Abstract
Background
Among HIV infected youth, the role of renal disease (RD) and its management has become more important as children/adolescents age into young adulthood. Identification of predictors of abnormal renal laboratory events (RLE) may be helpful in the management of their HIV infection and its associated renal complications.”
Methods
Data collected from HIV-infected children and youth followed for ≥48 months was analyzed to identify predictors of resolution versus persistence of RLE and determine the utility of RLE to predict the onset of RD. Analysis included descriptive and inferential methods using a multivariable extended Cox proportional hazards model.
Results
428 of 1874 at risk children (23%) developed RLE, which persisted in 229 of 428(54%). CD4<25% (hazard ratio[HR] 0.63, p<0.002) and HIV viral load>100,000 copies/ml (HR 0.31, p<0.01) were associated with reduced rates of resolution. Exposure to HAART/nephrotoxic HAART prior to or subsequent to RLE in most cases were not. Persistence of RLE was 88% sensitive for identifying new RD. Negative predictive values for RD were >95% for both the at risk cohort and in those with RLE.
Conclusions
Advanced HIV disease predicted persistence of RLE in HIV-infected youth. Persistent RLE were useful for identifying RD.
Keywords: HIV, HIV renal disease, HAART
Introduction
Among HIV-1 infected children and adolescents, renal disease may occur as a result of the complications of HIV-1 infection and as well as from highly active antiretroviral therapy (HAART) [1–6]. Treatment-dosing guidelines and new drug development have evolved over the years, resulting in exposure to antiretroviral agents from an increasing number of different classes for prolonged periods of time. Indeed, children and adolescents who acquired HIV infection perinatally or early in life as a result of parenteral or sexual exposure have started HAART earlier in the course of their disease and have been maintained on HAART for most of their lives [7]. HIV-associated nephropathy (HIVAN) was recognized previously as the leading cause of chronic kidney disease (CKD) in adults and children with HIV in the United States [8–10]. In 2004 nearly half of the cases of CKD in HIV-infected patients were reported as being due to HIVAN. Since then, however, the spectrum of CKD in HIV-infected patients has been changing with less HIVAN and more comorbid kidney disease, such as that caused by hypertension and diabetes being reported (11–14). Recently, the CDC’s Medical Monitoring Project (15) reported an association between renal disease with longer duration of HIV infection. Given the number of recent reports linking nephrotoxicity with certain antiretroviral agents and the length of such therapy in children, it is possible that among HIV-infected youth HAART may increasingly contribute to the development of CKD.
The data reported here are from a retrospective analysis of HIV-1 infected children in the United States enrolled in the Pediatric AIDS Clinical Trials Group (PACTG) Protocol 219/219C. In our initial report from this cohort, Andiman et al [6] described the incidence of renal laboratory abnormalities or renal laboratory events (RLE), which were defined as the last sequence of at least three abnormal values in at least one category; serum creatinine (Cr), urine persistent proteinuria (PP), or estimated glomerular filtration rate (eGFR). Twenty-two percent of the PACTG 219/219C cohort had at least one persistent RLE which was associated with older age, black race, Hispanic ethnicity, use of nephrotoxic antiretroviral drugs (tenofovir and indinavir) and other nephrotoxic antimicrobial agents previously known to be associated with these RLE’s. Subsequently, Purswani et al [16] reported kidney biopsy findings in the same cohort, and described both demographic and clinical risk factors for children with HIV associated CKD. The present report extends these previous findings by determining the sensitivity and specificity of RLE’s for new renal disease diagnoses as markers of new onset renal disease. We also attempted to identify predictors of resolution versus persistence of RLE’s in children with HIV infection followed in P219/219C.
Methods
Study Population
PACTG 219/219C was a multicenter prospective cohort whose main objective was to determine the consequences of HIV-1 infection and its treatment from infancy through childhood into adolescence. The study opened to enrollment in May 1993 and spanned what is now accepted as the pre-HAART era from 1993 to 1997, and the HAART era, from 1998 to May of 2007 [7], when it ended. Details of Protocol 219/219C have been previously described [6, 16]. Children enrolling at or after January 1, 1998 were considered to have enrolled during the HAART era.
Participants in this study were a subset of the 2,102 subjects reported by Andiman et al. [6] who had no abnormal labs or renal diagnosis at study entry. Abnormal renal lab events (RLE) were defined for this study as the first in the sequence of at least three abnormal values in at least one category; serum creatinine (Cr), urine persistent proteinuria (PP), or estimated glomerular filtration rate (eGFR). The date of onset of the abnormal renal lab event was the date of the earliest abnormal lab value, across categories. The timing definition used here contrasts with that used in Andiman et al [6], where the timing of the renal lab event was also the earliest date across categories but within a category the third rather than first sequential lab abnormality defined onset. Cutoff for increased urine protein excretion was trace or greater, while cutoffs for increased serum creatinine (Cr) were age-adjusted [14], and that for reduced eGFR was < 60 mL/min/1.73 m2. The Modified Schwartz-CKID formula was used to compute eGFR for those less than 18 years of age [17–18] and the Modification of Diet in Renal Disease (MDRD) formula for those over 18 years of age [19].
Study Outcome definitions
Renal Laboratory outcomes
Resolution of RLE
Study participants who were identified with RLE and for whom there were subsequently at least three sequential normal lab values in the given category. If abnormalities occurred in more than one domain, each had to resolve to meet this definition. The date of resolution was the latest of the three normal values, across categories.
Persistence of RLE
Study participants who were identified with RLE and in whom there was no resolution of abnormal measures were considered to have persistent RLE.
New onset renal diagnosis
Any subject with RLE in whom there was no renal diagnosis at P219C study entry, but who subsequently received a specific renal diagnosis was considered as having a new onset renal diagnosis.
Renal-related death
A participant death in which renal disease was either a primary cause or co-existing contributing cause of death.
Renal-toxic HAART
Medications recorded in the P219/219C database were considered in this analysis if they met the definition of renal-toxic medications as defined in Andiman (2009). HAART regimens which included either TDF or IDV were considered to be renal-toxic.
Only participants who had been on study for at least 48 months were included in the analysis in order to allow sufficient additional time (18 months) for a patient who previously had an RLE during the first 30 months on study to experience a resolution (three sequential normal lab values).
Renal Diagnoses
Clinical renal diagnoses were coded using the Medical Dictionary for Regulatory Activities (MedDRA®) v14.1, a hierarchical and multiaxial medical coding terminology for clinical events. Queries for renal events were done broadly using Preferred Terms associated with the renal and urinary system organ class (SOC). We also identified conditions via the Standardized MedDRA Queries (SMQ), the acute renal failure SMQ, the hypertension SMQ and the renovascular disorders SMQ. SMQs are predefined sets of related Preferred Terms.
Diagnoses were classified into broad types of renal problems (primary renal, other renal, urinary tract infections, hypertension, renal laboratory abnormalities, edema, hematuria). The earliest occurring episode was selected for further analysis. Information on participant deaths was identified in the source P219/219C database via the death case report form which collected death dates and major and contributing causes, among other information.
Statistical Analysis
Selected sensitivity and specificity analyses included the full study population (n=2102). The remaining analyses considered only those participants who experienced RLEs. Medication exposures were classified according to whether they occurred at or prior to the date of the RLE or after this time. Medication exposures after RLE resolution were not considered in the analysis. We classified children by their exposure status before and after RLE.
We compared personal and HIV disease laboratory markers for participants who enrolled in P219/219C in the pre-HAART to the HAART eras using standard statistical tests as appropriate: Fisher’s exact test, Pearson chi square, and the Wilcoxon rank sum test. We next explored the association between each medication exposure and RLE resolution with a Pearson chi square test, classifying outcomes according to whether participants with exposure to medication at or prior to their RLE were switched off medication after the event. We also computed the sensitivity, specificity, positive and negative predictive values for the RLE and its persistence in predicting primary renal diagnoses.
Finally, we fit multivariable extended Cox proportional hazards models to explore the associations between the resolution of the RLE and changing medication exposure during the course of the study, adjusting for personal (age at renal event, race, ethnicity, sex) and clinical characteristics (e.g., CDC HIV stage at P219/219C study entry, HIV viral load and CD4 percent). Study time began at the RLE and ended at either its resolution or censor (last study visit). HIV disease markers were considered in these models separately as they changed over time and as they were measured at the RLE (defined as the closest value at or before the time of the RLE). In addition to considering the continuous values of CD4 percent and HIV RNA viral load, we also used categorical values by classifying CD4 percent using a cutoff of 25% and HIV RNA viral load using a cutoff of 100,000 copies/ml.
We used a two-step modeling process. In the first step, we explored the univariable associations between covariates and RLE resolution. We then built a core multivariable model by including univariable covariates with a p < 0.20, retaining only those factors with adjusted p < 0.15. In the second step, we explored the associations between changing medication use and RLE resolution after adjusting for the core model variables. Each model included a separate medication exposure. Time-dependent HIV markers(viral load and CD4 percent) were the most recent values prior to the start of the interval for which changes in medication were reported. We performed secondary sensitivity analyses, focusing only on the association between these markers and RLE resolution. Finally, analyses were performed on the subset of participants who enrolled into P219/219C during the HAART era. SAS v9.2 was used for the analyses and values with two-sided p < 0.05 were considered to be statistically significant.
Results
Study population
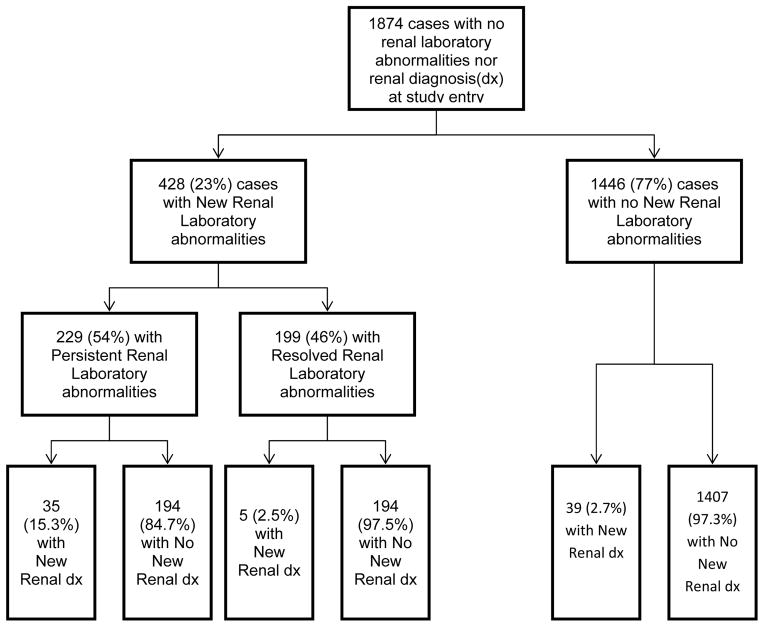
Figure 1 depicts a flow chart showing the breakdown of the 1874 participants who met study criteria and the subsequent distribution of those children who developed new renal lab abnormality or event (RLE) or new renal diagnoses. The majority of these children (1734, or 93%) were perinatally infected. Four hundred twenty-eight (428) of the 1874 subsequently had a new RLE either in serum creatinine (Cr), urine persistent proteinuria (PP) or estimated glomerular filtration rate (eGFR). All but 15 of these children were perinatally infected (96%).
Figure 1. Impact of persistent or resolved renal laboratory abnormalities on development of new renal disease diagnoses.
The Flow chart depicts the outcome in developing new renal diagnosis based on renal laboratory screening tests that persist or resolve. There were 1874 children in the study cohort with no renal laboratory abnormalities nor renal diagnosis at study entry. During follow-up, 428 (23%) developed new renal laboratory abnormalities while 1446 (77%) did not. Of those cases with new renal laboratory abnormalities, 229 (54%) had persistence of abnormal laboratory renal studies with 35 (15.3%) developing a new renal diagnosis. In contrast, 199 (46%) had resolution of their abnormal renal studies with only 5 (2.5%) developing a new renal diagnosis. In the 1446 (77%) cases that never developed new renal laboratory abnormalities, there were 39 (2.7%) who developed renal diagnosis.
Of the 428 subjects with new RLE, 199 resolved their RLE while 229 did not. Among those 229 who had persistent RLE, 35 (15.3%) developed a new renal diagnosis. There were 8 deaths in this later group; 4 of whom died of renal-related causes. There were an additional 8 deaths in the group without new renal diagnoses. None of these latter deaths were renal-related Among the 199 who resolved their abnormalities and the 1446 children who did not develop any new RLE, there was a much smaller percentage of children ( 2.5%[5/199] and 2.7%[39/1446] respectively) who developed a new renal diagnosis. Among the 1446 children, six of the 39 children with new renal diagnoses died; 3 of renal related causes. Forty-two of the other 1407 children died, only one being renal-related. That one renal-related death was counted as a “new renal diagnosis” for the purposes of the sensitivity/specificity analysis. There was one non-renal-related death in the group of 199 who resolved their RLE.
The frequency and severity of the new renal diagnoses were more pronounced among those children who had persistent abnormalities. While the frequency of renal tubular disorders was only minimally increased (1/199 (0.5%) among children who resolved their RLE versus 4/229 (1.7%) among children with persistent RLE), the frequency of proteinuria, renal parenchymal disease and renal failure were all markedly higher among children who had persistent RLE. In each of these three categories, the percentage among children who had persistent RLE was at least five times higher than that among children who experienced resolution of their RLE. Seventeen (7.4%) of the children with persistent RLE developed one of the following renal parenchymal diseases (nephrosis [11], focal segmental glomerulosclerosis [2], glomerulonephritis [2], and lupus nephritis[2]).
Demographic and clinical characteristics
Table 1 shows the demographic and clinical characteristics for the 428 participants in the analysis who had three consecutive RLE comparing those enrolled pre-HAART to those enrolled during the HAART era. Participants enrolled during the HAART era were slightly older at the time of the RLE and were followed (to resolution/last visit date) for less time compared to those enrolled during the pre-HAART era. As expected, there were fewer deaths among those enrolled during the HAART era. Overall, about 46% of the participants’ RLEs resolved. The number of participants who resolved their RLE was similar in both subgroups. Both CD4% and HIV RNA viral load values indicate that HAART-era participants were healthier at the time of the RLE than were those enrolled prior to the HAART era (all p < 0.05). Note that 84 participants, primarily among those enrolled during the pre-HAART era, had no HIV RNA viral load measured at the renal lab event time.
Table 1.
Characteristics of Patients who Developed New Renal Lab Events (RLE) during the Pre-HAART versus the HAART Eras.
| Enrollment during HAART era (1998 or after) | ||||
|---|---|---|---|---|
| Total (N=428) | No (N=299) | Yes (N=129) | P-Value | |
| Male, N (%) | 224 (52.3%) | 157 (52.5%) | 67 (51.9%) | 0.92 (a) |
| Race/Ethnicity, N (%) | 0.11 (a) | |||
| White, non-Hispanic | 47 (11.0%) | 37 (12.4%) | 10 (7.8%) | |
| Black, non-Hispanic | 222 (51.9%) | 144 (48.2%) | 78 (60.5%) | |
| Hispanic (any race) | 152 (35.5%) | 113 (37.8%) | 39 (30.2%) | |
| Other/Unknown | 7 (1.6%) | 5 (1.7%) | 2 (1.6%) | |
| CDC stage C at 219/219C study entry, N (%) | 109 (25.5%) | 69 (23.1%) | 40 (31.0%) | 0.09 (a) |
| Age, y at RLE, Median (Q1, Q3) | 10.53 (8.51, 12.18) | 10.33 (8.02, 12.08) | 11.22 (9.51, 12.84) | <.001 (b) |
| Months on study from RLE to resolution/or last patient contact, Median (Q1, Q3) | 38.27 (24.10, 58.52) | 42.40 (29.24, 61.64) | 28.88 (20.23, 44.08) | <.001 (b) |
| All lab abnormal sequences resolve, N (%) | 199 (46.5%) | 141 (47.2%) | 58 (45.0%) | 0.75 (a) |
| Death, N (%) | 17 (4.0%) | 16 (5.4%) | 1 (0.8%) | 0.03(a) |
| Renal-related primary or secondary cause of death, N(%) | 4 (23.5%) | 4 (25.0%) | 0 (0.0%) | 1.00 (a) |
| CD4 % at/prior to RLE | ||||
| Median (Q1, Q3) | 28 (20, 35) | 27 (19, 34) | 30 (22, 35) | 0.03 (b) |
| CD4 pct < 25, at/prior to RLE, N (%) | 167 (39.0%) | 128 (42.8%) | 39 (30.2%) | 0.02 (a) |
| Log RNA VL at/prior to RLE | ||||
| N | 344 | 218 | 126 | |
| Median (Q1, Q3) | 3.11 (2.60, 4.18) | 3.33 (2.60, 4.36) | 2.83 (2.60, 3.64) | 0.003 (b) |
| HIV RNA at/prior to RLE >100,000 copies, N (%) | 27 (7.8%) | 23 (10.6%) | 4 (3.2%) | 0.01 (a) |
Fisher’s Exact Test
Wilcoxon Test
Sensitivity and Specificity of Renal Lab Events for New Renal Diagnoses
Panel A in Figure 2 depicts the respective values for sensitivity, specificity, and positive and negative predictive values for an abnormal RLE for predicting a new renal diagnosis in children in the source population (1874). Panel B in Figure 2 depicts these same values but in this case for persistent abnormal RLE for predicting a new renal diagnosis among children who developed a new RLE (428). The sensitivity of RLE for predicting a new renal diagnosis for all children in the source population was only 50%, while the specificity for ruling out renal disease was high (78%). In contrast, the sensitivity of persistent RLE for predicting a new renal diagnosis among the 428 children with new onset of RLE was high (88%) while the specificity of persistent RLE was low (50%); absence of persistent RLE only identified half of those without renal diagnoses. In both groups, the positive predictive values of RLE and persistent RLE for a new renal diagnosis were low (about 10–15%) while the negative predictive values for both were greater than 95%. There was no marked difference in the figures for sensitivity, specificity, or negative predictive values when these values were determined for separate groups of children enrolled during the pre-HAART versus the HAART era. Though somewhat different, positive predictive values were low for both subgroups (<40%, data not shown). Hence, as a screening test for clinically significant renal disease, among those children with RLE, persistence of RLE captures almost 90% of those with renal diagnoses while, among all children in the source population, presence of an RLE identifies about half of those with renal diagnoses.
Figure 2.
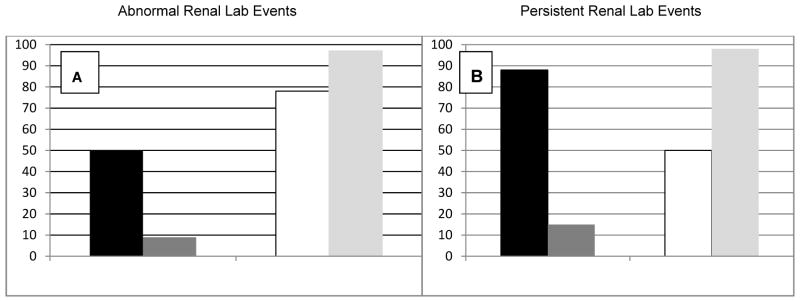
Sensitivity (Black bar), Specificity(White bar), Positive(Dark Gray bar) and Negative(Light Gray bar) Predictive Values of Abnormal Renal Lab Events(Panel A) in the source population(1874) and Persistent Renal Lab Events (Panel B) in the group with RLE (428) for New Renal Diagnoses.
Medication Exposures
In the total group of 428 participants, 88% of the children enrolled during the HAART era were exposed to HAART prior to the onset of their first renal lab event as opposed 61.5% of those enrolled during the pre-HAART era (p<.001, Fisher’s Exact Test) but this was likely due to the ready availability of HAART during the latter time period. There was no statistical difference relative to timing of exposure to renal-toxic HAART between children who enrolled during the two eras; 20% of HAART-era participants received renal-toxic HAART prior to the renal lab event compared to 14% of pre-HAART-era participants (p=0.11). Similarly, for the other classes of medications, exposure timing for HAART-era and pre-HAART-era participants did not differ. When we analyzed the timing of medication exposure and resolution outcome, the statistical results were heavily weighted by the number of subjects never exposed to the medications (Table 2). We then focused on those study participants receiving medications prior to the time of the abnormal renal lab event, cross classifying outcomes according to whether or not they were still exposed after the RLE. While 46% of those participants on HAART continuously did resolve their RLE, 55% of patients never treated with HAART also experienced resolution (Table 2, p=0.05). There is no evidence for increased rates of resolution when renal-toxic regimens were stopped. For example, of those participants who no longer had exposure to renal-toxic HAART after the RLE, 30% resolved the RLE as compared to 29.8% of those subjects who still remained on that class of medication. When this analysis was performed using hierarchical and mutually exclusive classification of medication exposure prior to the RLE (if they had any exposure to renal-toxic HAART and, if not, another HAART regimen, a non-HAART antiretroviral regimen, or no exposure to any antiretroviral agent), there was a suggestion that a lower resolution rate was associated with early renal-toxic HAART exposure (30%) as compared to the rate for those exposed to other antiretroviral regimens, ( 50%; p=0.03).
Table 2.
Timing of Medication Exposure for all Participants Relative to the Renal Lab Event
| All lab abnormal sequences resolve | ||||
|---|---|---|---|---|
| No (N=229) | Yes (N=199) | P-Value (a) | ||
| HAART, Exposure summary | Switched off | 7 (100.0%) | 0 (0.0%) | 0.05 |
| Switch on | 44 (52.4%) | 40 (47.6%) | ||
| Always Exposed | 157 (54.1%) | 133 (45.9%) | ||
| Never Exposed | 21 (44.7%) | 26 (55.3%) | ||
| Renal toxic HAART, Exposure summary | Switched off | 7 (70.0%) | 3 (30.0%) | 0.01 |
| Switched on | 36 (58.1%) | 26 (41.9%) | ||
| Always Exposed | 40 (70.2%) | 17 (29.8%) | ||
| Never Exposed | 146 (48.8%) | 153 (51.2%) | ||
| Renal toxic HAART/IDV, Exposure summary | Switched off | 15 (75.0%) | 5 (25.0%) | 0.27 |
| Switched on | 12 (54.5%) | 10 (45.5%) | ||
| Always Exposed | 15 (53.6%) | 13 (46.4%) | ||
| Never Exposed | 187 (52.2%) | 171 (47.8%) | ||
| Renal toxic HAART/TDF, Exposure summary | Switched off | 2 (100.0%) | 0 (0.0%) | <.001 |
| Switched on | 35 (64.8%) | 19 (35.2%) | ||
| Always Exposed | 20 (87.0%) | 3 (13.0%) | ||
| Never Exposed | 172 (49.3%) | 177 (50.7%) | ||
| IDV, Exposure summary | Switched off | 17 (77.3%) | 5 (22.7%) | 0.11 |
| Switched on | 9 (45.0%) | 11 (55.0%) | ||
| Always Exposed | 18 (58.1%) | 13 (41.9%) | ||
| Never Exposed | 185 (52.1%) | 170 (47.9%) | ||
| TDF, Exposure summary | Switched off | 2 (100.0%) | 0 (0.0%) | <.001 |
| Switched on | 36 (64.3%) | 20 (35.7%) | ||
| Always Exposed | 20 (87.0%) | 3 (13.0%) | ||
| Never Exposed | 171 (49.3%) | 176 (50.7%) | ||
Chi-Square Test
“Switched off” indicates medication exposure before RLE but not after.
“Switched on” indicates medication exposure after RLE but not before.
“Always exposed” indicates medication exposure both prior to and after RLE.
“Never exposed” indicates no medication exposure prior to end of followup or RLE resolution.
Exposure status considered until resolution, in cases where RLE resolved, or end of follow-up,
Time to Resolution of Abnormal Renal Lab Events
Table 3 shows, for the youths with renal laboratory abnormalities, the association between medication exposures and resolution, adjusting for time-varying CD4 percent, CDC stage C and Black race. (HIV RNA viral load measurements as they changed over time did not meet core model inclusion criteria). None of the medication exposures (renal toxic HAART, TDF, IDV, or non-antiretroviral renal toxic concomitant medications) were significantly associated with RLE resolution (p ≥0.53; results repeated as analysis set 1 of Table 4). We repeated the modeling process to adjust for CD4% (< 25), and HIV RNA viral load (> 100,000 copies) at the time of the first RLE (Table 4, analysis set 2). In addition to Black race and CDC Stage C, only HIV RNA viral load met model inclusion criteria. There were no significant associations between medication exposures and resolution (p>0.35). Since many participants were missing viral load data, we next developed a core model adjusting only for CD4 percent measured at the renal lab event. No other core model covariates met the inclusion criterion. There were no significant associations with medication exposure (p>0.63, Table 4, analysis set 3). Results for core model covariates suggested reduced rates of resolution for youths with CDC Stage C disease classification (p< 0.10), low time-varying CD4 percents (p=0.06), higher HIV RNA viral loads at RLE(p = 0.01), and low CD4 percent at RLE(p=0.002) (Table 5). There were also no significant associations with medication exposure, when we analyzed only the participants enrolled during the HAART era (p > 0.24, data not shown). For these participants, older age at renal lab events was associated with a longer time to RLE resolution (p=0.02; data not shown).
Table 3.
The association between personal and HIV disease characteristics and medication exposure with time to resolution of renal laboratory events.(RLE)†
| Parameter | Unadjusted Results1 | Adjusted Results2 | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-Value | |
| Core models: Personal characteristics and HIV disease markers | ||||
| Male | 0.96 (0.7,1.3) | 0.78 | -- | |
| Age, at RLE | 1.01 (1.0,1.1) | 0.51 | -- | |
| Race, Black | 0.82 (0.6,1.1) | 0.15 | 0.80 (0.6,1.1) | 0.15 |
| Ethnicity, Hispanic | 1.18 (0.9,1.6) | 0.26 | -- | |
| CDC Stage C | 0.73 (0.5,1.0) | 0.08 | 0.72 (0.5,1.1) | 0.09 |
| HIV RNA copies (Log10) at RLE | 0.74 (0.6,0.9) | <.001 * | -- | |
| CD4 % at RLE | 1.02 (1.0,1.0) | <.001 * | -- | |
| HIV RNA copies > 100,000 at RLE | 0.30 (0.1,0.7) | 0.004 * | -- | |
| CD4 % < 25 at RLE | 0.63 (0.5,0.8) | 0.002 * | -- | |
| CD4 % (time-varying) | 1.01 (1.0,1.0) | 0.03 * | -- | |
| CD4 % < 25 (time-varying) | 0.71 (0.5,1.0) | 0.03 * | 0.75 (0.6,1.0) | 0.06 |
| HIV RNA copies (Log10)(time-varying) | 0.95 (0.8,1.1) | 0.48 | -- | |
| HIV RNA, copies > 100K (time-varying) | 0.83 (0.5,1.3) | 0.38 | -- | |
| Antiretroviral medication exposures3 | ||||
| Renal toxic HAART (time-varying) | 0.89 (0.6,1.3) | 0.58 | 0.94 (0.6,1.4) | 0.79 |
| TDF Exposure (time-varying) | 0.93 (0.6,1.5) | 0.78 | 1.06 (0.6,1.8) | 0.82 |
| IDV Exposure (time-varying) | 0.86 (0.5,1.6) | 0.63 | 0.83 (0.5,1.5) | 0.53 |
| Renal toxic concomitant meds (time-varying) | 0.88 (0.3,2.4) | 0.81 | 1.06 (0.4,2.9) | 0.92 |
Unadjusted analyses include all potential confounders and antiretroviral medications. Each row represents a separate analysis.
Adjusted results include the time varying HIV disease markers and personal characteristics which made it through the core-model building process with p < 0.20 to include and p < 0.15 to remain.
For adjusted analyses, each row represents a separate analysis of a medication exposure, adjusting for core model covariates.
p < 0.05
This analysis was carried out on the primary derived dataset, formed using medication exposure observations as the framework, adding in CD4 and RNA viral load values.
Table 4.
The association between medication exposure and time to abnormal renal lab event (RLE) resolution for three separate analyses, considering time-varying HIV disease markers and HIV disease markers at the first RLE.
| Analysis set | Parameter | Unadjusted Results | Adjusted Results1 | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-Value | ||
| Analysis set 1: time varying CD4, RNA2 | Renal toxic HAART (time-varying) | 0.89 (0.6,1.3) | 0.58 | 0.94 (0.6,1.4) | 0.79 |
| TDF Exposure (time-varying) | 0.93 (0.6,1.5) | 0.78 | 1.06 (0.6,1.8) | 0.82 | |
| IDV Exposure (time-varying) | 0.86 (0.5,1.6) | 0.63 | 0.83 (0.5,1.5) | 0.53 | |
| Renal toxic concomitant meds (time-varying) | 0.88 (0.3,2.4) | 0.81 | 1.06 (0.4,2.9) | 0.92 | |
| Analysis set 2: CD4, RNA @ RLE3 | Renal toxic HAART (time-varying) | 0.89 (0.6,1.3) | 0.58 | 0.83 (0.5,1.3) | 0.42 |
| TDF Exposure (time-varying) | 0.93 (0.6,1.5) | 0.78 | 0.77 (0.5,1.3) | 0.35 | |
| IDV Exposure (time-varying) | 0.86 (0.5,1.6) | 0.63 | 0.88 (0.4,1.7) | 0.71 | |
| Renal toxic concomitant meds (time-varying) | 0.88 (0.3,2.4) | 0.81 | 1.34 (0.4,4.3) | 0.62 | |
| Analysis set 3: CD4 @ RLE4 | Renal toxic HAART (time-varying) | 0.89 (0.6,1.3) | 0.58 | 0.92 (0.6,1.4) | 0.68 |
| TDF Exposure (time-varying) | 0.93 (0.6,1.5) | 0.78 | 0.98 (0.6,1.6) | 0.94 | |
| IDV Exposure (time-varying) | 0.86 (0.5,1.6) | 0.63 | 0.87 (0.5,1.6) | 0.64 | |
| Renal toxic concomitant meds (time-varying) | 0.88 (0.3,2.4) | 0.81 | 1.04 (0.4,2.8) | 0.95 | |
These analyses were carried out on the primary derived dataset, formed using medication exposure observations as the framework, adding in CD4 and RNA viral load values.
Results adjust for the final core models, which were developed with p < 0.20 to include and p < 0.15 to remain. Each row represents a separate time to event multivariate regression analysis.
The core model for analysis 1 included Black race, CDC Stage C, CD4% < 25 (time varying)
The core model for analysis 2 included Black race, CDC Stage C, HIV RNA at 1st ARL > 100,000
The core model for analysis 3 included CD4% at 1st ARL< 25
Table 5.
The association between personal characteristics and HIV disease markers and time to abnormal renal lab (RLE) resolution for three separate analyses, considering time-varying HIV disease markers and HIV disease markers at the first RLE†.
| Analysis set | Parameter | Unadjusted Results | Adjusted Results | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-Value | ||
| Analysis 1:, time varying CD4, RNA | Race, Black | 0.82 (0.6,1.1) | 0.15 | 0.80 (0.6,1.1) | 0.15 |
| CDC Stage C | 0.73 (0.5,1.0) | 0.08 | 0.72 (0.5,1.1) | 0.09 | |
| CD4 pct < 25 (time-varying) | 0.71 (0.5,1.0) | 0.03 * | 0.75 (0.6,1.0) | 0.06 | |
| Analysis 2: CD4, RNA @ RLE | Race, Black | 0.82 (0.6,1.1) | 0.15 | 0.78 (0.6,1.1) | 0.13 |
| CDC Stage C | 0.73 (0.5,1.0) | 0.08 | 0.71 (0.5,1.0) | 0.08 | |
| HIV RNA copies > 100,000 at RLE | 0.30 (0.1,0.7) | 0.004 * | 0.31 (0.1,0.7) | 0.01 * | |
| Analysis 3: CD4 @ RLE | CD4 pct < 25 at RLE | 0.63 (0.5,0.8) | 0.002 * | 0.63 (0.5,0.8) | 0.002 * |
Note: These results only show core variable models. Core models were built with p < 0.20 to include and p < 0.15 to retain.
p < 0.05
These analyses were carried out on the primary derived dataset, formed using medication exposure observations as the framework, adding in CD4 and RNA viral load values.
Results of a sensitivity analysis focused solely on the relation between resolution and each CD4% and HIV RNA viral load measures (Supplemental Table 1) confirmed the reduced resolution rates for youths with low CD4 percentage and high HIV RNA viral loads. Additional analyses also confirmed the association between CDC Stage C disease classification and reduced resolution (p< 0.10; data not shown). In the sensitivity analysis of the HAART era-enrolled participants, the only significant findings were the association between older age at first RLE and reduced resolution rates (p=0.01; data not shown).
Discussion
Recent data have suggested that highly active antiretroviral therapy can change the natural history of HIVAN, not only by preventing its development but also by halting its progression once developed [11, 12]. While the time to–event analysis did not directly address the impact of non-renal toxic HAART on resolution of RLE, in descriptive analyses there appeared to be little impact of HAART on RLE resolution. We attempted to determine whether there was any association between persistent RLE and the use of renal-toxic HAART (as suggested recently by Leal et al, 2010 [20]). We once again found little evidence for a relationship between medication exposure and resolution of abnormal renal laboratory events. What this study did show, however, was that low CD4 percent values, higher HIV RNA viral load values, and older age at the time of the renal lab event were all associated with reduced rates of RLE resolution. A possible explanation is that patients with advanced HIV disease might have severe renal disease that does not respond to HAART. Since our analysis encompassed both the pre-HAART and HAART eras, data from the sicker pre-HAART era children is likely driving these findings. Nevertheless, some individual findings from our unadjusted, descriptive analyses suggest that continued exposure to renal toxic HAART may be associated with persistent RLE. The sensitivity and specificity analyses showed that three sequential abnormal renal labs are only modestly effective in predicting the occurrence of new primary renal diagnoses in HIV infected children and adolescents. It is noteworthy that for those participants with an existing RLE, finding persistence of these renal lab abnormalities was a good screening measure for these same outcomes. Overall, the negative predictive values of these two assessments were high. Therefore absence of RLE was a good indicator to rule out renal diagnoses, as would intuitively be expected. This suggests that when screening HIV-infected children and adolescents for renal disease, persistence of any of these three renal laboratory abnormalities should prompt referral to a nephrologist for a more thorough evaluation.
One limitation of our analyses was that PACTG 219/219C was not designed specifically to study HIV-renal related disease; consequently all renal related data collection was done as part of the child’s continuing care and not mandated prospectively as part of the study design. Nor did it include screening for microalbuminuria, or determining the microalbumin/Cr ratio. Despite these limitations, we have previously published two papers describing the nature of HIV-related renal disease using this same dataset (6, 5). A second limitation was that some of our retrospective analyses divided the total number of study subjects into two subgroups: those children enrolled in P219/219c during the pre-HAART era (1993–1997) versus those enrolled during the HAART era (1998–2007). The children enrolled during the pre-HAART era continued to be followed and had data collected during the latter time period when they were being treated with HAART. Hence, the results from this group may have been biased by the effects of untreated HIV infection prior to the time they started HAART. One additional limitation of our analysis was that we did not specifically assess for the potential nephrotoxic effects of either ritonavir-boosted atazanavir or lopinavir as suggested recently in a report by Ryom et al [ 21] based upon data from adult patients enrolled in the D:A:D study (Data Collection on Adverse events of Anti-HIV Drugs Study ).
Based upon recent data from adults, it is probable that the nature of HIV-associated renal disease is changing. The frequency of HIVAN may be decreasing as a result of the widespread availability of HAART which may be preventing the development of the early stages of HIVAN. At the same time there is a greater recognition of the potential nephrotoxicity of various antiretroviral agents which may be a concern among younger HIV-infected children whose renal function is still maturing. The difficulty here relates to how to distinguish the effects of the virus from the effects of the drugs. While proteinuria, especially persistent proteinuria, is a laboratory hallmark of HIVAN; additional laboratory abnormalities including alterations in serum Cr, eGFR, or other electrolyte abnormalities may be secondary to the effects of HIV or the medications (22). The ability to discern the etiology of renal lab abnormalities may be especially difficult in those patients who have more advanced HIV disease, and are being treated with HAART (especially if they are on nephrotoxic HAART). This may have influenced our results. Approximately 25% of the total group of children had AIDS (CDC Class C) at entry while 39% had a CD4% of less than 25% at or prior to the first RLE. The median age of the entire group at the first abnormal laboratory was 10.5 years; hence many of these children had been infected for at least a decade.
In summary, we analyzed 14 years of prospectively collected data from 1874 HIV-1 infected youth enrolled in PACTG 219/219C. We were able to document that persistent renal lab abnormalities are associated with an increased frequency and severity of primary renal diagnoses, and that advanced HIV renal disease is more likely to result in a lack of resolution of RLEs with HAART. Although the sensitivity and specificity analyses show that three sequential abnormal renal labs alone are only modestly effective in predicting primary renal diagnoses, in those participants with RLE, the persistence of these RLEs was a good predictor of these same outcomes. The negative predictive values of these same two assessments were also high.
Supplementary Material
MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).
MedDRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations(IFPMA).
Acknowledgments
The authors would like to thank the researchers and institutions involved in the conduct of 219C as well as the leadership and participants of the P219/219C protocol team and the 219C iDACS617 Working Group. The authors are grateful for the contributions of Joyce Kraimer, Carol Elgie, Barbara Heckman, Shirley Traite, and Nathan Tryon. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). This project has been funded in whole or in part with Federal fujnds from the National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200800014C. The authors also thank the individual staff members and sites who have participated in the conduct of this study, as provided in Appendix 3.
* James M Oleske MD MPH, Founding Chair and Russell Van Dyke MD, Chair, Mark Abzug MD and John Farley MD; Vice-Chairs, Mary Glen Fowler MD MPH, Michael Brady MD and Wayne Dankner MD; Past Vice-Chairs,
Protocol Team Members (versions 1 and 2 of PACTG-219): Mary Culnane MS CRNP, Elizabeth Hawkins, Lynne Mofenson MD, Yvonne J Bryson, MD, Edward M Connor MD, Lawrence D’Angelo MD MPH, Mark Mintz MD, Karen J O’Donnell PhD, Margaret Oxtoby, MD, Andrea Rubin Hale RN MPH, Richard D Gelber PhD, Steven Gortmaker PhD, William Lenderking PhD, Lynn Marrow, Christina Joy RN MSM, Colleen Clark MPH, Bethann Cunningham MS, Rhoda Sperling MD, Gwendolyn B Scott MD, Courtney Fletcher PharmD, Blake Caldwell MD, Dianne Donovan,
Protocol Team Members (versions 3 and 4 of PACTG-219C): Elizabeth Smith MD, Anne Fresia, Gregory Ciupak, Michelle Eagle PA, Dorothy R Smith MS CPNP, Paul Palumbo MD, John Sleasman MD, James Connor MD, Michael Hughes PhD, Rebecca Oyomopita MSc, George Johnson MD, Andrew Wiznia MD, Nancy Hutton MD, Andrea Kovacs MD, Mary Sawyer MD, Martin Anderson MD, Audrey Rogers PhD MPH, William Borkowsky MD, Jane Lindsey ScD, Jack Moye MD, Myron Levin MD, Marilyn Crain MD MPH, Paul Britto MS, Ruth Toumala MD, Joseph Cervia MD, Eileen Monagham, Kenneth Dominguez MD, Melody Higgins RN MS, George Seage DSc MPH, Denise Gaughan MPH, Phil Gona PhD, William Shearer MD PhD, Lois Howland DPH MS RN, Deborah Storm PhD RN, Kathleen Malee PhD, Wendy Mitchell MD, Carol Gore, Eve Powell, Michelle McConnell MD, Newana Beatty, Susan Brogly PhD, Jennifer Bryant CRA, Miriam Chernoff PhD, Barbara Heckman BS, Dawn English, Edward Handelsman MD, Patrick Jean-Philippe MD, Kathleen Kaiser, Joyce Kraimer MS, Linda Millar, Shirley Traite MSW, Paige Williams PhD, Elizabeth Woods MD MPH, Carol Worrell MD.
iDACS617 Working Group: Charles D. Mitchell, MD, MS, Miriam C. Chernoff, PhD, George R. Seage III, DSc, MPH, Murli U. Purswani, MD, Hans M.L. Spiegel, MD Barbara Heckman, James M. Oleske, MD, MPH, and Warren Andiman, M.D.
APPENDIX I
Participating institutions in the U.S.-based multisite cohort study, PACTG 219/219C, between 1993–2007.
The following institutions and clinical site investigators participated in PACTG 219/219C:
University of New Jersey Medical and Dental School - Department of Pediatrics, Division of Allergy, Immunology & Infectious Diseases: Dr. James Oleske, Dr. Arlene Bardeguez, Dr. Arry Dieudonne, Linda Bettica, Juliette Johnson, Boston Medical Center, Division of Pediatric Infectious Diseases: Dr. Stephen I. Pelton, Dr. Ellen R. Cooper, Lauren Kay, Ann Marie Regan, Med, Children’s Hospital LA - Department of Pediatrics, Division of Clinical Immunology & Allergy: Dr. Joseph A. Church, Theresa Dunaway, Long Beach Memorial Medical Center, Miller Children’s Hospital: Dr. Audra Deveikis, Dr. Jagmohan Batra, Susan Marks, Ilaisanee Fineanganofo, Harbor - UCLA Medical Center - Department of Pediatrics, Division of Infectious Diseases: Dr. Margaret A. Keller, Dr. Nasser Redjal, Spring Wettgen, Sheryl Sullivan, Johns Hopkins Hospital & Health System - Department of Pediatrics, Division of Infectious Diseases: Dr. Nancy Hutton, Beth Griffith, Mary Joyner, Carolyn Keifer, University of Maryland Medical Center, Division of Pediatric Immunology & Rheumatology: Dr. Douglas Watson, Dr. John Farley, Texas Children’s Hospital, Allergy & Immunology Clinic: Dr. Mary E. Paul, Chivon D. Jackson, Faith Minglana, Dr. Heidi Schwarzwald, Cook County Hospital: Dr. Kenneth M. Boyer, Dr. Jamie Martinez, Dr. James B. McAuley, Maureen Haak, Children’s Hospital of Columbus, Ohio: Dr. Michael Brady, Dr. Katalin Koranyi, Jane Hunkler, Charon Callaway, University of Miami Miller School of Medicine, Division of Pediatric Immunology & Infectious Disease: Dr. Gwendolyn B. Scott, Dr. Charles D. Mitchell, Dr. Claudia Florez, Joan Gamber, University of California San Francisco School of Medicine, Department of Pediatrics: Dr. Diane W. Wara, Dr. Ann Petru, Nicole Tilton, Mica Muscat, Children’s Hospital & Research Center Oakland, Pediatric Clinical Research Center & Research Lab: Dr. Ann Petru, Teresa Courville, Karen Gold, Katherine Eng, University of California San Diego Mother, Child & Adolescent HIV Program: Dr. Stephen A. Spector, Dr. Rolando M. Viani, Mary Caffery, Kimberly Norris, Duke University School of Medicine - Department of Pediatrics, Children’s Health Center: Margaret Donnelly, Dr. Kathleen McGann, Carole Mathison, John Swetnam, University of North Carolina at Chapel Hill School of Medicine - Department of Pediatrics, Division of Immunology and Infectious Diseases: Dr. Tom Belhorn, Jean Eddleman, Betsy Pitkin, Schneider Children’s Hospital: Dr. Vincent R. Bonagura, Dr. Susan Schuval, Dr. Blanka Kaplan, Dr. Constance Colter, Harlem Hospital Center: Dr. Elaine J. Abrams, Maxine Frere, Delia Calo, New York University School of Medicine, Division of Pediatric Infectious Diseases: Dr. William Borkowsky, Nagamah Deygoo, Maryam Minter, Seham Akleh, Children’s National Medical Center, George Washington University: Diana Dobbins, Deidre Wimbley, Dr. Lawrence D’Angelo, Hans Spiegel, University of Washington School of Medicine - Children’s Hospital and Regional Medical Center: Dr. Ann J. Melvin, Kathleen M. Mohan, Michele Acker, Suzanne Phelps, University of Illinois College of Medicine at Chicago, Department of Pediatrics: Dr. Kenneth C. Rich, Dr. Karen Hayani, Julia Camacho, Yale University School of Medicine - Department of Pediatrics, Division of Infectious Disease: Dr. Warren A. Andiman, Leslie Hurst, Dr. Janette de Jesus, Donna Schroeder, SUNY at Stony Brook School of Medicine, Division of Pediatric Infectious Diseases: Denise Ferraro, Jane Perillo, Michele Kelly, Howard University Hospital, Department of Pediatrics & Child Health: Dr. Sohail Rana, Dr. Helga Finke, Patricia Yu, Dr. Jhoanna Roa, LA County/University of Southern California Medical Center: Dr. Andrea Kovacs, Dr. James Homans, Dr. Michael Neely, Dr. La Shonda Spencer, University of Florida Health Science Center Jacksonville, Division of Pediatric Infectious Disease & Immunology: Dr. Mobeen H. Rathore, Dr. Ayesha Mirza, Kathy Thoma, Almer Mendoza, North Broward Hospital District, Children’s Diagnostic & Treatment Center: Dr. Ana M. Puga, Dr. Guillermo Talero, James Blood, Stefanie Juliano, University of Rochester Medical Center, Golisano Children’s Hospital: Dr. Geoffrey A. Weinberg, Barbra Murante, Susan Laverty, Dr. Francis Gigliotti, Medical College of Virginia: Dr. Suzanne R. Lavoie, Tima Y. Smith, St. Jude Children’s Research Hospital, Department of Infectious Diseases: Dr. Aditya Gaur, Dr. Katherine Knapp, Dr. Nehali Patel, Marion Donohoe, University of Puerto Rico, U. Children’s Hospital AIDS: Dr. Irma L. Febo, Dr. Licette Lugo, Ruth Santos, Ibet Heyer, Children’s Hospital of Philadelphia, Center for Pediatric & Adolescent AIDS: Dr. Steven D. Douglas, Dr. Richard M. Rutstein, Carol A. Vincent, Patricia C. Coburn, St. Christopher’s Hospital for Children/Drexel University College of Medicine: Dr. Jill Foster, Dr. Janet Chen, Dr. Daniel Conway, Dr. Roberta Laguerre, Bronx-Lebanon Hospital Center, Infectious Diseases: Dr. Emma Stuard, Caroline Nubel, Dr. Stefan Hagmann, Dr. Murli Purswani, New York Medical College/Metropolitan Hospital Center: Dr. Mahrukh Bamji, Dr. Indu Pathak, Dr. Savita Manwani, Dr. Ekta Patel, University of Massachusetts Memorial Children’s Medical School, Department of Pediatrics: Dr. Katherine Luzuriaga, Dr. Richard Moriarty, Baystate Health, Baystate Medical Center: Dr. Barbara W. Stechenberg, Dr. Donna J. Fisher, Dr. Alicia M. Johnston, Maripat Toye, Connecticut Children’s Medical Center: Dr. Juan C. Salazar, Kirsten Fullerton, Gail Karas, Medical College of Georgia School of Medicine, Department of Pediatrics, Division of Infectious Disease: Dr. Stuart Foshee, Dr. Chitra S. Mani, Dr. Deniis L. Murray, Dr. Christopher White, University of South Alabama College of Medicine, Southeast Pediatric ACTU: Dr. Mary Y. Mancao, Dr. Benjamin Estrada, LSU Health Sciences Center: Dr. Ronald D. Wilcox, Tulane University Health Sciences Center: Dr. Margarita Silio, Dr. Thomas Alchediak, Cheryl Borne, Shelia Bradford, St. Josephs Hospital and Medical Center, Cooper University Hospital - Children’s Hospital Boston, Division of Infectious Diseases, David Geffen School of Medicine at UCLA - Department of Pediatrics, Division of Infectious Diseases, Children’s Hospital of Orange County, Children’s Memorial Hospital - Department of Pediatrics, Division of Infectious Disease, University of Chicago - Department of Pediatrics, Division of Infectious Disease, Mt. Sinai Hospital Medical Center - Chicago, Women’s & Children’s HIV Program, Columbia University Medical Center, Pediatric ACTU, Incarnation Children’s Center, Cornell University, Division of Pediatric Infectious Diseases & Immunology, University of Miami Miller School of Medicine - Jackson Memorial Hospital, Bellevue Hospital (Pediatric), San Francisco General (Pediatric), Phoenix Children’s Hospital, Metropolitan Hospital Center (N.Y.), University of Cincinnati, SUNY Downstate Medical Center, Children’s Hospital at Downstate, North Shore University Hospital, Jacobi Medical Center, University of South Florida - Department of Pediatrics, Division of Infectious Diseases, Cornell University, Oregon Health & Science University - Department of Pediatrics, Division of Infectious Diseases, Children’s Hospital of the King’s Daughters, Infectious Disease, Lincoln Medical & Mental Health Center, Mt. Sinai School of Medicine, Division of Pediatric Infectious Diseases, Emory University Hospital, San Juan City Hospital, UMDNJ - Robert Wood Johnson, Ramon Ruiz Arnau University Hospital, Medical University of South Carolina, SUNY Upstate Medical University, Department of Pediatrics, Wayne State University School of Medicine, Children’s Hospital of Michigan, Children’s Hospital at Albany Medical Center, Children’s Medical Center of Dallas, Children’s Hospital - University of Colorado at Denver and Health Sciences, Center, Pediatric Infectious Diseases, Columbus Children’s Hospital, University of Florida College of Medicine - Department of Pediatrics, Division of Immunology, Infectious Diseases & Allergy, University of Mississippi Medical Center, Palm Beach County Health Department, Children’s Hospital LA - Department of Pediatrics, Division of Adolescent Medicine, Vanderbilt University Medical Center, Division of Pediatric Infectious Diseases, Washington University School of Medicine at St. Louis, St. Louis Children’s Hospital, Children’s Hospital & Medical Center, Seattle ACTU, Oregon Health Sciences University, St. Luke’s-Roosevelt Hospital Center, Montefiore Medical Center - Albert Einstein College of Medicine, Children’s Hospital, Washington, D.C., Children’s Hospital of the King’s Daughters, University of Alabama at Birmingham, Department of Pediatrics, Division of Infectious Diseases, Columbus Regional HealthCare System, The Medical Center, Sacred Heart Children’s Hospital/CMS of Florida, Bronx Municipal Hospital Center/Jacobi Medical Center.
References
- 1.Eley B. Metabolic complications of antiretroviral therapy in HIV-infected children. Expert opinion on drug metabolism & toxicology. 2008;4:37–49. doi: 10.1517/17425255.4.1.37. [DOI] [PubMed] [Google Scholar]
- 2.Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. The Pediatric infectious disease journal. 2012;31:592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachman SA, Chernoff M, Gona P, Van Dyke RB, Dankner WM, Seage GR, 3rd, et al. Incidence of noninfectious conditions in perinatally HIV-infected children and adolescents in the HAART era. Archives of pediatrics & adolescent medicine. 2009;163:164–171. doi: 10.1001/archpedi.163.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jao J, Wyatt CM. Antiretroviral medications: adverse effects on the kidney. Advances in chronic kidney disease. 2010;17:72–82. doi: 10.1053/j.ackd.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Purswani M, Patel K, Kopp JB, Seage GR, 3rd, Chernoff MC, Hazra R, et al. Tenofovir Treatment Duration Predicts Proteinuria in a Multi-Ethnic United States Cohort of Children and Adolescents with Perinatal HIV-1 Infection. The Pediatric infectious disease journal. 2012 doi: 10.1097/INF.0b013e31827f4eff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andiman WA, Chernoff MC, Mitchell C, Purswani M, Oleske J, Williams PL, et al. Incidence of persistent renal dysfunction in human immunodeficiency virus-infected children: associations with the use of antiretrovirals, and other nephrotoxic medications and risk factors. The Pediatric infectious disease journal. 2009;28:619–625. doi: 10.1097/INF.0b013e31819ca49a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brogly S, Williams P, Seage GR, 3rd, Oleske JM, Van Dyke R, McIntosh K. Antiretroviral treatment in pediatric HIV infection in the United States: from clinical trials to clinical practice. JAMA: the Journal of the American Medical Association. 2005;293:2213–2220. doi: 10.1001/jama.293.18.2213. [DOI] [PubMed] [Google Scholar]
- 8.Ray PE, Xu L, Rakusan T, Liu XH. A 20-year history of childhood HIV-associated nephropathy. Pediatric nephrology. 2004;19:1075–1092. doi: 10.1007/s00467-004-1558-1. [DOI] [PubMed] [Google Scholar]
- 9.Naicker S, Han TM, Fabian J. HIV/AIDS--dominant player in chronic kidney disease. Ethnicity & disease. 2006;16:S2-56-60. [PubMed] [Google Scholar]
- 10.Ramsuran D, Bhimma R, Ramdial PK, Naicker E, Adhikari M, Deonarain J, et al. The spectrum of HIV-related nephropathy in children. Pediatric nephrology. 2012;27:821–827. doi: 10.1007/s00467-011-2074-8. [DOI] [PubMed] [Google Scholar]
- 11.Atta MG. Diagnosis and natural history of HIV-associated nephropathy. Advances in chronic kidney disease. 2010;17:52–58. doi: 10.1053/j.ackd.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 13.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66(3):1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 14.Berliner AR, Fine DM, Lucas GM, et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28(3):478–486. doi: 10.1159/000112851. [DOI] [PubMed] [Google Scholar]
- 15.Shikha Garg, Carolyn Furlow-Parmley, Emma Frazier, Skarbinski Jacek for the Medical Monitoring Project; Centers for Disease Control and Prevention, Atlanta, GA. Prevalence of Chronic Kidney Disease Among HIV-infected Adults in Care in the United States Medical Monitoring Project, 2009: Among HIV-infected adults, factors associated with CKD included older age, female sex, duration of HIV ≥10 years, AIDS diagnosis, and recent CD4 count <350 cells/mm3. Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. March 3–6, 2013. [Google Scholar]
- 16.Purswani MU, Chernoff MC, Mitchell CD, Seage GR, 3rd, Zilleruelo G, Abitbol C, et al. Chronic kidney disease associated with perinatal HIV infection in children and adolescents. Pediatric nephrology. 2012;27:981–989. doi: 10.1007/s00467-011-2097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siberry GK, Iannone R, editors. Blood Chemistries/Body Fluids. The Harriet Lane Handbook: A Manual for Pediatric House Officers. 15. St. Louis: Mosby, Inc; 2000. p. 1042. [Google Scholar]
- 18.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 19.Gupta SK, Eustace JA, Winston JA, Boydstun II, Ahuja TS, Rodriguez RA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40:1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 20.Herlitz Leal C, Mohan Sumit, Stokes Michael B, Radhakrishnan Jai, D’Agati Vivette D, Markowitz Glen S. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney International. 2010;78:1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 21.Ryom L, Mocroft A, Kirk O, Worm S, Kamara D, Reiss P, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. Journal infectious diseases. 2013;207:1359–69. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhimma R, Purswani MU, Kala U. Kidney disease in children and adolescents with perinatal HIV-1 infection. J Int AIDS Soc. 2013 Jun;16:18596. doi: 10.7448/IAS.16.1.18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.