Abstract
Objective
Little is known about how patient-clinician communication leads to better outcomes. Among patients with diabetes, we describe patient-reported use of collaborative goal setting and evaluate whether perceived competency and physician trust mediate the association between collaborative goal setting and glycemic control.
Methods
Data from a patient survey administered in 2008 to a cohort of insured patients aged 18+ years with diabetes who initiated oral mono-therapy between 2000–2005 were joined with pharmaceutical claims data for the prior 12 months and laboratory data for the prior and subsequent 12 months (N=1,065). A structural equation model (SEM) was used to test mediation models controlling for baseline HbA1c.
Results
The hypothesized mediation model was supported. Patient-reported use of more collaborative goal setting was associated with greater perceived self-management competency and increased level of trust in the physician (p<0.05). In turn, both greater perceived competence and increased trust were associated with increased control (p< 0.05).
Conclusions
Findings indicate that engaging patients in collaborative goal setting during clinic encounters has potential to foster a trusting patient-clinician relationship as well as enhance patient perceived competence, thereby improving clinical control.
Practice Implications
Fostering collaborative goal setting may yield payoffs in improved clinical outcomes among patients with diabetes.
1. Introduction
Despite the availability of effective pharmacological and other treatments, clinical control, measured by Hemoglobin (Hb) A1c, is often not achieved among patients with diabetes.(1–2) Recently, interest has centered on how the features of patient-clinician communication may affect health outcomes.(3) Of particular interest is the role of active patient participation during clinical encounters.(4)
Previous observational(5–8) and interventional studies(9) have highlighted the benefits of active patient participation in medical encounters, although findings in the context of diabetes care are mixed.(10) One key component of active patient participation is collaborative goal setting.(11) Collaboratively setting a goal has been shown to lead to increased levels of goal commitment. (4, 12–17) Furthermore, collaboratively helping patients set and follow up on goals may be an effective way to help patients improve their self-efficacy, an important predecessor to effective self-management, and thus glycemic control and other patient-centered outcomes. (11, 18) Furthermore, the act of collaboratively setting goals may be beneficial to patient-clinician rapport, improving factors, such as patient trust, which have been shown to improve patient adherence to recommended treatment.(19) As such, the American Diabetes Association’s clinical practice guidelines acknowledge the importance of collaborative goal setting in diabetes care management.(20)
Among patients with diabetes, patients’ perceptions of collaborative care (including collaborative goal setting) have been shown to be associated with patients’ reported self-management(7) and, indirectly, with hypertension control.(5) However, the relationship between collaborative goal setting and clinical control among patients with diabetes remains poorly understood. Using a patient survey joined with laboratory data on HbA1c control among a sample of insured, primary care patients with diabetes, we describe patient-reported use of collaborative goal setting when receiving medical care for their diabetes, and evaluate the associations between patient reports of collaborative goal setting and subsequent glycemic control (as measured by HbA1c). As advocated by Street and colleagues,(3) we do so by evaluating plausible pathways through which communication may contribute to healing. Specifically, we evaluate whether patient perceived self-management competence and physician trust mediate the relationship between patient-reported use of collaborative goal setting when receiving medical care for their diabetes and subsequent glycemic control.
2. Methods
2.1 Patient selection
Survey-eligible patients were selected from a previously established cohort of insured patients aged 18 years and over who initiated oral mono-therapy between 2000 and 2005.(21) This cohort included all insured patients receiving diabetes care between 2000 and 2005 from a salaried, multi-specialty group practice in southeast Michigan. The medical group, which staffs 27 ambulatory clinics in Detroit and its surrounding suburbs, is owned by an integrated health system which maintains a large data repository that is commonly used for research purposes.
In October 2008 a subset of this original cohort was identified for survey administration. Survey-eligible patients were those with an office visit to a primary care physician or endocrinologist in the prior 6 months and who maintained their health insurance coverage with the health system-affiliated health plan. Because of the goals of the parent project that assembled the original cohort, patients with an insulin dispensing and those with no HbA1c testing in the prior year were excluded. Using each patient’s most recent HbA1c test result in the prior year, the survey was administered to all survey-eligible cohort members with an HbA1c >8% (N=418) plus a random sample of those with an HbA1c <l;8% (n=1,162), resulting in a survey cohort of N=1,580 patients.
2.2 Data sources
Automated Laboratory Data
All laboratory values used were obtained from the medical group’s affiliated clinical laboratory. Per study eligibility criteria, an HbA1c test result was available for all sample members at the time of survey administration (i.e., baseline). The first HbA1c test result available from the laboratory’s automated processing system in the 12 months following survey administration was used for the post HbA1c outcome.
Patient Survey
A mixed-mode mail/telephone survey was administered between January 22nd and May 3rd 2008. The survey included questions regarding the patient’s perceptions of their health care team’s use of collaborative goal setting,(5) their own perceived competence in managing their diabetes,(6) physician trust,(22) height, weight, and socio-demographic characteristics such as marital status, race, and educational attainment. Along with the survey and a stamped return envelope, the mailed survey packet included a $2 bill and a letter of study introduction signed by the principal investigator. The letter, which explained the study and contained elements of informed consent (including an opt-out option), was sent on Health System letterhead. Survey administration followed a modified Dillman process.(23) Nonresponders to the mailed survey were contacted via telephone and asked if they would complete a telephone interview. Survey responders, regardless of mode, received $20 cash.
Pharmaceutical Claims Data
Outpatient pharmaceutical claims data from the 12-month period prior to survey administration were used to identify and control for the number of different types of oral anti-diabetic medications dispensed to the patient.
2.3 Measures
Primary Outcome
The primary outcome of interest was the first HbA1c test result on record in the health system’s clinical laboratory in the 12-month period following survey administration. Only test results that were at least 90 days following the baseline HbA1c value, but still within 12 months of survey administration, were considered.
2.3.1 Collaborative goal setting
Patients’ perception of collaborative goal setting when receiving medical care for their diabetes was evaluated using three items from the Patient Assessment of Chronic Illness Care (PACIC) instrument.(24–25) For each of these items (i.e., (1) asked to talk about my goals in caring for my diabetes; (2) helped to set specific goals to improve my eating or exercise; and (3) set a goal together with my team for what I could do to manage my diabetes) participants are asked to rate how often each event occurred over the past six months using a scale that ranges from 1 (Never) to 5 (Very Often). The reliability of the resulting factor (latent variable) was confirmed in the current sample: the Cronbach’s Alpha internal consistency estimate of collaborative goal setting scale was high (0.83).
2.3.2 Perceived Competence
Survey respondents who reported having an HbA1c target level were also asked to report the degree to which they feel able to manage the daily aspects of diabetes care using the previously validated Perceived Competence for Diabetes Scale.(6) Using a seven-point Likert format (where 1 reflects “not at all true” and 7 reflects “very true”), this scale asks respondents to indicate how true four statements are for them. The statements address the patient’s ability to manage disease, feeling capable of handling their disease, ability to do own routine care, and feeling able to meet challenges of controlling diabetes. Because survey respondents who did not report having a target level for their HbA1c control were not asked to complete this scale, for analyses we created a binary variable reflective of having an HbA1c target level and reporting high perceived competence (i.e., a score greater than or equal to 20) vs. other (which included both respondents who reported low perceived competence [i.e., a score less 20] and those with no target level for their HbA1c).
2.3.3 Physician trust
Patient-reported interpersonal physician trust was measured using the item “I trust this doctor’s judgments about my medical care,”(22) with a Likert format ranging from 1=Strongly disagree to 7=Strongly agree.
2.3.4 Controls
In addition to these endogenous factors, we considered a number of exogenous factors. These included patient age, gender, race, marital status, and educational attainment. We also controlled for body mass index > 40 (as calculated from self-reported height and weight) as well as the number of different oral anti-diabetic medications dispensed to the patient at the time of survey administration.
2.4 Statistical methods
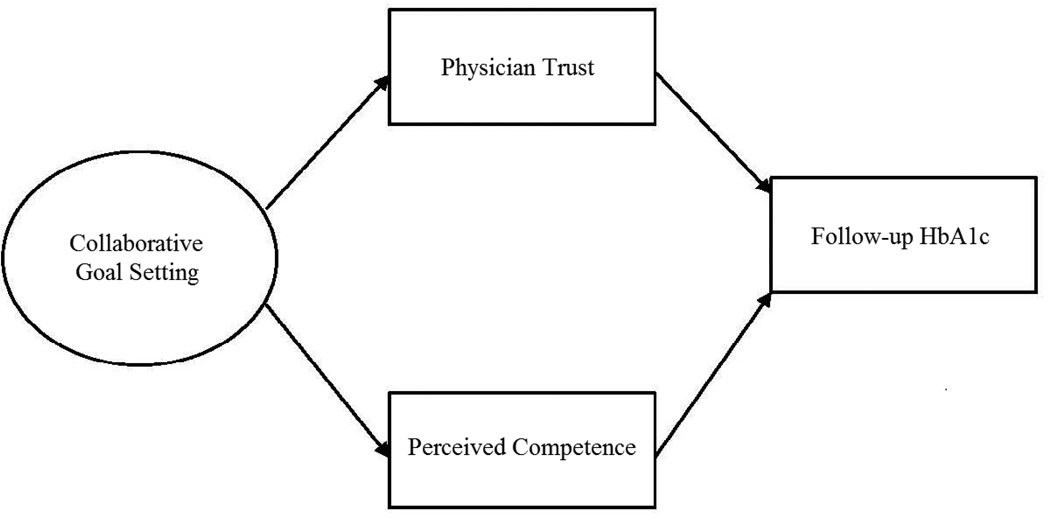
Structural equation modeling (SEM) was used to test the mediational roles of physician trust and perceived competence on the relationship between collaborative goal setting and subsequent HbA1c, controlling for baseline HbA1c. In the model, collaborative goal setting was specified as a latent variable using the 3 items from the PACIC as described above. The hypothesized mediation model, depicted in Figure 1, was fit with and without covariates. Covariates included the exogenous factors (i.e., patient age, gender, race, educational attainment, marital status, BMI, and anti-diabetic medication use) as described above. Due to the nesting of patients within primary care physicians, the TYPE=COMPLEX procedure in Mplus (v. 6.11) was used to obtain the correct fit statistics and standard errors.
Figure 1.
Mediation Model of Collaborative Goal Setting and Glycemic Control Among Patients with Diabetes
3. Results
3.1 Sample characteristics
Among the N=1,580 patients contacted for survey administration, n=190 were ineligible (n=17 were deceased, 5 did not speak English, 27 reported not having diabetes and 104 reported currently using insulin and n=37 did not have valid contact information). One thousand and sixty-five (n=1,065) eligible respondents completed the survey (n=951 via mail and n=114 via telephone) resulting in an overall response rate of 77% (i.e., 1065/(1580–190)). Those who reported not seeing a primary care physician (i.e., a general internist or family medicine physician) for their diabetes care (n=58), or did not have an HbA1c test in the 12 months following survey administration (n=44) are excluded from the current analyses. This resulted in N=963 individuals eligible for inclusion in the current study. Responders and non-responders did not differ significantly (p<0.05) in terms of gender or age, but they were less likely to be African American (21.0% vs. 31.5%) and to have a lower HbA1c at baseline (7.3% vs. 7.5%).
Characteristics of the sample are presented in Table 1. The average age of the cohort was 68 years (SD=11.14, range, 30–96) with 48% being female, 60% White, and 31% Black. At the time of survey administration, the mean HbA1c value among the sample was 7.3% (SD=1.22; range 4.8%–13.7%). Almost two thirds of the sample (62%) reported being married, 15% reported having a college degree or more education, and 10% had a BMI of 40 or higher. The average number of oral anti-diabetic medications dispensed to the sample at the time of survey administration was 1.12 (SD=0.34).
Table 1.
Sample Characteristics at time of Survey Administration (N=963)
| Percent | |
|---|---|
| Socio-demographic Characteristics | |
| Mean Age in Years (SD) | 68 (11.14) |
| Female | 48 |
| Race | |
| Black | 31 |
| White | 60 |
| Other | 8 |
| College Degree or More Education | 15 |
| Currently Married | 62 |
| Body Mass Index (BMI) ≥ 40 | 10 |
| Mean No. Oral Anti-diabetic Agents (SD) | 1.12 (0.34) |
| Mean Hemoglobin (Hb) A1c (SD) | 7.3% (1.22) |
On average, patients reported engaging in collaborative goal setting when receiving medical care for their diabetes over the past 6 months ‘sometimes’ (mean = 3.1, range 1 [never] to 5 [always]) (Table 2). Patients reported high levels of physician trust (mean= 6.1, SD=1.49, range 1 [Strongly Disagree] to 7 [Strongly Agree]). Among patients reporting an HbA1c target level (n=963), reports of perceived competence ranged from 4 to 28, with a mean =22.5 (SD=5.49). This resulted in 41% of survey respondents being classified as having a target HbA1c and high perceived competence.
Table 2.
Collaborative Goal Setting, Perceived Competence, and Physician Trust
| Mean Collaborative Goal Setting Score (SD) | |
| Asked to talk about my goals in caring for my diabetes | 3.3 (1.29) |
| Helped to set specific goals to improve eating or exercise | 3.5 (1.20) |
| Set a goal together with my team for what I could do to manage my diabetes | 2.6 (1.37) |
| Mean Factor Score* | 3.1 (1.11) |
| Percent with target HbA1c Reporting High Perceived Competence | 41 |
| Mean Physician Trust (SD) | 6.1 (1.49) |
Cronbach Alpha = 0.83; Omega = 1.11
The mean HbA1c for the post survey period (i.e., the outcome of interest) was 7.3% (sd=1.41, range 4.8 – 15.3). The average time interval between the Post- and Pre-HbA1c was 222.4 days (sd=97.7, range 90 – 648).
3.2 The relationships among collaborative goal setting, self-efficacy, trust and HbA1c
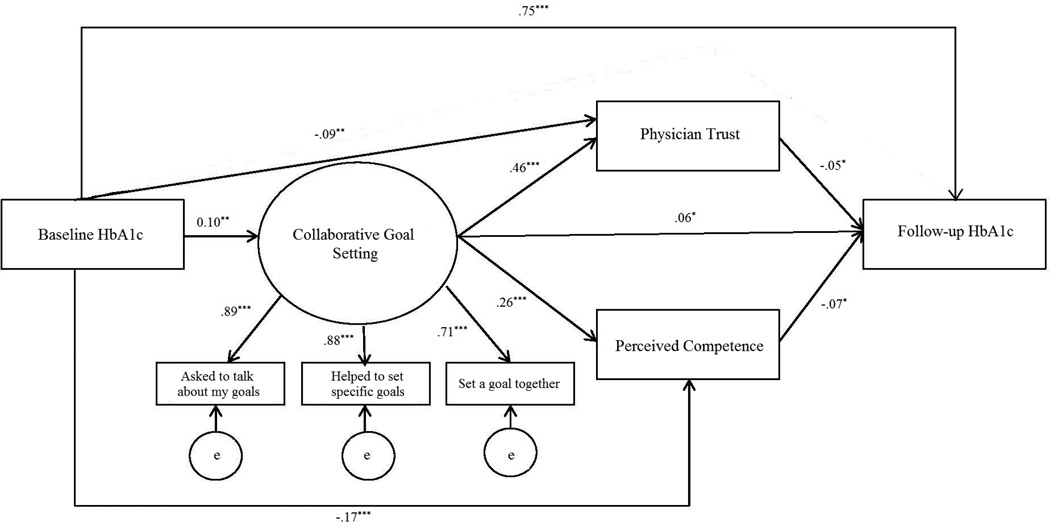
The unadjusted mediation model fit was good (χ2 = 27.4; df=8; p <.001; RMSEA (90% CI) = 0.05 (0.03 – 0.07); CFI=1.11; and TLI=0.99). The standardized factor loadings for the collaborative goal setting latent variable ranged from .66 to .86. Among the five path coefficients of interest, all were statistically significant (p <0.05): the effect of collaborative goal setting on perceived competence; the effect of collaborative goal setting on physician trust; and the effect of collaborative goal setting, trust, and perceived competence on HbA1c. Standardized parameter estimates are given in Figure 2.
Figure 2.
Unadjusted Structural Equation Model (SEM) Results
We tested the significance of the two mediation pathways: (a) collaborative goal setting → perceived competence → HbA1c and (b) collaborative goal setting → physician trust → HbA1c. Results supported that both perceived competence and physician trust are significant mediators of the relationship between collaborative goal setting and HbA1c (p <0 .05). Furthermore, the significance between collaborative goal setting and HbA1c implies that this relationship is only partially mediated by perceived competence and physician trust.
After controlling for other patient characteristics, the mediational relationships of both perceived competence and trust between collaborative goal setting and better glycemic control remained significant (p<0.05), as did the direct and positive relationship between collaborative goal setting and HbA1c (p<0.01). Covariate effects are provided in Table 3. As indicated in Table 3, patients with at least a college education and those with a BMI greater than or equal to 40 reported less collaborative goal setting as compared to their counterparts. High perceived competence was significantly and negatively associated with a BMI greater than or equal to 40, but significantly and positively associated with greater educational attainment whereas trust was significantly associated with increased patient age. In addition to being significantly associated with high perceived competence and trust, HbA1c was significantly associated with decreasing age, but positively associated with the number of oral agents dispensed to the patient.
Table 3.
Adjusted Structural Equation Model (SEM) Results: Covariate Effects
| Collaborative Goal Setting |
Perceived Competence |
Trust | Follow-up A1c |
|
|---|---|---|---|---|
| Socio-demographic | ||||
| Age | −0.05 | −0.07 | 0.13* | −0.09* |
| Female | −0.02 | .001 | −0.05 | 0.01 |
| Black | 0.01 | −0.02 | 0.04 | 0.02 |
| College or More | −0.11† | 0.21* | −0.01 | −0.05 |
| Married | 0.02 | 0.01 | −0.01 | −0.03 |
| BMI ≥ 40 | −0.12* | −0.21* | −0.01 | 0.01 |
| Oral Anti-diabetic Agents | −.001 | −0.05 | −0.05 | 0.48* |
p<0.01
p=0.05
4. Discussion and Conclusion
4.1 Discussion
Findings here illustrate that, controlling for patient characteristics including the patient’s prior HbA1c control, the more patients with diabetes report engaging in collaborative goal setting when receiving medical care for their diabetes, the more likely they are to report both increased trust in their physician and high perceived competence, which in turn are associated with better glycemic control. As such, these findings indicate that engaging patients with diabetes in collaborative goal setting during clinical encounters has the potential to foster a trusting patient-clinician relationship as well as enhance patient perceived competence, thereby potentially improving clinical control.
The indirect relationship between collaborative communication and clinical outcomes has previously been illustrated in the context of diabetes care. For example, Naik and colleagues found that collaborative goal setting and patient activation, while not directly associated with hypertension control among patients with diabetes, were associated with patients proactively communicating their self-monitoring results with their physicians, which led to improvements in hypertension control.(5) Others have found improved diabetes outcomes when patients and their providers are in agreement regarding treatment goals.(26)
Findings here also point to the fact that patients with higher HbA1c levels to begin with are those more likely to report engaging in more collaborative goal setting, perhaps implying that collaborative goal setting is often not used in clinical care until problems, such as elevated HbA1c levels, are identified and need to be addressed. In fact, our findings support only a partial mediation model with a direct and positive relationship between collaborative goal setting and subsequent HbA1c. This finding supports the idea that not all ‘collaborative goal setting’ is equal in its ability to improve patient outcomes. It may be that the potential benefits of a collaborative goal setting process are not achieved if the communication exchanges used in the process do not facilitate the building of positive patient-clinician rapport or a patient’s confidence to execute any goals set during the exchange. As such, while our findings, on one hand, support previously demonstrated benefits of active patient participation during office visits,(27) they also continue to illustrate the challenges in understanding the mechanisms through which active participation lead to these benefits and how best to foster productive participation processes during clinical encounters(3) as well as how to do so in a timely, proactive fashion.
The role patient-clinician communication can play in specifically fostering patients’ perceived self management competence has rarely been studied. One study’s findings suggest that physician support of patient’s autonomous self-regulation for medication use and perceived competence for diabetes self-management result in better subsequent quality of life and clinical control among patients with diabetes.(18) Furthermore, self-efficacy, which is closely linked to perceived competence, has been shown to influence analytical thinking and problem solving on complex tasks,(28–29) and to be associated with setting higher goals.(29–30) Thus, it would seem important to further understand the ways in which patient-clinician communication can enhance patients’ perceived competence as well as potential strategies for achieving this.
From our study, we also found that patient reports of using collaborative goal setting when receiving medical care for their diabetes were associated with improved physician trust and, in turn, better glycemic control. Previous studies have found that trust in one’s physician is positively correlated with symptom improvement(31) and quality of life.(32) Taken together, these findings arguably support using collaborative goal setting not only to foster a trusting relationship between patients and their physicians, but also through that process of trust building, as a means to potentially improve patient health.
Two additional findings, both regarding obesity, seem worthy of particular note. The first is that obese patients report lower perceived competence, an important input to self-management and thus clinical control, relative to those who are not obese. (18) The second is that obese patients also report engaging in less collaborative goal setting during their clinical visits than their non-obese counterparts. Whether this latter finding is due to differences in perceptions of what does and does not reflect collaborative goal setting, differences in patient preferences, inherent provider bias,(33–34) or some combination of such factors is not known. However, combined these findings imply that despite the potential for collaborative goal setting processes to offer a much needed boost to their perceived competence, obese individuals represent a subgroup of patients for whom the exposure to collaborative processes during clinical encounters remains relatively infrequent.
Results should be considered in the context of a number of important limitations. First, while a measure of glycemic control was available prior to survey administration, the measures of self-efficacy, trust, and collaborative goal setting were all obtained from one patient survey. Although the stem to the self-efficacy and trust survey items instructs the respondent to indicate their confidence at the time of survey completion, and the stem for the collaborative goal setting survey items directs the respondent to consider care “over the past 6 months,” the responses from any given individual may be temporally intertwined. Furthermore, because of a skip pattern in the survey, a binary measure of perceived competence was used in place of the validated construct (for which scores range) from 7–28. Additionally, there may be other important components of collaborative goal setting, trust or perceived competence not measured by the survey, or other important factors that impact clinical control (such as treatment adherence and intensification, disease duration or co-morbidities) that are not included in the model. In addition, survey respondents were limited to insured patients receiving care in one integrated delivery system; hence, results may not generalize beyond the population studied here. Finally, as tight glycemic control remains controversial, particularly among older patients, it is plausible that collaborative goal setting may, among some patients, result in increased HbA1c levels.
4.2 Conclusions
This study found that patients’ perceptions of more collaborative goal setting when receiving medical care for diabetes are associated with improvements in glycemic control through improvements in patients’ diabetes care perceived competence and physician trust. As such, results here provide additional empirical evidence of the potential benefits of patient-centered communication during clinical encounters.
4.3 Practice Implications
Fostering collaborative goal setting during office visits may yield payoffs in improved clinical outcomes such as glycemic control among patients with diabetes.
Acknowledgements
Funding: The database used was developed under a contract from Sanofi-Aventis.
Footnotes
J.E.L, E.D., and H.L.M. worked on the concept and design. J.E.L. and E.D. collected the data. J.E.L., H.L.M., and L.D. analyzed the data. J.E.L., H.L.M., E.D., M.H., R.W., and L.D. interpreted the data, reviewed/edited the manuscript, and gave final approval. The study was funded by Sanofi-Aventis. Representatives from the funding agency did not play any role in the study design, data collection, analyses or interpretation. The Institutional Review Board of the participating medical group approved all aspects of the study protocol.
Contributor Information
Jennifer Elston Lafata, Social and Behavioral Health, School of Medicine, Virginia Commonwealth University.
Heather L. Morris, Social and Behavioral Health, School of Medicine, Virginia Commonwealth University.
Elizabeth Dobie, Center for Health Policy and Health Services Research, Henry Ford Health System.
Michele Heisler, Internal Medicine and Health Behavior and Health Education, University of Michigan.
Rachel M. Werner, Department of Medicine, Philadelphia VA Medical Center and University of Pennsylvania.
Levent Dumenci, Social and Behavioral Health, School of Medicine, Virginia Commonwealth University.
References
- 1.Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Annals Of Internal Medicine. 2006;144(7):465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Grant RW, Buse JB, Meigs JB. Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28(2):337–342. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Street RL, Jr, Makoul G, Arora NK, Epstein RM. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Education & Counseling. 2009;74(3):295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Street RL. Analyzing communication in medical consultations: Do behavioral measures correspond to patients' perceptions? Medical Care. 1992;30(11):976–988. doi: 10.1097/00005650-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Naik AD, Kallen MA, Walder A, Street RL. Improving hypertension control in diabetes mellitus: The effects of collaborative and proactive health communication. Circulation. 2008;117(11):1361–1368. doi: 10.1161/CIRCULATIONAHA.107.724005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 7.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The Relative Importance of Physician Communication, Participatory Decision Making, and Patient Understanding in Diabetes Self-management. JGIM: Journal of General Internal Medicine. 2002;17(4):243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams GC, McGregor H, Zeldman A, Freedman ZR, Deci EL, Elder D. Promoting glycemic control through diabetes self-management: evaluating a patient activation intervention. Patient Education & Counseling. 2005;56(1):28–34. doi: 10.1016/j.pec.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Rost KM, Flavin KS, Cole K, McGill JB. Change in metabolic control and functional status after hospitalization. Impact of patient activation invervention in diabetic patients. Diabetes Care. 1991;14(10):881–889. doi: 10.2337/diacare.14.10.881. [DOI] [PubMed] [Google Scholar]
- 10.Street RL, Jr, Piziak VK, Carpentier WS, Herzog J, Hejl J, Skinner G, et al. Provider-patient communication and metabolic control. Diabetes Care. 1993;16(5):714–721. doi: 10.2337/diacare.16.5.714. [DOI] [PubMed] [Google Scholar]
- 11.Langford AT, Sawyer DR, Gioimo S, Brownson CA, O'Toole ML. Patient-Centered Goal Setting as a Tool to Improve Diabetes Self-Management. The Diabetes Educator. 2007;33(Supplement 6):139S–144S. doi: 10.1177/0145721707304475. [DOI] [PubMed] [Google Scholar]
- 12.Heisler M, Cole I, Weir D, Kerr EA, Hayward RA. Does physician communication influence older patients' diabetes self-management and glycemic control? Results from the Health and Retirement Study (HRS) Journals of Gerontology Series A: Biological Sciences & Medical Sciences. 2007;62A(12):1435–1442. doi: 10.1093/gerona/62.12.1435. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 14.Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 15.Oprea L, Braunack-Mayer A, Rogers WA, Stocks N. An ethical justification for the Chronic Care Model (CCM) Health Expectations. 2010;13(1):55–64. doi: 10.1111/j.1369-7625.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health Affairs. 2009;28(1):75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangione CM, Gerzoff RB, Williamson DF, Steers WN, Kerr EA, Brown AF, et al. The association between quality of care and the intensity of diabetes disease management programs. Annals Of Internal Medicine. 2006;145(2):107–116. doi: 10.7326/0003-4819-145-2-200607180-00008. [DOI] [PubMed] [Google Scholar]
- 18.Williams GC, Patrick H, Niemiec CP, Williams LK, Divine G, Lafata JE, et al. Reducing the health risks of diabetes: How self-determination theory may help improve medication adherence and quality of life. Diabetes Educator. 2009;35(3):484–492. doi: 10.1177/0145721709333856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funnell MM, Anderson RM. Empowerment and Self-Management of Diabetes. Clinical Diabetes. 2004;22(3):123–127. [Google Scholar]
- 20.Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, et al. National standards for diabetes self-management education. Diabetes Care. 2008;31(Suppl 1):S97–S104. doi: 10.2337/dc10-S097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lafata JE, Dobie EA, Divine GW, Ulcickas Yood ME, McCarthy BD. Sustained hyperglycemia among patients with diabetes: what matters when action is needed? Diabetes Care. 2009;32(8):1447–1452. doi: 10.2337/dc08-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flocke SA. Measuring attributes of primary care: development of a new instrument. The Journal Of Family Practice. 1997;45(1):64–74. [PubMed] [Google Scholar]
- 23.Dillman DA. Mail and internet surveys: The tailored design method. Hoboken: John Wiley & Sons, Inc.; 2007. [Google Scholar]
- 24.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC) Medical Care. 2005;43(5):436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 25.Glasgow RE, Whitesides H, Nelson CC, King DK. Use of the Patient Assessment of Chronic Illness Care (PACIC) with diabetic patients: relationship to patient characteristics, receipt of care, and self-management. Diabetes Care. 2005;28(11):2655–2661. doi: 10.2337/diacare.28.11.2655. [DOI] [PubMed] [Google Scholar]
- 26.Heisler M, Vijan S, Anderson R, Ubel P, Bernstein S, Hofer T. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? Journal of General Internal Medicine. 2003;18:893–902. doi: 10.1046/j.1525-1497.2003.21132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ: Canadian Medical Association Journal = Journal De L'association Medicale Canadienne. 1995;152(9):1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 28.Bandura A, Wood R. Effect of perceived controllability and performance standards on self-regulation of complex decision making. Journal Of Personality And Social Psychology. 1989;56(5):805–814. doi: 10.1037//0022-3514.56.5.805. [DOI] [PubMed] [Google Scholar]
- 29.Wood R, Bandura A, Bailey T. Mechanisms Governing Organizational Performance in Complex Decision-Making Environments. Organizational Behavior & Human Decision Processes. 1990;46(2):181. [Google Scholar]
- 30.Bandura A, Cervone D. Differential Engagement of Self-Reactive Influences in Cognitive Motivation. Organizational Behavior & Human Decision Processes. 1986;38(1):92. [Google Scholar]
- 31.Thom DH, Kravitz RL, Bell RA, Krupat E, Azari R. Patient trust in the physician: Relationship to patient requests. Family Practice. 2002;19(5):476–483. doi: 10.1093/fampra/19.5.476. [DOI] [PubMed] [Google Scholar]
- 32.Preau M, Leport C, Salmon-Ceron D, Carrieri P, Portier H, Chene G. Health-related quality of life and patient-provider relationships in HIV-infected patients during the first three years after starting PI-containing anti-retroviral treatment. AIDS Care. 2004;16(5):649–661. doi: 10.1080/09540120410001716441. [DOI] [PubMed] [Google Scholar]
- 33.Hebl MR, Xu J. Weighing the care: physicians' reactions to the size of a patient. Int J Obes Relat Metab Disord. 2001;25(8):1246–1252. doi: 10.1038/sj.ijo.0801681. [DOI] [PubMed] [Google Scholar]
- 34.Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes Relat Metab Disord. 2001;25(10):1525–1531. doi: 10.1038/sj.ijo.0801745. [DOI] [PubMed] [Google Scholar]