Abstract
IMPORTANCE
Sentinel lymph node biopsy has been proposed as an alternative to up-front elective neck dissection (END) for determination of pathologic nodal status in patients undergoing surgical treatment for oral cavity squamous cell carcinoma (OSCC) with clinically negative neck (cN0). Sentinel lymph node biopsy using current standard tracer agents and imaging adjuncts such as radiolabeled sulfur-colloid and planar lymphoscintigraphy (LS), however, is associated with several drawbacks.
OBJECTIVE
To assess the preliminary utility of technetium Tc 99m(99mTc)-tilmanocept, a novel molecular imaging agent for sentinel lymph node (SLN) mapping, in OSCC.
DESIGN, SETTING, AND PARTICIPANTS
Prospective, nonrandomized, single-arm, part of an ongoing phase 3 clinical trial. Patients had previously untreated, clinically and radiographically node-negative OSCC (T1-4aN0M0) at an academic tertiary referral center.
INTERVENTIONS
Patients received a single dose of 50 μg 99mTc-tilmanocept injected peritumorally followed by dynamic planar LS and fused single-photon emission computed tomography/computed tomography (SPECT/CT) prior to surgery. Surgical intervention consisted of excision of the primary tumor and radioguided SLN dissection followed by planned END. The excised lymph nodes (SLNs and non-SLNs) underwent histopathologic evaluation for presence of metastatic disease.
MAIN OUTCOMES AND MEASURES
False-negative rate and negative predictive value of SLNB using 99mTc-tilmanocept and comparison of planar LS with SPECT/CT in SLN localization.
RESULTS
Twelve of 20 patients (60%) had metastatic neck disease on pathologic examination. All 12 had at least 1 SLN positive for metastases. No patients had a positive END node who did not have at least 1 positive SLN. These data yield a false-negative rate of 0% and negative predictive value of 100% using 99mTc-tilmanocept in this setting. Dynamic planar LS and SPECT/CT revealed a mean (range) number of hot spots per patient of 2.9 (1-7) and 3.7 (1-12), respectively. Compared with planar LS, SPECT/CT identified additional putative SLNs in 11 of 20 cases (55%).
CONCLUSIONS AND RELEVANCE
The high negative predictive value and low false-negative rate in identification of occult metastases shows 99mTc-tilmanocept to be a promising agent in SLN identification in patients with OSCC. Use of SPECT/CT improves preoperative SLN localization including delineation of SLN locations near the primary tumor when compared with planar LS imaging.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00911326
Elective neck dissection (END) is the current gold standard for both staging and treatment of the clinically and radiographically negative (cN0) neck in patients undergoing surgical management for oral cavity squamous cell carcinoma (OSCC). However, END is associated with considerable potential morbidity and can be argued as unnecessary for the approximately three-quarters of patients with early-stage oral cancer who ultimately prove to have no nodal metastases on histopathologic assessment.1 As such, sentinel lymph node biopsy (SLNB) has emerged as a promising method to allow determination of the presence or absence of nodal metastases in patients with cN0 OSCC with the goal of reducing the invasiveness and morbidity of the procedure compared with END.
Despite reasonable overall accuracy of SLNB in OSCC, false-negative results for SLNB procedures using traditional agents, such as technetium Tc 99m (99mTc) radiolabeled sulfur colloid, have been reported with varying range up to 10%.2-4 Several mechanisms thought to contribute to false-negative SLNB results include obscuring of relevant sentinel lymph nodes (SLNs) by persistent radioactive signal of the primary tumor (ie, shine-through due to primary tumor proximity), lymphatic obstruction by gross tumor, uneven radionuclide injection, and lesser surgeon experience.2,3,5 Of note, because of its particulate nature and unstandardized variation in preparation (100-1000 nm diameter), 99mTc radiolabeled sulfur colloid is retained for prolonged periods within the injection site, which in turn contributes to the phenomenon of shine-through effect.6 As such, the issue of shine-through effect remains relevant during SLNB procedures, especially in patients with floor of mouth tumors in which tumor proximity to relevant level I lymphatics increases the challenge of accurate intraoperative SLN localization and identification.2,4 Furthermore, standard perioperative imaging techniques such as 2-dimensional dynamic planar lymphoscintigraphy (LS), commonly used in association with SLNB, are of limited value in such situations because planar LS is also hampered by phenomena such as tumor shine-through. In addition, planar LS offers limited relevant anatomic detail that might be of benefit to the surgeon during operative planning.7 Fused single-photon emission computed tomography/computed tomography (SPECT/CT) may afford the surgeon better topographical orientation and delineation of SLNs against the surrounding anatomy and thus provide an advantage over planar LS, although prior studies have not consistently shown SPECT/CT results to influence the outcome of SLNB.2,8
Technologies that aid in improving the accuracy of SLN procedures through the development of improved SLN tracer mapping agents, when used in conjunction with more advanced perioperative imaging and detection modalities, may prove useful in overcoming some of the above obstacles. 99mTc–diethylenetriamine penta-acetic acid–mannosyl–dextran (99mTc-tilmanocept; Navidea Biopharmaceuticals Inc) is anovel targeted radiopharmaceutical agent intended for use in SLN mapping and intraoperative procedures in an effort to address drawbacks associated with LS agents, including 99mTc sulfur colloid. The design of 99mTc-tilmanocept (synthetic molecule with a dextran-10 backbone with multiple mannose side moieties, making it a CD206 receptor–targeted radiopharmaceutical) allows it to exhibit 2 features that may favor its utility in the management of OSCC: (1) more rapid clearance from the injection site and (2) sustained SLN uptake without distal lymph node accumulation due to binding specificity to lymphatic tissues.9,10
The efficacy of 99mTc-tilmanocept has been previously reported in trials examining its use in patients with cutaneous melanoma and breast cancer.9-11 Its efficacy for use in patients with head and neck squamous cell carcinoma (HNSCC) is currently the subject of an ongoing phase 3 multi-institutional trial from which this study derives. In the present study, we report our preliminary single-institution experience within the setting of this ongoing trial assessing the utility of 99mTc-tilmanocept as an SLN mapping agent in patients with cN0 OSCC, as well as that of perioperative imaging adjuncts including both planar LS and SPECT/CT, examining preoperative imaging data, intraoperative findings, and histopathologic data from surgical procedures.
Methods
Institutional Review
A phase 3 clinical trial of 99mTc-tilmanocept for SLNB in patients with HNSCC (oral, cutaneous) with cN0 neck status is currently accruing patients in a multi-institutional setting including at The Ohio State University Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Columbus, Ohio. The protocol received the approval of the Western Institutional Review Board.
Patient Enrollment
During the period August 2009 through June 2012, 20 patients presenting for primary treatment of cN0 OSCC were prospectively enrolled in the study at this site. Patients were defined as having cN0 status on the basis of physical examination results and contrast-enhanced high-resolution helical computed tomography or magnetic resonance imaging of the neck. Pregnant and lactating women, patients with known metastatic disease, and patients currently enrolled in another protocol were excluded from this study.
Radiopharmaceutical Injection and Lymphoscintigraphy
All patients received a single dose of 50 μg of tilmanocept, radiolabeled with either 1.8 × 107-Bq or 7.4 × 107-Bq 99mTc for injection either on the day of surgery or on the day before surgery, respectively. The timing of the radiopharmaceutical injection was at the surgeon’s discretion except in patients with floor of mouth tumors, in whom 99mTc-tilmanocept injection was required to be performed the day before surgery to allow for improved clearance of the radiopharmaceutical from the primary injection site. Fifteen patients received day of surgery injection and 5 patients received the day before surgery injection. In all patients, injection of 99mTc-tilmanocept was performed using a total of 50.1-mL aliquots with 4 injections placed peritumorally (ie, 12-, 3-, 6-, and 9-o’clock positions at the periphery of the tumor) and 1 injection placed deep to the primary tumor. Following injection and prior to surgery, all patients underwent LS imaging per institutional protocol. At this institution, patients typically underwent dynamic planar LS over the initial 60 minutes following radiopharmaceutical injection including subsequent planar spot views in various projections followed by additional SPECT/CT imaging acquisition. In patients undergoing day before surgery injection, SPECT/CT typically occurred the following day just prior to surgery.
Radiologic Review
A retrospective review of the planar LS and SPECT/CT imaging data was performed by a blinded board-certified nuclear medicine physician. Total focal areas of increased radiopharmaceutical accumulation thought to represent putative SLNs were documented for planar imaging (dynamic and spot), as well as with SPECT/CT images.
Sentinel Lymph Node Biopsy
Surgery occurred within 1 to 30 hours following 99mTc-tilmanocept injection. For patients undergoing day of surgery injection or day before surgery injection, surgery was required within 1 to 15 or 15 to 30 hours following injection, respectively. At surgery, excision of the primary tumor was performed prior to SLNB. An intraoperative handheld gamma probe (Neoprobe; Devicor Medical Products Inc) was used to conduct an initial detection survey of the entire cervical lymph node basin to identify the area(s) of increased radioactivity representing putative SLNs. Sentinel lymph node biopsy was performed through an incision along the planned incision for an END using the handheld gamma probe to localize and identify radioactive lymph nodes. Radioactivity of lymph nodes was quantified with the gamma probe by either the mean of 3 separate 2-second duration counts or a single 10-second duration count. Lymph nodes with radioactivity counts that exceeded 3 standard deviations above the mean normal tissue background count (3 σ counting rule) were considered SLNs and excised. After removal of all SLNs meeting these criteria, the gamma probe was placed back within the nodal basin to ensure that no substantial residual radioactivity remained. After removal of all SLNs, patients underwent planned END. Bilateral ENDs were performed when the primary lesion involved the midline or tumors less than 1 cm from midline with evidence of contralateral drainage on LS, or per the surgeon’s discretion.
Histopathologic Analysis of Lymph Nodes
Histopathologic assessment carried out on SLNs, as well as non-SLNs (ie, those obtained during END), was specified per protocol requirements. Relevant tissue blocks, slides, and/or images were additionally submitted to a central laboratory designated by the trial sponsor for additional specimen processing and/or review, which included serial sectioning and immunohistochemical assessment. In short, following formalin fixation, all SLNs were cut in a transverse fashion along the longest axis every 2 mm. Slides from these sections underwent initial evaluation by means of standard hematoxylin-eosin (H&E) staining. Sentinel lymph nodes with findings of metastases on H&E assessment were deemed positive nodes. Sentinel lymph nodes that were negative for metastases on H&E assessment were then further evaluated at the central laboratory with immunohistochemical staining for pancytokeratin markers (eg, AE1/AE3, CK8/CK18, MNF 116). The non-SLNs (ie, those obtained during END) were assessed by means of H&E staining of a single section carried out along the longitudinal axis of the node. Lymph node metastatic deposits were classified as either macrometastases (>2 mm in diameter) or micrometastases (≤2 mm in diameter).
Results
A total of 20 patients with newly diagnosed OSCC with cN0 necks were enrolled in the study. The cohort consisted of 15 men (75%) and 5women (25%). The mean (range) age at diagnosis was 62 (46-87) years. The site of the primary lesion was the oral tongue in 12 cases (60%), the floor of the mouth in 5 cases (25%), the alveolar ridge in 2 cases (10%), and the mucosal lip in 1 case (5%). Of the 20 patients, 7 (35%) presented with category T1, 11 (55%) with category T2, and 2 (10%) with category T3 disease (Table 1).
Table 1. Patient Characteristics.
| Characteristic | Patients (N = 20) |
|---|---|
| Sex, No. (%) | |
| Male | 15 (75) |
| Female | 5 (25) |
| Age, median (range), y | 62 (46-87) |
| Primary tumor site, No. (%) | |
| Oral tongue | 12 (60) |
| Floor of mouth | 5 (25) |
| Alveolar ridge | 2 (10) |
| Mucosal lip | 1 (5) |
| T classification, No. (%) | |
| 1 | 7 (35) |
| 2 | 11 (55) |
| 3 | 2 (10) |
Fifteen patients underwent radiotracer injection on the same day as surgery, and 5 patients received radiotracer injection the day prior to surgery. Both planar LS and SPECT/CT revealed focal areas of increased radiopharmaceutical accumulation in all cases, with the exception of participant 16, for whom a registration error occurred during the acquisition of the dynamic planar LS images (Table 2). Planar LS revealed a mean (range; median) of 2.9 (1-7; 3) hot spots, and SPECT/CT, 3.7 (1-12; 4) hot spots. We found that SPECT/CT revealed more hot spots than dynamic planar LS in 11 cases (58%), the same number of hot spots in 5 cases (26%), and fewer hot spots in 3 cases (16%). The difference in the number SLNs identified between planar LS and SPECT/CT, however, was not statistically significant (P = .27).
Table 2. Summary of Radiological, Surgical, and Pathological Findings.
| Patient No./Location | SLNs, No. | SLN Location on SPECT/CT | SLNs on SLNB, No. | SLN Surgical Location | Positive SLNs, No. (Location) | Positive LNs on END, Proportion | Metastatic Deposit | |
|---|---|---|---|---|---|---|---|---|
| On LS | On SPECT/CT | |||||||
| 1/Tongue | 4 | 4 | Right levels I-IV | 4 | Right levels I, II, V | 1 (Right level I) | 0/15 | Micro |
| 2/Tongue | 2 | 4 | Left levels II, III, V | 6 | Left levels I-III, V | 1 (Left level II) | 0/12 | Micro |
| 3/Tongue | 3 | 5 | Right levels II, IV; left level IV | 5 | Right levels II, V; left level V | 1 (Right level V) | 0/11 | Micro |
| 4/FOM | 1 | 2 | Left levels I, III | 1 | Left level V | 0 | 0/17 | None |
| 5/FOM | 4 | 4 | Right levels I, II; left levels I, III | 5 | Right levels I, II; left levels I, III | 3 (Right levels I, II; left level III) | 1/72 | Macro |
| 6/Tongue | 1 | 2 | Right levels II, IV | 3 | Right levels II, III | 1 (Right level II) | 0/10 | Macro |
| 7/Tongue | 2 | 2 | Right level III; left level III | 4 | Right levels II-IV | 1 (Right level III) | 0/20 | Macro |
| 8/Tongue | 2 | 3 | Left levels I, III | 4 | Left levels II-IV | 0 | 0/20 | None |
| 9/Alveolus | 4 | 12 | Right levels I-IV; right parotid; left levels I-III | 11 | Right levels I-III; left levels I-III | 2 (Right levels I, II) | 0/7 | Macro |
| 10/Tongue | 1 | 3 | Right levels II, III | 5 | Right levels II, III | 3 (Right levels II, III) | 0/11 | Micro |
| 11/Tongue | 1 | 4 | Right levels II-IV | 4 | Right levels II, III | 0 | 0/51 | None |
| 12/FOM | 1 | 4 | Right levels I-III | 3 | Right levels I, II | 3 (Right levels I, II) | 0/26 | Micro |
| 13/Tongue | 5 | 1 | Left level II | 9 | Right level IV; left levels I-III | 2 (Left levels II, III) | 0/26 | Macro |
| 14/FOM | 5 | 6 | Right levels II-IV; left levels II, III | 4 | Left levels I-III; right level II | 0 | 0/43 | None |
| 15/Tongue | 3 | 2 | Right levels II, III | 3 | Right levels II, III; left level III | 1 (Right level II) | 0/28 | Micro |
| 16/Alveolus | Error | 4 | Left levels II, IV, V | 5 | Left levels I, II, V | 0 | 0/19 | None |
| 17/Tongue | 4 | 4 | Right levels II, IV | 7 | Right levels II-IV | 3 (Right levels II, III) | 0/15 | Macro |
| 18/Mucosal lip | 4 | 5 | Right levels I, II; left level I | 7 | Right levels I, III; left level I | 0 | 0/19 | None |
| 19/FOM | 7 | 1 | Left level II | 2 | Left levels III, IV | 0 | 0/61 | None |
| 20/Tongue | 2 | 2 | Right levels IV, V | 7 | Right levels II, III | 0 | 0/22 | None |
Abbreviations: END, elective neck dissection; FOM, floor of mouth; LN, lymph node; LS, lymphoscintigraphy; macro, macrometastases; micro, micrometastases; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; SPECT/CT, single-photon emission computed tomography/computed tomography.
Identification of at least 1 SLN during surgery was achieved in all 20 participants. The mean (range; median) number of lymph nodes excised during SLNB was 5.0 (1-11; 4.5). The anatomic location of each SLN according to SPECT/CT, as well as intraoperative findings, is shown in Table 2. The degree of correlation between surgical findings and preoperative imaging results was higher for SPECT/CT than for dynamic planar LS (0.55 and 0.27, respectively; P = .16) (Figure 1).
Figure 1. Correlation Plot.
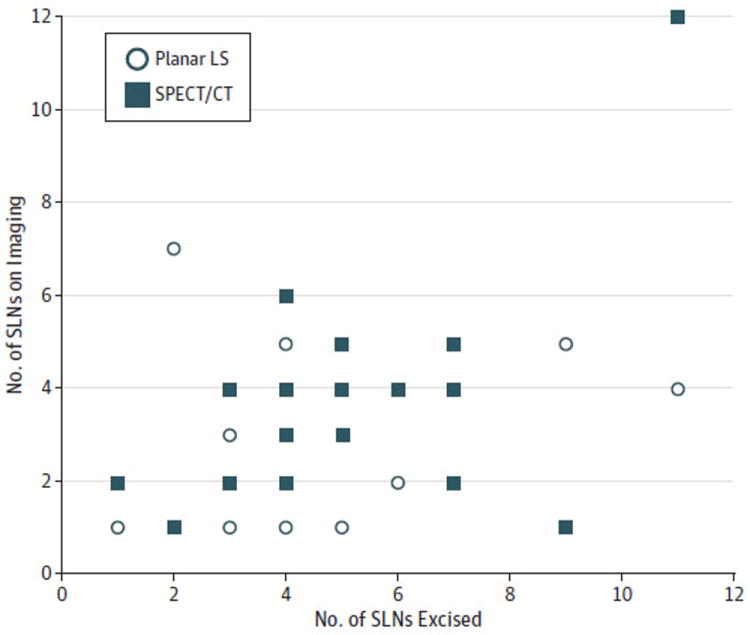
Correlation plot comparing the number of sentinel lymph nodes (SLNs) visualized on each imaging modality (dynamic planar lymphoscintigraphy [LS] and fused single-photon emission computed tomography/computed tomography [SPECT/CT]) and number of SLNs excised during SLN biopsy.
Twelve of the 20 participants (60%) were identified with occult positive nodal metastases. Two patients in the day before surgery 99mTc-tilmanocept injection group (40%) had positive lymph node metastases. Of the 15 patients with day of surgery 99mTc-tilmanocept injections, 10 (67%) had positive lymph node metastases. For all 12 of these participants, histopathologic assessment of the SLNs was positive for occult metastases in at least 1 SLN. No patients had a positive END node who did not have at least 1 positive SLN. The mean (range; median) number of positive SLNs was 1.8 (1-3; 1.5). Of the 12 patients with metastatic cervical nodes, 6 (50%) had macrometastases and 6 (50%) had micrometastases (Table 2). These results yield a negative predictive value for a negative SLNB result of 100% and a false-negative rate of 0%.
Discussion
Minimal access surgical approaches are becoming more common to reduce surgical morbidity. The same is true for OSCC, in which SLNB is being evaluated as a possible alternative to up-front END in patients with cN0 necks. Sentinel lymph node biopsy seeks to identify relevant tissue for diagnosis while minimizing dissection, thereby sparing relevant structures. In a comparison of quality of function, morbidity, and quality of life following SLNB and END, Schiefke et al12 found statistically significantly improved outcomes following SLNB in swallowing function, tactile and pain sensation, scar appearance, shoulder constant score, and fear of disease progression.
Our study aimed to evaluate the utility of 99mTc-tilmanocept, a novel radiotracer in SLNB for participants with cN0 OSCC, as well as the relative utility of perioperative imaging adjuncts, which may assist with preoperative localization and correlation with intraoperative findings. As mentioned, the present analysis of our single-institution experience is performed within the context of an ongoing multi-institutional trial examining the efficacy of 99mTc-tilmanocept for SLNB for patients with cN0 HNSCC.
Despite its prospective nature, it is important to acknowledge potential limitations of the present study. As a single-arm study that seeks primarily to examine efficacy measures of 99mTc-tilmanocept, this study does not compare the efficacy of 99mTc-tilmanocept with that of 99mTc sulfur colloid, the agent traditionally used in most presently performed SNLB procedures. The efficacy of 99mTc-tilmanocept in OSCC, however, has not been characterized, and as such, examination of variables describing accuracy including the rate of false-negative results and negative predictive value in a single-arm study is appropriate. To accomplish this goal, results of SLNB in conjunction with END remains the gold standard with which to measure such variables when assessing new SLN agents including 99mTc-tilmanocept, as was carried out in the present study.
An additional drawback to the present study pertains to the fact that lymph nodes obtained during the END (ie, non-SLNs) did not undergo the same degree of histopathological analysis as the SLNs (step sectioning and immunohistochemical staining). As such, there exists the potential for underestimation of the rate of false-negative results. Other studies, however, have examined non-SLNs such as those obtained via END using such detailed methods, identifying the rate of occult disease in non-SLNs to be quite low (2%).13 Such methodology obviously also poses a substantial practical issue, and thus in the absence of established efficacy, routine use of similar detailed measures for non-SLNs becomes difficult to justify.
The present series identified a 60% incidence of occult nodal metastases. Although admittedly in a relatively small group of patients, this rate is considerably higher than previously reported rates of occult neck disease (20%-30%) in patients with cN0 early-stage OSCC.2,3 This may be in part the result of more detailed histopathologic assessment including additional step sectioning and immunohistochemical staining performed on SLNs that is typically beyond the standard H&E staining of a single section performed at most institutions on an END specimen. Atula et al14 similarly showed an increased rate of positive SLNs with the addition of step sectioning and cytokeratin staining when compared with standard histologic evaluation (33% vs 22%, respectively).
In light of these sorts of discrepancies, the Second International Conference on Sentinel Node Biopsy in Mucosal Head and Neck Cancer has recommended that all SLNs undergo evaluation with step sectioning, H&E, and cytokeratin staining to prevent missed micrometastases.15 Of note, the present series also included 2 participants with larger category T3 primary tumors, both of whom were subsequently identified with occult metastatic neck disease via SLNB and/or END, which likely also served to increase the observed rate of neck disease identified in the whole group.
In the present series, we observed a negative predictive value of 100% for negative SLNB result and a false-negative rate of 0% for all OSCC tumor subsites. This includes the floor of mouth tumor subsite, for which previous authors have reported lower SLN identification rates and higher false-negative rates2,3,5; albeit the sample size of patients with floor of mouth tumors in our series was small (5 participants). Overall, these preliminary results at our institution indicate that 99mTc-tilmanocept used in conjunction with SLNB to predict regional nodal status seems promising.
Interestingly, we found that for the patients with identified occult metastatic disease, not only was this predicted by at least 1 positive sentinel node in all 12 participants, but in 11 of these participants (92%) the only positive node(s) identified were the SLNs, with no non-SLNs yielding additional metastatic foci. In the absence of a prospective study, it would be premature and inappropriate to suggest therapeutic potential for SLNB alone in OSCC on the basis of such findings, especially because the non-SLNs in this and most prior studies have not been subjected to the same degree of histologic scrutiny. Nonetheless, this finding is consistent with previous reports by others13 and seems to support the ability of 99mTc-tilmanocept to achieve accurate SLN mapping and correctly identify anatomic metastatic pathways for a disease entity with substantial potential for unpredictable behavior due to factors including variable lymphatic drainage pattern and occurrence of “skip” metastases. Furthermore, such findings indicate aspects of tumor behavior that may not be adequately encompassed by empirical END carried out in the absence of preoperative LS and/or SLNB procedures. Whereas END maintains a well-established role in the management of the cN0 neck at present, these findings may suggest a role for lymphatic mapping agents such as 99mTc-tilmanocept as a useful adjunct even when END procedures are planned (ie, to guide extent) and thus merit prospective study.
In the present study, more SLNs were detected with the use of SPECT/CT than with planar LS (mean, 3.7 vs 2.9 nodes, respectively; P = .26). Although the difference was not statistically significant, it is important to note that the single institutional site data are likely underpowered to detect such a difference. We did also note improved correlation between results obtained from SPECT/CT images and intraoperative SLNB findings when compared with results obtained from planar LS images (correlation coefficient, 0.55 and 0.27, respectively). Of note, SPECT/CT images provided additional SLN data beyond those of planar LS in 11 patients. This is comparable to results obtained by Wagner et al,16 who found that 11 of 49 SLNs were detected only by means of SPECT/CT, and Bilde et al,8 who reported 47% more SLNs identified by means of SPECT/CT compared with planar LS. Furthermore, although difficult to objectively quantify, we observed that specific anatomic information regarding exact SLN location, including relation to surrounding relevant anatomic structures depicted on SPECT/CT, was far superior to that provided by planar LS. This observation is characteristically exemplified in Figure 2, where images from dynamic planar LS and SPECT/CT of participant 5 can be compared. This example was characteristic of the difference between the results obtained from both types of imaging for many of the participants in the present series.
Figure 2. Comparison of 2 Imaging Methods.
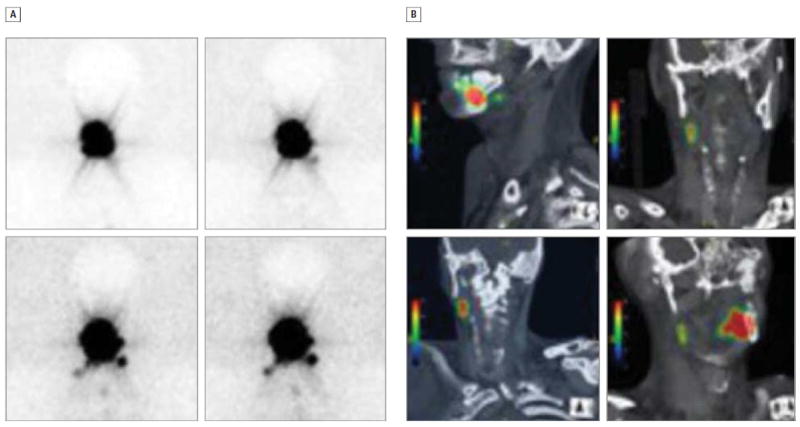
Dynamic planar lymphoscintigraphy (A) and fused single-photon emission computed tomography/computed tomography (B) images from participant 5 demonstrating the delineation of sentinel lymph node location in relationship to adjacent structures in the 2 imaging modalities.
Of note, we observed that in 3 patients (13, 15, and 19), dynamic planar LS did reveal more putative SLN regions than were noted on SPECT/CT, and in 1 additional patient (18), an individual hot-spot focus was found on planar LS that was missed on SPECT/CT. On further evaluation of these 4 patients, we found that no SLNs were missed in participant 13; however, there was improved delineation of individual SLNs within the left neck on planar LS when compared with SPECT/CT, resulting in the higher overall SLN count. The finding of additional SLNs on planar LS in this patient did not alter the surgical plan because all of the SLNs were located in the left level II neck. Participant 15 was found to have a radioactive focus within the left neck on planar LS that was not present on SPECT/CT. This patient underwent variance in perioperative imaging timing compared with most other participants in that SPECT/CT was obtained at 20 minutes after 99mTc-tilmanocept injection and prior to the acquisition of the final dynamic planar LS images. It is conceivable that at the time of SPECT/CT acquisition insufficient radiotracer accumulation within the SLNs had occurred. Participant 18 had a right level V hot spot that was visualized on planar LS but not on SPECT/CT. The presence of this hot spot was not confirmed with the intraoperative gamma probe. The same was true for participant 19, for whom multiple bilateral hot spots were apparent on planar LS but were not confirmed on SPECT/CT or the intraoperative gamma probe. This participant’s injection occurred the day prior to surgery. Although this variability is not easily explained, it is conceivable that the timing of planar LS, SPECT/CT, and surgery with respect to radiotracer injection may explain the variability of observed results in these patients. Admittedly, it is feasible that the delayed acquisition of SPECT/CT imaging relative to planar LS in the majority of participants also could explain in part the comparative difference in SLN identification between the 2 techniques.
The utility of SPECT/CT in this study was particularly evident in the preoperative localization of level I SLNs—an area that has proven challenging to assess because of the proximity of these lymph nodes to the primary site of disease and high levels of radioactive shine-through. In the present study, putative level I SLNs were identified on SPECT/CT in 7 patients and confirmed intraoperatively with the gamma probe and subsequent excision in 5 of these patients. Three of these participants in whom SPECT/CT localized level I SLNs presented with floor of mouth OSCC, and this tumor location is one that involves notably close proximity of the primary site to these lymph nodes. Overall, these findings of SPECT/CT correlation with intraoperative findings, high level of anatomic detail including topographical orientation and delineation of SLNs with respect to surrounding surgically relevant structures, and ability to allow level I SLN delineation support a potential role of SPECT/CT in conjunction with 99mTc-tilmanocept for SLN mapping to assist with preoperative planning of incisions and localization of SLNs intraoperatively.
Finally, the stipulation that patients with floor of mouth tumors undergo radiotracer injection the day prior to surgery (to improve radiotracer clearance from the primary site) afforded us the opportunity to informally evaluate the extent of distal accumulation of 99mTc-tilmanocept. Five patients (4, 5, 12, 14, and 19) underwent radiotracer injection the day prior to surgery. These patients underwent dynamic planar LS over 60 minutes immediately following radiotracer injection followed by SPECT/CT carried out on the day of surgery (because this would provide important information likely to correspond with intraoperative gamma probe use). Examination of these participants revealed that the number of radioactive putative SLN sites detected on planar LS immediately following injection was similar to that identified on subsequent SPECT/CT obtained the day following injection (3.6 vs 3.4 sites, respectively; P = .80). Admittedly, the use of 2 differing imaging modalities on each day (planar LS and SPECT/CT), as well as the small sample size, makes formal comparison and conclusions difficult because this would necessitate use of similar imaging methods on both days (ie, SPECT/CT), as well as a larger sample size. Nonetheless, this informal observation does seem to support previous findings reporting stability of 99mTc-tilmanocept within SLNs for a prolonged period after injection (up to 30 hours).6,17,18 The sustained uptake and stable pattern of 99mTc-tilmanocept uptake within SLNs and its ability to be reliably detected and localized the day following injection may aid in overcoming the logistical challenges and inefficiencies often associated with SLNB procedures using agents such as 99mTc sulfur colloid. Such agents often involve injection the same day as surgery, as well as more careful coordination and timing of nuclear medicine injection and imaging with relation to surgery.
Conclusions
The use of 99mTc-tilmanocept in conjunction with SLNB in patients with cN0 OSCC is promising and seems to be supported by the results of this preliminary study with low observed false-negative rate (0%) and high negative predictive value (100%). Adjunctive use of detailed imaging including SPECT/CT along with 99mTc-tilmanocept may be of benefit in preoperative planning by providing the surgeon with improved spatial resolution and localization of relevant nodes and seems to better correlate with intraoperative findings when compared with the use of planar LS. In addition, use of 99mTc-tilmanocept for SLNB may simplify operating room logistics and efficiency by allowing for a broader window and flexibility of timing of preoperative procedures and operating room scheduling.
Acknowledgments
Funding/Support: The data from the present study derive in part from multi-institutional trial NEO3-06 sponsored by Navidea Biopharmaceuticals Inc (Dublin, Ohio).
Role of the Sponsor: Navidea Biopharmaceuticals provided no funding for this article. The authors have no relevant financial or other relationship with Navidea Biopharmaceuticals.
Footnotes
Author Contributions: Drs Marcinow and Agrawal had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Teknos, Old, Agrawal.
Acquisition of data: Hall, Byrum, Teknos, Old, Agrawal.
Analysis and interpretation of data: Marcinow, Hall, Teknos, Old, Agrawal.
Drafting of the manuscript: Marcinow, Hall, Agrawal.
Critical revision of the manuscript for important intellectual content: Hall, Byrum, Teknos, Old, Agrawal.
Statistical analysis: Marcinow, Byrum, Agrawal.
Administrative, technical, or material support: Hall, Teknos, Old, Agrawal.
Study supervision: Hall, Teknos, Old, Agrawal.
Additional Contributions: Navidea Biopharmaceuticals provided the use of a model 2100 intraoperative radioisotope detector.
Previous Presentation: This study was presented at the American Head and Neck Society Eighth International Conference on Head and Neck Cancer; July 23, 2012; Toronto, Ontario, Canada.
Conflict of Interest Disclosures: None reported.
99mTc–tilmanocept is a registered trademark of Navidea Biopharmaceuticals.
Contributor Information
Anna M. Marcinow, Department of Otolaryngology–Head and Neck Surgery, The Ohio State University College of Medicine, Columbus.
Nathan Hall, Department of Radiology, The Ohio State University College of Medicine, Columbus.
Eric Byrum, Department of Radiology, The Ohio State University College of Medicine, Columbus.
Theodoros N. Teknos, Department of Otolaryngology–Head and Neck Surgery, The Ohio State University College of Medicine, Columbus.
Matthew O. Old, Department of Otolaryngology–Head and Neck Surgery, The Ohio State University College of Medicine, Columbus.
Amit Agrawal, Department of Otolaryngology–Head and Neck Surgery, The Ohio State University College of Medicine, Columbus.
References
- 1.Kligerman J, Lima RA, Soares JR, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg. 1994;168(5):391–394. doi: 10.1016/s0002-9610(05)80082-0. [DOI] [PubMed] [Google Scholar]
- 2.Ross GL, Soutar DS, Gordon MacDonald D, et al. Sentinel node biopsy in head and neck cancer: preliminary results of a multicenter trial. Ann Surg Oncol. 2004;11(7):690–696. doi: 10.1245/ASO.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Civantos F, Jr, Zitsch R, Bared A, Amin A. Sentinel node biopsy for squamous cell carcinoma of the head and neck. J Surg Oncol. 2008;97(8):683–690. doi: 10.1002/jso.21015. [DOI] [PubMed] [Google Scholar]
- 4.Civantos FJ, Zitsch RP, Schuller DE, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol. 2010;28(8):1395–1400. doi: 10.1200/JCO.2008.20.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkureishi LW, Ross GL, Shoaib T, et al. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann Surg Oncol. 2010;17(9):2459–2464. doi: 10.1245/s10434-010-1111-3. [DOI] [PubMed] [Google Scholar]
- 6.Wallace AM, Hoh CK, Limmer KK, Darrah DD, Schulteis G, Vera DR. Sentinel lymph node accumulation of Lymphoseek and Tc-99m-sulfur colloid using a “2-day” protocol. Nucl Med Biol. 2009;36(6):687–692. doi: 10.1016/j.nucmedbio.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoeckli SJ. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope. 2007;117(9):1539–1551. doi: 10.1097/MLG.0b013e318093ee67. [DOI] [PubMed] [Google Scholar]
- 8.Bilde A, Von Buchwald C, Mortensen J, et al. The role of SPECT-CT in the lymphoscintigraphic identification of sentinel nodes in patients with oral cancer. Acta Otolaryngol. 2006;126(10):1096–1103. doi: 10.1080/00016480600794453. [DOI] [PubMed] [Google Scholar]
- 9.Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: a molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14(2):913–921. doi: 10.1245/s10434-006-9099-4. [DOI] [PubMed] [Google Scholar]
- 10.Ellner SJ, Hoh CK, Vera DR, Darrah DD, Schulteis G, Wallace AM. Dose-dependent biodistribution of [(99m)Tc]DTPA-mannosyldextran for breast cancer sentinel lymph node mapping. Nucl Med Biol. 2003;30(8):805–810. doi: 10.1016/j.nucmedbio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Sondak VK, King DS, Kager JS, et al. Combined analysis of phase III trials evaluating [99mTc]tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node-negative cutaneous melanoma. Ann Surg Oncol. 2013;20(2):680–688. doi: 10.1245/s10434-012-2612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiefke F, Akdemir M, Weber A, Akdemir D, Singer S, Frerich B. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck. 2009;31(4):503–512. doi: 10.1002/hed.21001. [DOI] [PubMed] [Google Scholar]
- 13.Christensen A, Bilde A, Therkildsen MH, et al. The prevalence of occult metastases in nonsentinel lymph nodes after step-serial sectioning and immunohistochemistry in cN0 oral squamous cell carcinoma. Laryngoscope. 2011;121(2):294–298. doi: 10.1002/lary.21375. [DOI] [PubMed] [Google Scholar]
- 14.Atula T, Hunter KD, Cooper LA, Shoaib T, Ross GL, Soutar DS. Micrometastases and isolated tumour cells in sentinel lymph nodes in oral and oropharyngeal squamous cell carcinoma. Eur J Surg Oncol. 2009;35(5):532–538. doi: 10.1016/j.ejso.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Stoeckli SJ, Pfaltz M, Ross GL, et al. The second international conference on sentinel node biopsy in mucosal head and neck cancer. Ann Surg Oncol. 2005;12(11):919–924. doi: 10.1245/ASO.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Wagner A, Schicho K, Glaser C, et al. SPECT-CT for topographic mapping of sentinel lymph nodes prior to gamma probe-guided biopsy in head and neck squamous cell carcinoma. J Craniomaxillofac Surg. 2004;32(6):343–349. doi: 10.1016/j.jcms.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Mendez J, Wallace AM, Hoh CK, Vera DR. Detection of gastric and colonic sentinel nodes through endoscopic administration of 99mTc-DTPA-mannosyl-dextran in pigs. J Nucl Med. 2003;44(10):1677–1681. [PubMed] [Google Scholar]
- 18.Salem CE, Hoh CK, Wallace AM, Vera DR. A preclinical study of prostate sentinel lymph node mapping with [99MTC]diethylenetetramine pentaacetic acid-mannosyl-dextran. J Urol. 2006;175(2):744–748. doi: 10.1016/S0022-5347(05)00139-4. [DOI] [PubMed] [Google Scholar]


