Abstract
Land use and land cover (LULC) is now recognized as an important driver of disease. For emerging or re-emerging infectious diseases, LULC offers context and serves as a likely proximate driver of risk particularly when considering vector-borne or zoonotic diseases. Ontological differences embedded within disciplinary structures impede progress limiting the ultimate potential of both LULC data and land change theory within disease research. Geography, space, and time serve as effective complements to traditional health and place organizational and disease-research strategies. Improved systemic clarity is obtained if one orients the disease relationship to particular contexts and if the scales of the relationships are clearly defined.
Introduction
Land use and cover matter
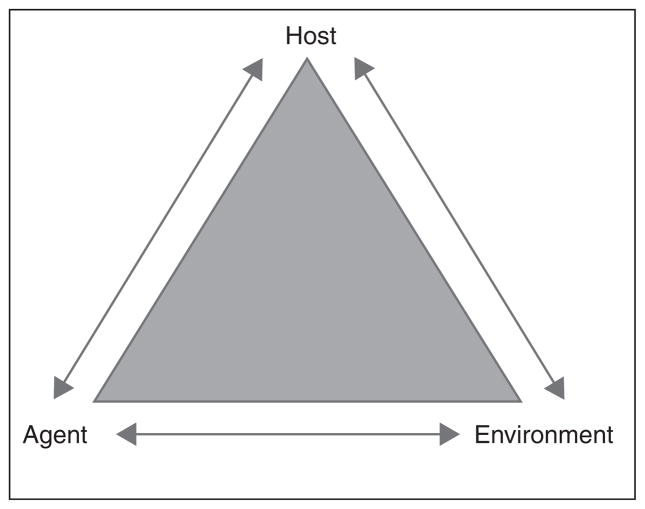
It is often cited as the most important factor in the emergence or re-emergence of infectious disease [1]. The role of land use and land cover (LULC) as cause, contributor, or facilitator of health and disease systems is known, but it curiously remains a distant third wheel of the epidemiological triangle model of disease causation (Figure 1). It might be simply that the chain of transmission emphasizes factors related to host and agent. But we assert that the ontological differences between the health research and land change science communities substantively impede relevant basic science. Land change models best fit epidemiological evidence structures when firmly rooted in ecologic studies (Figure 2). To be clear, we define ecologic studies, specifically within the epidemiological evidence framework, as those where the unit of analysis is a population rather than an individual. This is a precise definition and distinct from what might be considered ‘ecologic(al)’ in the land change science community, particularly, the lack of any ‘biotic’ reference. These are ontological differences.
Figure 1.
The epidemiological triangle model of disease causation.
Figure 2.
LULC models are best used in ecologic scale study designs. Although potentially appropriate, there are far fewer good applications of LULC models in case series, cross-sectional, or case control applications. We believe that the near term potential for the expansion of LULC and health research is within cross-sectional designs.
It is important to recognize that land use and cover models are abstractions often only loosely, or more commonly indirectly, tied to relevant drivers of health and disease. This abstraction and associated causation problem permeates the consumers of land change data for health research. Understanding the relationship between LULC data and disease requires intimate knowledge of the dynamic relationship between the environment, people, and pathogens. This becomes increasingly complex as disease lifecycles often alternate between humans and nonhuman vertebrates, invertebrates or other mediating living organisms. Among the several, fine reviews previously conducted linking humans and altered landscapes to increased disease risk [2–5] practical generalizations have proven rare. Further, the emergence of the focus on human–environment relationships in health research mediated by LULC has largely driven the thematic questions by region. For example, most studies in the tropics focus on rapidly emerging diseases due to increased human–vector/animal contacts, but in temperate regions far more research explores climate change impacts or otherwise small changes that allow subpopulations (animal/human/vector) to be exposed and begin infective/epidemic cycles. While sensitive to the human–environment theoretical core, space and time should serve as complementary to traditional health and place organizational strategies. Until space and time become equal to place and health (agent and host), land change science and related data products will remain relegated to ecologic scale research questions.
Framework
The International Geosphere–Biosphere Program (IGBP) and the International Human Dimensions Program have offered a categorization of land change models: comparative land use dynamics models used to improve understanding of the relationships among human decision making across scales, empirical diagnostic models that rely on remotely sensed observations of spatial and temporal land cover dynamics, and integrative assessment models emergent through the development of land use and cover change models, which explicitly incorporate prediction and scenario development. All three classes of models are found distributed among health research papers incorporating meaningful representations of land surface systems. Land use and cover change systems are often manifestations of synergistic relationships, where causality is bi-directional in that LULC not only affects sociocultural norms, but these norms influence LULC [6]. Unfortunately, it is almost always the case that health literatures focus on only one side of this relationship with disease response as a dependent variable in a larger group-level model with LULC, in some form, a one-way predictor.
Group-level models, also commonly called ecologic studies, specifically apply if the population, rather than the individual, is the unit of study and some group-level factor is the disease or, more frequently, the exposure. These studies often use routinely collected data at the scale of a community or political entity. Despite a long tradition of using population-level data for descriptive science questions, these data are less frequently used for hypothesis testing because of the ecologic fallacy and modifiable areal unit problems. With the notable exception of community randomized trials, specific impediments for LULC application among the health community, include difficulty managing confounding and interaction effects, and arguably most relevant today, the rise of the biomedical paradigm [7]. Today, from within the land use and cover change community, we see the continuing epidemiological conflict [8] as one between scientists who dispute the contention over whether the health of a community is more than the summation of the health of its individual members. Besides the aforementioned ontological issues, confusion emerges among land change modelers and epidemiologists because most parameterizations of LULC models are not summary measures of individual-level variables and do not have corresponding individual-level values. Ecological fallacy issues arise when these integral variables are subsequently modeled at the individual level. What appears in print then are often papers in which LULC conditions are modeled at increasingly fine spatial resolution in an attempt to circumvent this problem. As is well known though, resolution is often a trade of time for space and, as we see in many of the health and disease papers published today, this negotiation often emerges. As vector-borne and zoonotic diseases such as malaria, Lyme, dengue, rabies, and sleeping sickness emerge, re-emerge or remain in a state of continued endemicity, relevant questions of how land-disease modeling approaches are implemented are increasingly important. Is land use/cover change associated with emerging and/or endemic strains of a disease? If so, why and how? What can be done? While we do not answer these specific questions, the land change science community needs to be better integrated into epidemiological research. This brief review provides recent examples of the increasing importance of land use and cover modeling toward better understanding the transmission of the vector-borne diseases malaria and trypanosomiasis.
Vector-borne diseases
Vector-borne diseases are those in which transmission between an infected and susceptible human or animal is dependent on another living organism or vector. Vectors can be biological with pathogens reproducing within the vector and transmitted (usually) during feeding, or mechanical with pathogens migrating between hosts by attaching themselves to vectors. The epidemiology of a vector-borne disease is predicated primarily on the life-cycle, preferred habitat and feeding behavior of a vector species. These vector bionomics are strongly correlated with existing LULC and many vector-borne diseases have distributions mediated by LULC (Table 1). Below we describe vector bionomics and transmission risks for malaria and trypanosomiasis, which are chosen based on their historical importance, clear link to LULC, and potential future impact.
Table 1.
Select disease vectors with known land use and cover relationships
| Vector-borne and zoonotic diseases | Important LULC and disease relationship | Sample reference |
|---|---|---|
| Dengue | Aedes mosquitoes are found primarily in urban and peri-urban areas, spatially clustered near areas with standing water, such as rice paddies, marsh/swamps, poor urban housing, and gasoline stations. | [9] |
| Hanta virus | Rare reservoir populations are geographically restricted in patches of suitable habitat defined by characteristics of elevation, slope, aspect, and land cover. | [10] |
| Japanese encephalitis | The spatial pattern of JE cases during the 2005 epidemic in Nepal was significantly associated with low precipitation and the percentage of irrigated land. | [11] |
| Leishmaniasis | Constructed risk maps for the presence of Leishmania infantum vector, P. perniciosus. Altitude, land use and drainage features were the only effective predictors for the species distribution. | [12] |
| Lyme disease | The niche model described in this study was intended to identify and delineate the geographic range of Ixodes tick and B. burgdorferi occurrence at a regional scale. Ixodes tick habitat is characterized by low lying vegetated areas with abundant leaf litter such as high grass and brush areas, and presence of a nearby water source for the tick to prevent desiccation. | [13] |
| Malaria | Agricultural development, road construction, mining and other land exploitation activities are strongly related to Anopheline density as well as increasing human–vector interactions. | [14] |
| Meningitis | Land cover particularly associated with barren and semi-arid lands — deforestation feedbacks to risk of the disease. | [15] |
| Nipah Virus | Agricultural intensification particularly through the mixed agricultural land use of mango plantations to swine production increases risk. | [16] |
| Onchocerciasis | Interesting synergistic discussion presented where the disease is most often found in areas of good agricultural potential, but drives people from these areas. | [17] |
| Plague | Epizootic activity is most likely to occur in areas where multiple populations of susceptible hosts are living at high densities in diverse habitats. Land cover diversity as opposed to a single determinant class is the principal interesting outcome. | [18] |
| Rabies | Locations with high arable agriculture land cover were at greater risk of reporting rabies while those with higher elevation and tree cover were at lower risk of reporting disease. | [19] |
| Rift Valley Fever | In semiarid areas, precipitation and green vegetation abundance are major disease determinants. There is a close relationship between green vegetation development and breeding and upsurge patterns of disease vectors. | [20] |
| Schistosomiasis | This paper supports the hypothesis that the distribution of schistosomiasis is not random and that locations of cases are linked to anthropogenic activities that modify landscape structures. | [21] |
| Trachoma | Trachoma risk was found to be associated with spatial access to water and savanna and shrub cropland land covers. | [22] |
| Trypanosomiasis | Synergistic population environment disease model presented linking climate change to future disease risk. | [23•] |
| West Nile Virus | Habitat conversion both agricultural and urbanization both linked as drivers of the spread of WNV. | [24] |
| Yellow Fever | Yellow fever and dengue have similar landscape drivers as the vectors are similar sylvatic mosquitos. | [25] |
Malaria
Malaria is endemic in more than 100 countries with more than 3.3 billion people at risk. Its continued impact across generations has made it one of the most widely studied diseases in human history. Malaria is transmitted by the female Anopheles mosquito and is the result of infection due to the presence of Plasmodium parasites, primarily P. vivax, P. falciparum, P. malariae, and P. ovale. Studies of malaria epidemiology are vast and risk is multidimensional involving human population growth and mobility, climate and land change, societal infrastructure and development, and vector competence and bionomics. It is not surprising that researchers have taken vastly different approaches to better understand risk factors of infection. For example, there is a well-established link between climate variation and seasonal malaria risk; however, the long-term and intraregional distribution of risk remains contentious [26••,27] as climate–malaria links often are made at very large scales [28,29••] limiting model predictability across space and time. Disciplinarity is problematic particularly for malaria as few studies have embraced the complex coupling between LULC and climate or between humans and LULC that is recognized in earth, climate and population sciences. Although these factors have been separately evaluated, the mutually exclusive approaches often fail to detect signals from coupled relationships [30••,27].
The relationship of LULC with malaria is rooted in vector bionomics. Only about 70 Anopheles species (of over 450) can transmit malaria to humans and only ~27 are effective transmitters [31••]. In the Amazon, the primary malaria vector is An. darlingi Root, 1926 [32•]. An. darlingi is highly dependent on water for its survival and breeding habitat, is a typical riverine species that inhabits jungle and forest environments, and is mostly distributed in low altitude regions (<500 m above sea level) with high relative humidity [33••]. Although vector competence is highly dependent on temperature and humidity [31••], the microhabitats where the oviposition, feeding and resting occurs are mediated by the surrounding LULC. Suitable habitats for these behaviors are dominated by ecologically altered landscapes (deforested, secondary forest, grass/cropland) and forest fringes as well as natural and artificial bodies of water (fish farms, rice fields, irrigation canals, etc.) [34•,35•].
Trypanosomiasis
African trypanosomiasis (sleeping sickness) is a zoonotic, parasitic infection of wildlife, domesticated animals, and humans. The causative agents (parasites of the Trypanosoma brucei species complex) are transmitted by the bite of the tsetse fly (genus Glossina). Approximately 8.5 million km2 in 36 sub-Saharan Africa countries are infested with tsetse [36], resulting in approximately 70 million people with exposure risk [37,38]. In recent years, African trypanosomiasis has emerged in new locations due to anthropogenic land use and cover changes and broader climate changes [23•]. As a k-strategist insect vector dependent on land cover, tsetse flies are particularly well suited for dynamic spatial models [39]. Preferred ecological conditions vary by tsetse species, but temperatures that are too low (e.g. <17C for the Glossina morsitans group) limit tsetse presence in the southern reaches of their distributions (i.e. southern Mozambique, Zimbabwe, Botswana, Angola, and southern Zambia), as well as at higher elevations. Warmer temperatures and dryer conditions lead to the utilization of vegetation cover to mitigate the effects of such conditions. As these ecological conditions vary according to season, fly distributions and associated human risk across sub-Saharan Africa likewise vary. American trypanosomiasis (Chagas Disease) is an infection with Trypanosoma cruzi, which is spread through bites of Reduviidae vectors (Triatominae family). It is considered to have the largest social and economic impact in Latin America [40,41], accounting for almost all global infections. Chagas consists of an acute phase of mild symptoms, followed by a chronic phase with more severe cardiovascular, enteric and abdominal ailments. Control of Chagas was initially focused on rural areas, but massive rural–urban migration altered the epidemiology to make it both a rural and urban disease, with urban transmission occurring primarily via blood transfusion. Vector foci vary from housing structures, cleared or degraded land, and palm trees [42–44]. The relationship with LULC is particularly problematic as sylvatic foci of Chagas exist throughout Latin America [45], meaning that the distribution of T. cruzi will not only follow human–vector habitat, but also animal–vector habitat.
Conclusions
Ontological differences particularly around space and time continue to impede basic health and land change science. Further, the land change science community tends to focus on continuous, synoptic space and discrete time, while the health community tends to focus on discrete space and continuous time. The dominant land change science paradigm will continue to promote the production of models with restricted applicability in health research. Despite this, within the broader health community, land surface models do play an increasingly important role for helping explain disease causation. The most common role of land change models within the papers reviewed here is as a proximal driver of the fundamental niche of the insect (or animal) vector. This is distinct from and often superior to presence only sampling models where land cover is reduced to mere context. The next logical step forward though is with the development of vector distribution models. Humans alter landscapes and animal/vector biodiversity changes with altered landscapes. Thus simultaneous or stepwise modeling approaches where land cover/change and species distribution are jointly predicted by human/structure/etc. and land/climate/etc., which lead to disease predicted by vector–host interactions are the next logical, but unfortunately, distant step. Multilevel models are a step in this direction, and include both individual-level (individual exposure) and group-level (e.g. species distribution) variables simultaneously. The popularity of multilevel studies is growing rapidly [46] due to the return of interest community-level influences and the increasing availability of tools to analyze multilevel data. Other approaches offer similar advantages, such as structural equation models where individuals are linked to latent (group-level) structural factors [47] or multiscale, population linked, spatial clustering [48••].
As we noted in the beginning, geography, space, and time ought to serve as complementary to traditional health and place organizational strategies. Challenges remain due to the execution of land and health science, which often have disparate methods ranging from study design to analysis. Improved clarity is obtained if one orients the relationship to particular LULC contexts, and if the scales of the relationships are clearly defined. We focused this review on a narrow set of vector-borne diseases, but as shown in Table 1 many other disease systems are applicable. Significant interest also can be found for developed world disease and land cover/human health relationships, particularly around direct climate change impacts. Few of these developed world human health and land cover papers advance the state of the science for integrated ecologic scale models. This is not directly a testament to quality, but rather that individual-level data are much more widely available and LULC data are again reduced to one-way context. Health scientists interested in using land use or cover models as data in explanatory models must be aware of that LULC data are modeled products. Beyond production limitations, uncertainty and error, the important ‘fitness-for-use’ paradigm [49,50] should play an important role in the disease model construction. Specific disciplinary expertise resides at each corner of the epidemiological triangle model of disease causation and as our understanding of disease becomes ever more systemic, it is incumbent that all three be meaningfully represented.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Woolhouse M. How to make predictions about future infectious disease risks. Philos Trans R Soc B Biol Sci. 2011;366:2045–2054. doi: 10.1098/rstb.2010.0387. http://dx.doi.org/10.1098/rstb.2010.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patz JA, Daszak P, Tabor GM, Alonso Aguirre A, Pearl M, Epstein J, Wolfe ND, Marm Kilpatrick A, Foufopoulos J, Moyneux D, Bradle DJ. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patz JA, Olson SH, Uejio CK, Gibbs HK. Disease emergence from global climate and land use change. Med Clin North Am. 2008;92:1473–1491. doi: 10.1016/j.mcna.2008.07.007. http://dx.doi.org/10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Vora N. Impact of anthropogenic environmental alterations on vector-borne diseases. Medscape J Med. 2008;10:238. [PMC free article] [PubMed] [Google Scholar]
- 5.Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V. Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr. 2010;9:54. doi: 10.1186/1476-072X-9-54. http://dx.doi.org/10.1186/1476-072X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verburg P, Erb, Mertz, Espindola Land transformations: between global challenges and local impacts. Curr Opin Environ Sustain. 2013 doi: 10.1016/j.cosust.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foladori G. The challenge of infectious diseases to the biomedical paradigm. Bull Sci Technol Soc. 2005;25:145–158. http://dx.doi.org/10.1177/0270467604274337. [Google Scholar]
- 8.Poole C. Ecologic analysis as outlook and method. Am J Public Health. 1994;84:715–716. doi: 10.2105/ajph.84.5.715. http://dx.doi.org/10.2105/AJPH.84.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarfraz MS, Tripathi NK, Tipdecho T, Thongbu T, Kerdthong P, Souris M. Analyzing the spatio-temporal relationship between dengue vector larval density and land-use using factor analysis and spatial ring mapping. BMC Public Health. 2012;12:853. doi: 10.1186/1471-2458-12-853. http://dx.doi.org/10.1186/1471-2458-12-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass GE, Shields T, Cai B, Yates TL, Parmenter R. Persistently highest risk areas for hantavirus pulmonary syndrome: potential sites for refugia. Ecol Appl. 2007;17:129–139. doi: 10.1890/1051-0761(2007)017[0129:phrafh]2.0.co;2. http://dx.doi.org/10.1890/1051-0761(2007)017[0129:PHRAFH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Impoinvil DE, Solomon T, Schluter WW, Rayamajhi A, Bichha RP, Shakya G, Caminade C, Baylis M. The spatial heterogeneity between japanese encephalitis incidence distribution and environmental variables in Nepal. PLoS ONE. 2011;6:e22192. doi: 10.1371/journal.pone.0022192. http://dx.doi.org/10.1371/journal.pone.0022192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barón SD, Morillas-Márquez F, Morales-Yuste M, Díaz-Sáez V, Irigaray C, Martín-Sánchez J. Risk maps for the presence and absence of Phlebotomus perniciosus in an endemic area of leishmaniasis in southern Spain: implications for the control of the disease. Parasitology. 2011;138:1234–1244. doi: 10.1017/S0031182011000953. http://dx.doi.org/10.1017/S0031182011000953. [DOI] [PubMed] [Google Scholar]
- 13.Mak S, Morshed M, Henry B. Ecological niche modeling of lyme disease in British Columbia. Can J Med Entomol. 2010;47:99–105. doi: 10.1603/033.047.0114. [DOI] [PubMed] [Google Scholar]
- 14.Yasuoka J, Levins R. Impact of deforestation and agricultural development on Anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–460. [PubMed] [Google Scholar]
- 15.Palmgren H. Meningococcal disease and climate. Global Health Action. 2009 doi: 10.3402/gha.v2i0.2061. http://dx.doi.org/10.3402/gha.v2i0.2061. [DOI] [PMC free article] [PubMed]
- 16.Pulliam JRC, Epstein JH, Dushoff J, Rahman SA, Bunning M, Jamaluddin AA, Hyatt AD, Field HE, Dobson AP, Daszak P, et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface. 2011;9:89–101. doi: 10.1098/rsif.2011.0223. http://dx.doi.org/10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barro AS, Oyana TJ. Predictive and epidemiologic modeling of the spatial risk of human onchocerciasis using biophysical factors: a case study of Ghana and Burundi. Spat Spacio-Temporal Epidemiol. 2012;3:273–285. doi: 10.1016/j.sste.2012.08.001. http://dx.doi.org/10.1016/j.sste.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Rebecca JE, Enscore RE, Biggerstaff BJ, Reynolds PJ, Ettestad P, Brown T, Pape J, Tanda D, Levy CE, Engelthaler DM, Cheek J, Bueno R, Targhetta J, Montenieri JA, Gage KL. Human plague in the Southwestern United States, 1957–2004: spatial models of elevated risk of human exposure to Yersinia pestis. J Med Entomol. 2007;44:530–537. doi: 10.1603/0022-2585(2007)44[530:hpitsu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Tenzin, Dhand NK, Warda MP. Anthropogenic and environmental risk factors for rabies occurrence in Bhutan. Prev Vet Med. 2012;107:21–26. doi: 10.1016/j.prevetmed.2012.05.003. http://dx.doi.org/10.1016/j.prevetmed.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Anyamba A, Chretien JP, Small J, Tucker CJ, Formenty PB, Richardson JH, Britch SC, Schnabel DC, Erickson RL, Linthicum KJ. Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci U S A. 2009;106:955–959. doi: 10.1073/pnas.0806490106. http://dx.doi.org/10.1073/pnas.0806490106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anaruma Filho F, Moreno Sant’Ana J, Ferreira dos Santos R, Castagna CL. Environmental inducers of schistosomiasis mansoni in Campinas, Brazil. Geospat Health. 2010;5:79–91. doi: 10.4081/gh.2010.189. [DOI] [PubMed] [Google Scholar]
- 22.Clements ACA, Kur LW, Gatpan G, Ngondi JM, Emerson PM, Lado M, Sabasio A, Kolaczinski JH. Targeting trachoma control through risk mapping: the example of Southern Sudan. PLoS Negl Trop Dis. 2010;4:e799. doi: 10.1371/journal.pntd.0000799. http://dx.doi.org/10.1371/journal.pntd.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Messina JP, Moore NJ, DeVisser MH, McCord PF, Walker E. Climate change and risk projection: dynamic spatial models of African trypanosomiasis in Kenya. Ann Assoc Am Geogr. 2012;102:1038–1048. doi: 10.1080/00045608.2012.671134. http://dx.doi.org/10.1080/00045608.2012.671134This study details the creation of a model combining climate change, population, and importantly for vectorborne disease research, a species distribution model, as opposed to a fundamental niche model. Uncertainty is discussed in detail. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpatrick AM. Globalization, land use, and the invasion of West Nile virus. Science. 2011;334:323–327. doi: 10.1126/science.1201010. http://dx.doi.org/10.1126/science.1201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gubler DJ. The changing epidemiology of yellow fever and dengue 1900 to 2003: full circle? Comp Immunol Microbiol Infect Dis. 2004;27:319–330. doi: 10.1016/j.cimid.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 26••.Campbell-Lendrum D, Woodruff R. Comparative risk assessment of the burden of disease from climate change. Environ Health Perspect. 2006;114:1935–1941. doi: 10.1289/ehp.8432. This study describes the strengths and limitations of the WHO comparative risk assessment approach to evaluating the impact of climate change on health. On the one hand, the approach provides the current best approach for presenting policy makers with evidence for a climate impact across multiple disease outcomes, on the other hand, the approach is limited in its ability to incorporate mediators such as vulnerability, development, and adaptation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gage KL, Burkot TR, Eisen RJ, Hayes EB. Climate and vectorborne diseases. Am J Prev Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. http://dx.doi.org/10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Bouma MJ, Dye C. Cycles of malaria associated with El Niño in Venezuela. JAMA. 1997;278:1772–1774. http://dx.doi.org/10.1001/jama.1997.03550210070041. [PubMed] [Google Scholar]
- 29••.Ermert V, Fink AH, Morse AP, Paeth H. The impact of regional climate change on malaria risk due to greenhouse forcing and land-use changes in tropical Africa. Environ Health Perspect. 2012;120:77–84. doi: 10.1289/ehp.1103681. http://dx.doi.org/10.1289/ehp.1103681Large-scale modeling study to look at the impact of climate change scenarios (REMO, A1B, and B1) on future malaria risk. The model uses 0.5° grid cells and shows that malaria incidence will increase in Sahel and low altitudes, but also become epidemic in high altitudes. The models only use temperature and rainfall as the scenarios are modeled using expected LU change. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Hay SI, Rogers DJ, Randolph SE, Stern DI, Cox J, Shanks GD, Snow RW. Hot topic or hot air? Climate change and malaria resurgence in East African highlands. Trends Parasitol. 2002;18:530–534. doi: 10.1016/s1471-4922(02)02374-7. http://dx.doi.org/10.1016/S1471-4922(02)02374-7This paper provides four plausible explanations for the increase in malaria incidence in the East African Highlands in contrast to changes in climate, which did not change over time. These include drug resistance, failures in vector control, decline in health services, and changes in land cover. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Wernsdorfer WH. Global challenges of changing epidemiological patterns of malaria. Acta Trop. 2012;121:158–165. doi: 10.1016/j.actatropica.2011.06.014. Excellent review of the microbiological, entomological, and environmental factors of malaria that have evolved over time. This paper also discusses quantitative tools used to predict transmission as well as new and emerging control methods to reduce burden. [DOI] [PubMed] [Google Scholar]
- 32•.Tadei WP, Dutary Thatcher B. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop São Paulo. 2000;42:87–94. doi: 10.1590/s0036-46652000000200005. This study collected 11,895 Anopheles in 15 locations in the Brazilian Amazon with known high malaria incidence between 1994 and 1998. Vector densities and infection with three different plasmodium species was tested. Results demonstrate the importance of An. darlingi as well as other species, but argues strongly for focus on An. darlingi in vector control programs. [DOI] [PubMed] [Google Scholar]
- 33••.Hiwat H, Bretas G. Ecology of Anopheles darlingi root with respect to vector importance: a review. Parasites Vectors. 2011;4:177. doi: 10.1186/1756-3305-4-177. http://dx.doi.org/10.1186/1756-3305-4-177Comprehensive review of the behavior and ecology of An. darlingi, reaffirming the belief that it is the key malaria vector in the Amazon. The review also identifies several shortcomings in our knowledge for specific aspects of vectorial capacity of darlingi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Sánchez-Lozano W, Pinedo VV, Salas-Cobos E, Flores S, Patz JA. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81:5–12. This study presents results of a 12-month longitudinal study to examine ecological characteristics of Anopheles darlingi breeding sites and demonstrates that forest clearing is related to increased likelihood of finding An. darlingi mosquitoes. [PMC free article] [PubMed] [Google Scholar]
- 35•.Barros FSM, Honório NA, Arruda ME. Temporal and spatial distribution of malaria within an agricultural settlement of the Brazilian Amazon. J Vector Ecol. 2011;36:159–169. doi: 10.1111/j.1948-7134.2011.00153.x. http://dx.doi.org/10.1111/j.1948-7134.2011.00153.xThis paper is a comprehensive assessment of relationship between humans and Anopheles (larva and adults) in an agricultural zone of the Amazon. The paper itself has a few analytical issues, but it is methodologically strong and is an example of how studies should incorporate multiple factors into the assessment of malaria risk. [DOI] [PubMed] [Google Scholar]
- 36.Allsopp R. Options for vector control against trypanosomiasis in Africa. Trends Parasitol. 2001;17:15–19. doi: 10.1016/s1471-4922(00)01828-6. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization (WHO) [last accessed 27.11.12];Trypanosomiasis, Human African (Sleeping Sickness) 2012 http://www.who.int/mediacentre/factsheets/fs259/en/
- 38.Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, Fèvre EM, Mattioli RC, Jannin JG. Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis. 2012;6:e1859. doi: 10.1371/journal.pntd.0001859. http://dx.doi.org/10.1371/journal.pntd.0001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeVisser MH, Messina JP, Moore NJ, Lusch DP, Maitima J. A dynamic species distribution model of Glossina subgenus Morsitans: the identification of tsetse reservoirs and refugia. Ecosphere. 2010:1. http://dx.doi.org/10.1890/ES10-00006.1 (art 6)
- 40.Dias JC. Elimination of Chagas disease transmission: perspectives. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):41–45. doi: 10.1590/s0074-02762009000900007. [DOI] [PubMed] [Google Scholar]
- 41.Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira AR, Monteiro PS, Rebelo JM, Argañaraz ER, Vieira D, Lauria-Pires L, Nascimento R, Vexenat CA, Silva AR, Ault SK, Costa JM. Emerging Chagas disease: trophic network and cycle of transmission of Trypanosoma cruzi from palm trees in the Amazon. Emerg Infect Dis. 2001;7:100–112. doi: 10.3201/eid0701.700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abad-Franch F, Vega MC, Rolón MS, Santos WS, de Arias AR. Community participation in Chagas disease vector surveillance: systematic review. PLoS Negl Trop Dis. 2011;5:e1207. doi: 10.1371/journal.pntd.0001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottdenker NL, Calzada JE, Saldana A, Carroll CR. Association of anthropogenic land use change and increased abundance of the Chagas disease vector Rhodnius pallescens in a rural landscape of Panama. Am J Trop Med Hyg. 2011;84:70–77. doi: 10.4269/ajtmh.2011.10-0041. http://dx.doi.org/10.4269/ajtmh.2011.10-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceballos LA, Piccinali RV, Marcet PL, Vazquez-Prokopec GM, Cardinal MV, Schachter-Broide J, Dujardin JP, Dotson EM, Kitron U, Gürtler RE. Hidden sylvatic foci of the main vector of Chagas disease Triatoma Infestans: threats to the vector elimination campaign? PLoS Negl Trop Dis. 2011;5:e1365. doi: 10.1371/journal.pntd.0001365. http://dx.doi.org/10.1371/journal.pntd.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grady SC, Messina JP, McCord PF. Population vulnerability and disability in Kenya’s tsetse fly habitats. PLoS Negl Trop Dis. 2011;5:e957. doi: 10.1371/journal.pntd.0000957. http://dx.doi.org/10.1371/journal.pntd.0000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bollen KA. Structural Equations with Latent Variables. John Wiley & Sons; 1989. [Google Scholar]
- 48••.Salje H, Lessler J, Endy TP, Curriero FC, Gibbons RV, Nisalak A, Nimmannitya S, Kalayanarooj S, Jarman RG, Thomas SJ, Burke DS, Cummings DAT. Revealing the Microscale Spatial Signature of Dengue Transmission and Immunity in an Urban Population. Proc Natl Acad Sci U S A. 2012;109:9535–9538. doi: 10.1073/pnas.1120621109. This study is one of the first to demonstrate small-scale spatial dependencies of dengue infection in that dengue infection is highly localized even when the population at risk is mobile, lives in an area of high population density, and exhibits immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devillers R, Bédard Y, Jeansoulin R, Moulin B. Towards spatial data quality information analysis tools for experts assessing the fitness for use of spatial data. Int J Geogr Inf Sci. 2007;21:261–282. http://dx.doi.org/10.1080/13658810600911879. [Google Scholar]
- 50.Goodchild MF, Shortridge AM, Fohl P. Encapsulating simulation models with geospatial data sets. In: Lowell K, Jaton A, editors. Spatial Accuracy Assessment: Land Information Uncertainty in Natural Resources. Ann Arbor, MI: Ann Arbor Press; 1999. pp. 123–130. [Google Scholar]