Abstract
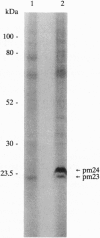
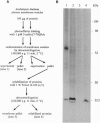
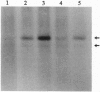
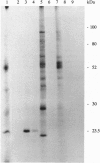
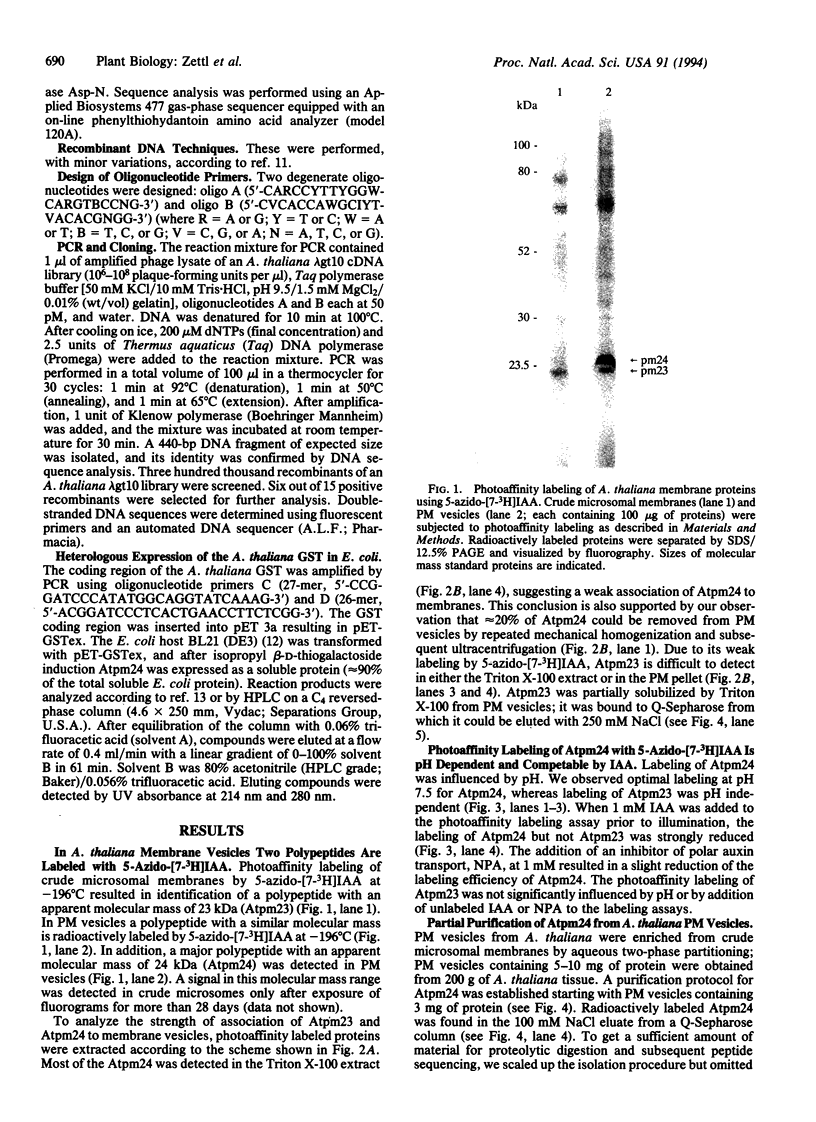
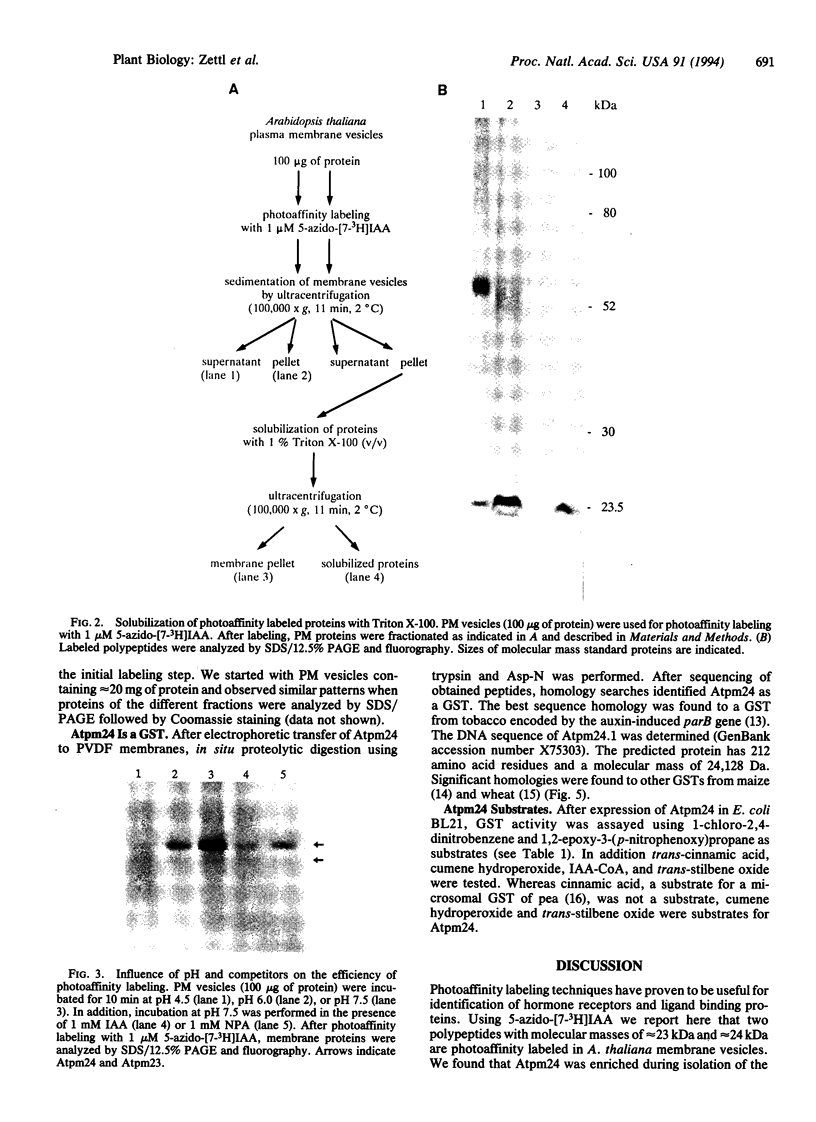
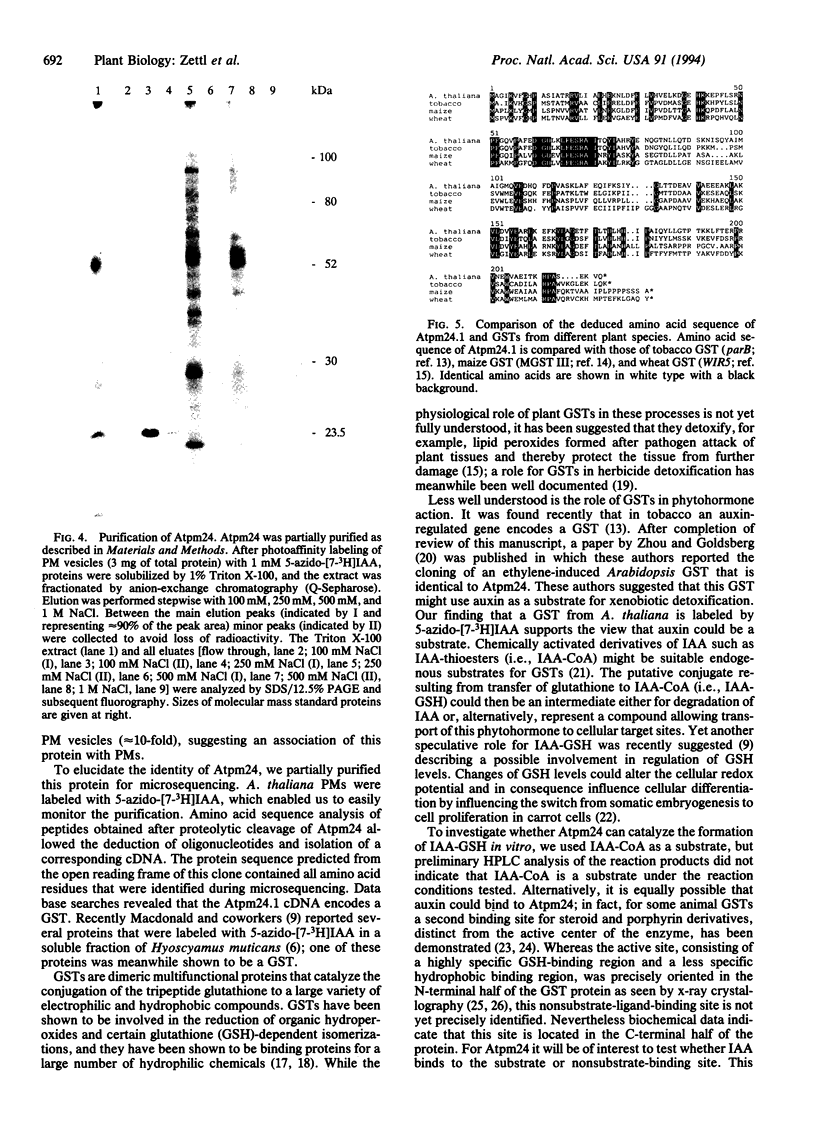
We used 5-azido-[7-3H]indole-3-acetic acid (5-azido-[7-3H]IAA), a photoaffinity analogue of the plant hormone indole-3-acetic acid (IAA), to search for auxin-binding proteins in Arabidopsis thaliana membranes. We identified an auxin-binding protein with a molecular mass of 24 kDa (Atpm24) in microsomes as well as in plasma membrane vesicles. Atpm24 was solubilized by 1% Triton X-100 and partially purified. A cDNA clone (Atpm24.1) corresponding to Atpm24 was isolated. The amino acid sequence predicted from the Atpm24.1 cDNA contains 212 amino acid residues with a relative molecular mass of 24,128 Da. Data base searches revealed that the predicted protein has homology to glutathione S-transferases (GSTs; EC 2.5.1.18). When Atpm24.1 was expressed in Escherichia coli, we found a high level of GST activity in the bacterial extracts. We have analyzed the substrate specificity of this protein and found that cumene hydroperoxide and trans-stilbene oxide but not trans-cinnamic acid or IAA-CoA were substrates. A role for this GST in physiological processes of plants is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhargava M. M., Listowsky I., Arias I. M. Studies on subunit structure and evidence that ligandin is a heterodimer. J Biol Chem. 1978 Jun 25;253(12):4116–4119. [PubMed] [Google Scholar]
- Bilang J., Macdonald H., King P. J., Sturm A. A soluble auxin-binding protein from Hyoscyamus muticus is a glutathione S-transferase. Plant Physiol. 1993 May;102(1):29–34. doi: 10.1104/pp.102.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong J. L., Morgenstern R., Jörnvall H., DePierre J. W., Tu C. P. Gene expression of rat and human microsomal glutathione S-transferases. J Biol Chem. 1988 Jun 15;263(17):8430–8436. [PubMed] [Google Scholar]
- Dudler R., Hertig C., Rebmann G., Bull J., Mauch F. A pathogen-induced wheat gene encodes a protein homologous to glutathione-S-transferases. Mol Plant Microbe Interact. 1991 Jan-Feb;4(1):14–18. doi: 10.1094/mpmi-4-014. [DOI] [PubMed] [Google Scholar]
- Feldwisch J., Zettl R., Hesse F., Schell J., Palme K. An auxin-binding protein is localized to the plasma membrane of maize coleoptile cells: identification by photoaffinity labeling and purification of a 23-kda polypeptide. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):475–479. doi: 10.1073/pnas.89.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove G., Zarlengo R. P., Timmerman K. P., Li N. Q., Tam M. F., Tu C. P. Characterization and heterospecific expression of cDNA clones of genes in the maize GSH S-transferase multigene family. Nucleic Acids Res. 1988 Jan 25;16(2):425–438. doi: 10.1093/nar/16.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Jones A. M., Lomax T. L. Specific photoaffinity labeling of two plasma membrane polypeptides with an azido auxin. Proc Natl Acad Sci U S A. 1989 Jul;86:4948–4952. doi: 10.1073/pnas.86.13.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks G. R., Rayle D. L., Lomax T. L. The diageotropica mutant of tomato lacks high specific activity auxin binding sites. Science. 1989 Jul 7;245:52–54. doi: 10.1126/science.245.4913.52. [DOI] [PubMed] [Google Scholar]
- Jones A. M., Venis M. A. Photoaffinity labeling of indole-3-acetic acid-binding proteins in maize. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6153–6156. doi: 10.1073/pnas.86.16.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald H., Jones A. M., King P. J. Photoaffinity labeling of soluble auxin-binding proteins. J Biol Chem. 1991 Apr 25;266(12):7393–7399. [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W. Microsomal glutathione transferase. Purification in unactivated form and further characterization of the activation process, substrate specificity and amino acid composition. Eur J Biochem. 1983 Aug 15;134(3):591–597. doi: 10.1111/j.1432-1033.1983.tb07607.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Morgenstern R., Meijer J., Depierre J. W., Ernster L. Characterization of rat-liver microsomal glutathione S-transferase activity. Eur J Biochem. 1980 Feb;104(1):167–174. doi: 10.1111/j.1432-1033.1980.tb04412.x. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Huber R., Lo Bello M., Federici G., Parker M. W. Three-dimensional structure of class pi glutathione S-transferase from human placenta in complex with S-hexylglutathione at 2.8 A resolution. J Mol Biol. 1992 Sep 5;227(1):214–226. doi: 10.1016/0022-2836(92)90692-d. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Schäffer J., Gallay O., Huber R. The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 1991 Aug;10(8):1997–2005. doi: 10.1002/j.1460-2075.1991.tb07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Nagata T. parB: an auxin-regulated gene encoding glutathione S-transferase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):56–59. doi: 10.1073/pnas.89.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Goldsbrough P. B. An Arabidopsis gene with homology to glutathione S-transferases is regulated by ethylene. Plant Mol Biol. 1993 Jun;22(3):517–523. doi: 10.1007/BF00015980. [DOI] [PubMed] [Google Scholar]