoral Training Grant (H133P110012).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■ ■ of outstanding interest
- 1.Bartlett J, Brunner M, Gough K. Deliberate poisoning with dinitrophenol (DNP): an unlicensed weight loss pill. Emerg Med J. 2010;27:159–160. doi: 10.1136/emj.2008.069401. [DOI] [PubMed] [Google Scholar]
- 2.van Marken Lichtenbelt W, Vanhommerig J, Smulders N, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 3.Virtanen K, Lidell M, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM LS, Williams G, et al. Identification and importance of brown, adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 6.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zingaretti M, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 8.Frontini A, Vitali A, Perugini J, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta. 2013;1831:950–959. doi: 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Mitschke M, Hoffmann L, Gnad T, et al. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 2013;27:1621–1630. doi: 10.1096/fj.12-221580. [DOI] [PubMed] [Google Scholar]
- 10.Petrovic N, Walden T, Shabalina I, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11■■.Shabalina I, Petrovic N, de Jong J, et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. This study elegantly demonstrates that following cold exposure, BeAT mitochondria exhibit functional characteristics of BAT mitochondria. These data show that UCP1 expression in WAT is of functional significance.
- 12.Shabalina I, Ost M, Petrovic N, et al. Uncoupling protein-1 is not leaky. Biochim Biophys Acta. 2010;1797:773–784. doi: 10.1016/j.bbabio.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 14.Rolfe D, Brown G. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 15.Nicholls D, Bernson V, Heaton G. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl. 1978;32:89–93. doi: 10.1007/978-3-0348-5559-4_9. [DOI] [PubMed] [Google Scholar]
- 16■.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta. 2013;1831:943–949. doi: 10.1016/j.bbalip.2013.01.009. This study discusses the disassociation in UCP1 levels and thermogenic capacity in BAT/BeAT, arguing that UCP1 mRNA levels alone may be misleading when trying to determine the thermogenic capacity of BAT/BeAT.
- 17.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:249–258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 18.Nedergaard J, Matthias A, Golozoubova V, et al. UCP1: The original uncoupling protein - and perhaps the only one? J Bioenerg Biomembr. 1999;31:475–491. doi: 10.1023/a:1005400507802. [DOI] [PubMed] [Google Scholar]
- 19.Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20■.van der Lans A, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. This study shows that 10 days of mild cold exposure, while activating BAT, has no impact on WAT. This is important, as it shows that mild cold exposure protocols, which are known to activate existing BAT in humans, do not result in browning of WAT.
- 21.Orava J, Nuutila P, Lidell M, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Quellet V, Labbe S, Blondin D, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 ■.Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue activation improves glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014 doi: 10.2337/db14-0746. Epub ahead of print. Our recent data, which are currently in press, show for the first time that acute activation of BAT alters whole-body glucose metabolism and insulin sensitivity. This could have far reaching implications in the setting of metabolic syndrome/diabetes.
- 24.Sidossis L, Porter C, Saraf M, et al. Browning of subcutaneous adipose tissue in humans after severe adrenergic stress. FASEB J. 2014 doi: 10.1016/j.cmet.2015.06.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]