Abstract
An association between hepatitis C virus (HCV) infection and insulin resistance (IR) has been recently reported. However, causality has not been established. The cross-sectional nature of most reported studies and varying degrees of fibrosis have limited definitive conclusions about the independent role of HCV in development of IR. We sought to evaluate whether HCV induces IR by prospectively analyzing a cohort of adult liver transplant (LT) recipients. A total of 34 adults (14 HCV(+) and 20 HCV(−)) who underwent consecutive LT were followed during the first year posttransplantation. IR was estimated using the homeostasis model assessment (HOMA). Univariate and multivariate repeated measures analyses and Cox regression models were used. There were no significant differences between the groups with respect to age, body mass index (BMI), family history of diabetes, alcohol consumption, or laboratory indices. The cohort had no or minimal fibrosis. There was lower prednisone use in the HCV(+) group, and no difference in the use of tacrolimus between the two groups was found. IR was 77% higher in HCV(+) subjects during the first year post-LT when controlling for BMI (P = 0.035). Subjects with high HCV ribonucleic acid (RNA) levels reached high HOMA-IR significantly earlier than those with lower HCV RNA (P = 0.03). Following the first month post-LT, HCV(+) subjects were 4 times more likely to become diabetic than HCV(−) controls (P < 0.01). In conclusion, there is significantly higher IR in the HCV(+) group during the first year post-LT. This cannot be explained by differences in BMI, medications used, alcohol consumption, or degree of fibrosis. Higher HCV RNA levels were associated with earlier elevations in HOMA-IR. Collectively, these results provide strong evidence that HCV induces the development of IR.
Hepatitis C virus (HCV) infection is a major public health concern, infecting 3.3% of the world's population. Recently, HCV has been associated with diabetes mellitus.1 This association is based on the finding of increased insulin resistance (IR) among HCV-infected subjects.2,3 IR is not only a precursor to diabetes, but is also itself associated with significant morbidity.4 IR is a major component of nonalcoholic fatty liver disease and other conditions such as polycystic ovarian syndrome.5 Insulin resistance, independent of dysglycemia, has been associated with a 2-fold increase in the risk of hypertension, a 3-fold increased risk of coronary heart disease, and an 8-fold increased risk of type 2 diabetes. These undesirable health effects can develop in a period of less than 15 years.6 In a study by Bonora et al.,7 among 1,326 individuals with type 2 diabetes followed for 4.5 years, IR was an independent predictor of cardiovascular disease. Furthermore, fasting hyperinsulinemia8 and IR have also been shown to predict overall mortality.9
The presence of IR in the setting of HCV infection is of particular importance because hyperinsulinemia appears to play a role in the progression of HCV-liver disease. In 2003, Hickman et al.10 evaluated 160 patients with chronic HCV. Hickman et al.10 concluded that hyperinsulinemia contributes to the development of fibrosis and is not merely a consequence of liver injury in HCV-infected individuals. Hui et al.3 evaluated 260 HCV-infected individuals using multivariate analysis and found IR to be an independent predictor of degree of fibrosis and rate of fibrosis progression. Recent investigations at the cellular level have provided additional evidence for a direct role of hyperinsulinemia in the development of fibrosis.11,12
Furthermore, evidence also suggests that IR may contribute to the development of fibrosis in HCV infection indirectly through steatosis. Hepatic steatosis is considered a common histological feature of chronic liver disease and is present in up to 30% to 70% of patients with HCV infection.13 The relationship between IR and steatosis in nonalcoholic steatohepatitis is well established.14 IR is associated with an increased flux of dietary and stored free fatty acids away from adipose tissue and into the liver, leading to the development of hepatic steatosis.15 Although viral factors may play a role in the development of steatosis, specifically in genotype 3a infection,16,17 other elements, including risk factors for nonalcoholic steatohepatitis and abdominal fat mass, have been independently associated with steatosis in HCV-infected subjects.18,19 This suggests that, as in nonalcoholic fatty liver disease, IR is important in the pathogenesis of obesity-related steatosis in chronic HCV infection.20 Given the independent association between HCV and IR it is, therefore, likely that hyperinsulinemia contributes to the development of steatosis even in the nonobese HCV-infected population. Steatosis itself has been noted by multiple investigators to be strongly associated with fibrosis progression in HCV infection and the mechanisms of fibrosis in HCV-associated steatosis likely share similarities with those involved in nonalcoholic fatty liver disease.17,19-21
Results previously reported by our group2 and by Hui et al.3 suggest that increasing viremia likely leads to greater IR, but the cross-sectional nature of these studies has thus far precluded establishing a causal role for HCV in the development of IR. Most importantly, previous studies have not been able to clearly evaluate changes in viremia independently of histology, which has been a major impediment in clarifying the relationship between hepatitis C infection and IR. A number of reports have discussed the association between HCV infection and diabetes and/or IR over the past few years. However, definitive conclusions about the independent role of HCV in development of IR continue to be limited by confounding factors. Therefore, we selected a cohort of liver transplant (LT) recipients followed immediately after transplantation to serve as a plausible model of de novo HCV liver disease. We hypothesized that HCV-infected subjects develop greater degrees of IR over time than uninfected controls during the first year posttransplantation, and that IR would correlate with levels of HCV ribonucleic acid (RNA) level independently of fibrosis.
PATIENTS AND METHODS
Patient Population
Our cohort consisted of consecutive adult patients (18-year-old or older) who underwent orthotopic LT at Massachusetts General Hospital between January 2002 and March 2003. A total of 49 patients underwent LT during this period. Of these, 8 subjects were not eligible to participate in our study (pediatric patients), 3 patients were missed and not enrolled, 2 patients refused to participate, and 2 had significant complications postoperatively and died within 3 months after transplantation. Thus, 34 subjects were included in our study. Subjects were followed longitudinally from the time of LT until 1 year post-LT. Data including patient characteristics and medical history were obtained at time of transplantation, and at 1, 3, 6, 9, and 12 months following transplantation. Anthropometric measurements and blood sample collections were obtained at 1, 3, 6, 9, and 12 months following transplantation.
The diagnosis of HCV infection was established by serological markers and confirmed in all cases by HCV RNA testing. The HCV(+) group included 14 subjects with confirmed HCV infection pretransplantation. Comorbidities noted in the HCV(+) group included history of alcohol abuse pretransplantation (n = 7), hepatocellular carcinoma (n = 3), and sickle cell disease (n = 1). The HCV(−) group consisted of 20 subjects with negative tests for HCV RNA who had other causes of liver failure necessitating transplantation. This group included patients with end-stage liver disease caused by alcoholic cirrhosis (n = 10), hepatocellular carcinoma (n = 5), hepatitis B virus infection (n = 2), cryptogenic cirrhosis (n = 4), primary biliary cirrhosis (n = 1), vasoactive intestinal peptide-producing tumor (n = 1), and hemochromatosis (n = 1). Institutional review board approval was obtained and all patients provided written informed consent for participation in this study.
Patient Characteristics
Patients’ demographic characteristics including gender, age, and self-reported ethnicity were reviewed. Family history of diabetes mellitus was obtained from patient interviews. Anthropometric data including weight and height were obtained at the time of blood sample collection. Total lifetime history of alcohol consumption was assessed by interview using an abbreviated version of a previously validated questionnaire.22,23
Immunosuppressive medications were recorded at each time point of interest. Immunosuppressive regimens for post-LT recipients at Massachusetts General Hospital have been described previously by Baid et al.24 Maintenance immunosuppressive regimens at Massachusetts General Hospital included prednisone, tacrolimus, and azathioprine, or mycophenolate mofetil. In general, mycophenolate mofetil was not used in the HCV(+) group because of concerns regarding increased HCV RNA levels and potential for early graft recurrence.25,26 Information regarding participants’ use of glucose-lowering agents, antihypertensive drugs, and lipid-lowering medications was obtained from patients’ charts. Subjects who were taking medications to lower blood pressure or those with a systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg (stage I classification of the Joint National Committee) on more than 1 occasion were considered hypertensive. Allograft liver biopsies obtained for clinical purposes were reviewed by a blinded pathologist. Fibrosis scores were based on the scale of Ishak et al.27 Steatosis scores were based on the scale described by Brunt et al.28
Laboratory Determinations
Laboratory data, including all chemistries and HCV RNA levels, were obtained at 5 different time points (1 month posttransplant, and then every 3 months following transplantation for a period of 1 yr). Venous blood samples were collected after an overnight fast (minimum of 8 hours). Plasma glucose concentration was measured using the Hitachi 917 (Hitachi, Yokohama, Japan) Roche reagent hexokinase method. Fasting plasma insulin was measured by radioimmunoassay.
Serum aminotransferase, bilirubin, fasting total cholesterol, and triglyceride levels were obtained by standard assays. High-density lipoprotein levels were measured using polyethylene glycol-modified enzymes, and low-density lipoprotein levels were calculated using the Friedewald formula. Hepatitis C viral RNA levels were assessed by reverse-transcription polymerase chain reaction detection of HCV RNA using Roche COBAS Amplicor 2.0 (Roche Molecular Diagnostics, Branchburg, NJ) and expressed as International Units per milliliter (IU/mL). This assay had a lower limit of detection of 600 IU/mL and an upper limit of 850,000 IU/mL. For samples with HCV RNA <600 IU/mL, HCV RNA qualitative polymerase chain reaction was performed using Roche Amplicor 2.0 (Roche Molecular Diagnostics). This assay had a lower limit of detection of 60 IU/mL.
Calculations
IR was calculated using the homeostasis model assessment (HOMA), a validated model derived from normal volunteers, expressed in the following equation: IR = fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5.29 In addition, HOMA2-IR was also calculated in all patients. This is a model that more accurately reflects IR, particularly in subjects with higher insulin levels. HOMA2-IR was calculated using a publicly available program.30 Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Total lifetime alcohol consumption was calculated as follows: lifetime alcohol consumption = number of days/month × average drinks/day (gm ethanol) × period of time. Each drink was estimated to contain 10 gm of alcohol.22,23
Statistical Analysis
The SAS program (SAS Institute, Cary, NC) was used for all statistical analyses. Continuous variables were expressed as medians with ranges. Observations based on continuous variables were compared using Wilcoxon rank tests. Categorical variables were compared using Fisher's exact tests. The following variables were evaluated: HCV status, gender, ethnicity, age, BMI, family history of diabetes, alcohol consumption, HCV geno-type, use of immunosuppressives and antiviral medications, liver enzymes, bilirubin levels, lipids, hemoglobin A1c, fasting glucose, and fasting insulin levels. Finally, we evaluated all available liver biopsies performed during the study period.
Tacrolimus and prednisone use over the follow-up period were compared using logistic regression models. The correlations between use of and dose of interferon as well as ribavirin with the natural log of HOMA (HOMA-IR and HOMA2-IR) over time were estimated through mixed-effects models to adjust for repeated observations on the same individuals.
The natural log of HOMA across time was used as the primary outcome of interest based on the characteristics of the distribution of this variable. All statistical analyses were performed using HOMA-IR and were repeated using HOMA2-IR. A mixed-effects model was used to evaluate the factors that contributed most significantly to IR across time. Variables considered in the model were the presence of HCV infection, BMI, high-density lipoprotein level (previously found to be a significant predictor of IR2), and medications used. Variables identified as confounders and those that were clinically relevant were entered in the final model. Steatosis and fibrosis scores were not entered into the final model because only patients with abnormal liver function tests underwent liver biopsies.
Cox models were used to evaluate the time to development of high HOMA (defined as the level above the medians of HOMA-IR and HOMA2-IR) as a function of HCV RNA. For each subject, HCV RNA levels were categorized as low (<600 IU/mL and >60 IU/mL, i.e., undetectable by quantitative but detectable by qualitative assay), medium range (≥600 IU/mL and ≤500,000 IU/mL), or high (>500,000 IU/mL). Then, an integrated value per subject was obtained using the mean categorical HCV RNA value from the initial time point (month 1) until reaching high HOMA. Cox regression analysis was used to establish whether subjects with higher integrated HCV RNA levels reached high HOMA levels ealier. BMI was also considered in the model.
The development of diabetes during the study period was evaluated. Subjects with fasting glucose level ≥125 mg/dL or who were treated with hypoglycemic medications were defined as diabetics. The prevalence of diabetes at month 0 after transplantation was calculated and compared between the HCV(+) and HCV(−) groups. In addition, logistic regression was used to assess the incidence or rate of diabetes over time for each of the groups of interest while controlling for initial diabetic status at month 0 post-LT.
RESULTS
Patient Characteristics
Patients’ baseline demographics characteristics are summarized in Table 1. There were 5 women in the HCV(−) group and none in the HCV(+) group, with no statistically significant difference between the two groups. The vast majority of participants in both groups were Caucasian. There were no significant differences between the two groups with respect to age and BMI at transplantation. Although a slightly higher percentage of patients with HCV infection were considered diabetic prior to transplantation, the difference in prevalence of diabetes pre-orthotopic LT between the two groups did not reach statistical significance. There was no difference in family history of diabetes or reported history of total lifetime alcohol consumption between the two groups. There were no subjects treated with lipid lowering agents in either group during the course of the study. The majority of HCV(+) patients had the 1a genotype. A total of 5 subjects, all HCV(+), underwent liver biopsies for elevated liver enzymes. Among these, all but 1 patient had fibrosis scores equal to or less than 1 on the Ishak scale.27 One patient had a fibrosis score of 3 at 10 months post-LT.
TABLE 1.
Demographic Characteristics at Time of Transplantation
| HCV(−) (n = 20) | HCV(+) (n = 14) | ||||
|---|---|---|---|---|---|
| Median (range) | Frequency | Median (range) | Frequency | P value | |
| Gender (M/F) | - | 15M/5F | 14 M/ 0 F | 0.06 | |
| Race/ethnicity | - | 2 A, 1 AA, 1 H, 16 W | - | 0 A, 1 AA, 3 H, 10 W | 0.5 |
| Age (yr) | 54.5 (30−71) | - | 51.5 (25−66) | - | 0.35 |
| BMI (kg/m2) | 24.9 (21.5−40.9) | - | 24.2 (18.8−28.6) | - | 0.2 |
| History of DM prior to OLT | - | n = 6 (30%) | - | n = 5 (36%) | 0.27 |
| Family history of DM | - | n = 9 (45%) | - | n = 7 (50%) | 0.8 |
| EtOH (gm/lifetime) | 1584 (0−6186) | - | 1548 (0−8280) | - | 0.86 |
| HCV genotype | - | - | - | 6 (1a), 2 (1b), 1 (2b), 2(3a), 1(4c/d), 2 (unknown) | NA |
Abbreviations: M, Male; F, Female; A, Asian; AA, African American; H, Hispanic; W, non-Hispanic white; DM, diabetes mellitus; EtOH, ethanol; NA, not applicable.
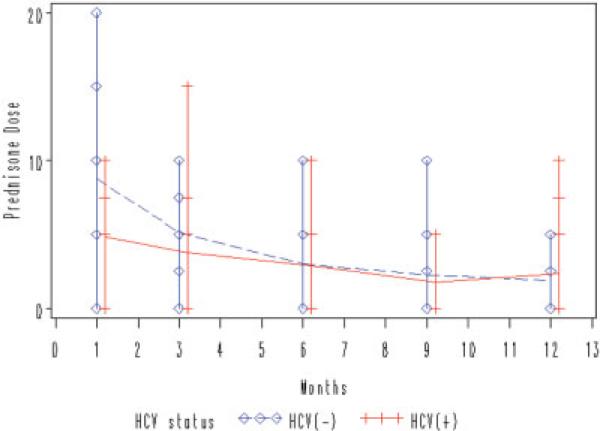
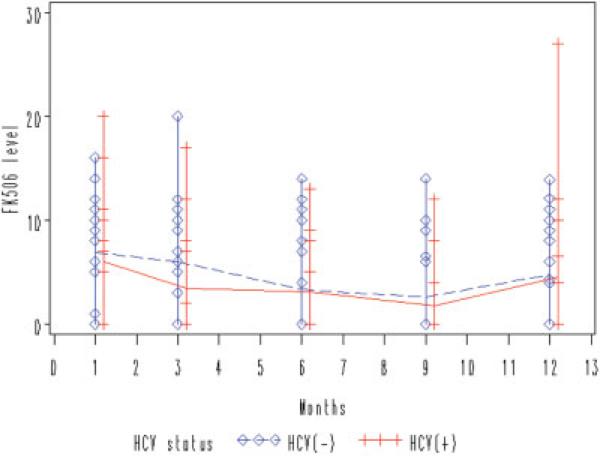
All participants were treated with prednisone and tacrolimus. Figures 1 and 2 demonstrate the median prednisone dose and tacrolimus level over time for each group of interest. There was a trend toward lower prednisone use in the HCV(+) group compared to the HCV(−) group, reflecting concerted efforts to minimize prednisone in the chronic HCV group at our institution (P = 0.06). There was no significant difference in the use of tacrolimus between the two groups over time. Table 2 summarizes the use of and response to antiviral treatment during the course of the study. A total of 12 HCV(+) subjects were treated with pegylated interferon and ribavirin at some point during the first yr post-LT. The other 2 subjects were not treated because of contraindications to treatment (anemia). A total of 5 of the 14 subjects were considered “responders” (achieved HCV RNA undetectability at month 12 post-orthotopic LT). There were no correlations between use or dose of interferon or ribavirin and HOMA-IR over time (data not shown). There was no difference between the 2 groups with respect to the rate of hypertension during the study period (7/14 HCV(+) patients and 9/20 HCV(−) patients met criteria for hypertension; P = not significant), and a minimal number of subjects in the entire cohort were treated with antihypertensive medications.
Figure 1.
Trend toward lower prednisone use in HCV(+) subjects. Using analysis of maximum likelihood ratio, there is no significant difference in mean daily prednisone dose between HCV(+) and HCV(−) groups over time, although data suggests a trend toward lower prednisone use in the HCV(+) group (P = 0.06). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 2.
Comparable use of tacrolimus (FK506) over time. Using analysis of maximum likelihood ratio, there is no significant difference in tacrolimus levels between HCV(+) and HCV(−) groups over time (P = 0.09). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE 2.
Summary of Antiviral Therapy (PEG IFN and Ribavirin)
| Subject ID no. | Time of OLT | Month 1 post-OLT | Month 3 post-OLT | Month 6 post-OLT | Month 9 post-OLT | Month 12 post-OLT | Response to antiviral treatment |
|---|---|---|---|---|---|---|---|
| 70 | – | – | – | R | R | ||
| 446 | – | R | R | ||||
| 599 | – | D | D | N | |||
| 604 | – | N | |||||
| 608 | – | N | |||||
| 610 | – | N | |||||
| 612 | – | R | R | ||||
| 621 | – | D | N | ||||
| 629 | – | – | – | – | – | – | N/A |
| 636 | – | – | – | N | |||
| 638 | – | – | – | – | – | – | N/A |
| 639 | – | R | R | R | R | ||
| 640 | – | – | N | ||||
| 645 | – | R | R | R |
NOTE: Shaded areas indicate antiviral treatment.
Abbreviations: PEG IFN, pegylated interferon; D, deceased; N, nonresponder (detectable HCV RNA); N/A, not applicable; R, responder (undetectable HCV RNA).
-, indicates that no treatment was being provided at the specified time.
Table 3 shows the results of laboratory testing obtained at the initial post-LT time point (month 1 post-LT) of the study. There was no statistically significant difference between the two groups in any of the chemistries evaluated.
TABLE 3.
Chemistries at Initial Post-LT Time Point (Month 1)
| HCV(−) (n = 20) Median (range) | HCV(+) (n = 14) Median (range) | P value | |
|---|---|---|---|
| AST (U/L) | 22 (13−124) | 23 (10−38) | 0.9 |
| ALT (U/L) | 28 (8−195) | 23.5 (5−43) | 0.4 |
| Total bilirubin (mg/dL) | 0.7 (0.1−3.6) | 1.05 (0.4−8.6) | 0.2 |
| Direct bilirubin (mg/dL) | 0.45 (0.1−2.3) | 0.65 (0.2−5.1) | 0.4 |
| Cholesterol (mg/dL) | 127.5 (61−264) | 123 (90−202) | 0.7 |
| HDL (mg/dL) | 24 (5−73) | 28 (8−40) | 0.98 |
| LDL (mg/dL) | 80.5 (18−157) | 73 (48−137) | 0.98 |
| Triglycerides (mg/dL) | 136.5 (51−280) | 114.5 (82−261) | 0.4 |
| HbA1c | 5.35 (3.8−7.3) | 5.1 (3.7−7.6) | 0.6 |
| Glucose (mg/dL) | 106 (83−282) | 102 (70−283) | 0.8 |
| Insulin (mg/dL) | 10.2 (5.8−17.8) | 12.1 (7.5−167.6) | 0.2 |
| C-peptide (pmol/L) | 1,000 (130−3500) | 1,250 (33−3100) | 0.8 |
| HOMA IR (mU)(mmol)/L2 | 2.9 (1.3−7.1) | 3.1 (1.8−66.6) | 0.4 |
| HOMA2 IR (pmol)(mmol)/L2 | 1.49 (0.8−2.3) | 1.6 (1−19.2) | 0.2 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high density lipoprotein; LDL, low density lipoprotein; HbA1c, hemoglobin A1c.
HCV Is Independently Associated With Increased IR During the First Yr Posttransplantation
In the initial phase, HCV status and fasting insulin over time were evaluated in univariate analyses. There was a statistically significantly higher fasting insulin level over time for HCV(+) as compared to HCV(−) subjects (estimate = 0.24, standard error = 0.12, P = 0.049). This association was even stronger when controlling for BMI (estimate = 0.36, standard error = 0.13, P = 0.007). High-density lipoprotein was later added to our multiple regression model given that this had been a selected variable in our prior cross-sectional cohorts.2 High-density lipoprotein level was not significantly associated with insulin levels over time while both HCV and BMI remained strongly associated with fasting insulin.
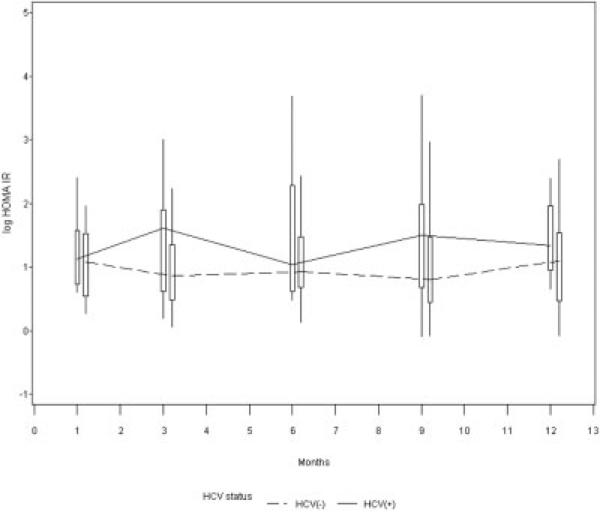
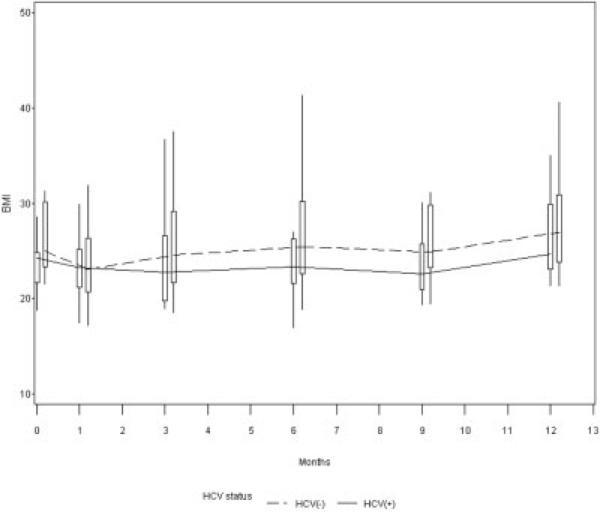
When evaluating HOMA, only BMI and HCV infection were significantly associated with IR over time. The univariate association between HCV status and HOMA-IR over time was first evaluated. There was a trend towards higher HOMA-IR over time for the HCV(+) than the HCV(−) group (estimate = 0.24, standard error = 0.18, P = 0.19). These results are depicted in Figure 3. Similar results were obtained when using HOMA2-IR as the outcome (not shown). We found lower BMI levels over time in the HCV(+) as compared to the HCV(−) group (P < 0.01, Fig. 4) and BMI was noted to be a confounder in the association between HCV infection and IR. In other words, when we controlled for BMI, the presence of HCV infection was independently associated with increased IR. Our final model included the variables HCV, BMI, and high high-density lipoprotein. HCV infection was associated with a 77% (= e0.57)increase in IR (P = 0.0035) and an 1-unit increase in BMI was associated with a 7% (= e0.07) increase in IR (P = 0.004) (Table 4). Results of analysis using HOMA2-IR were similar (not shown).
Figure 3.
Trend toward higher HOMA-IR over time in HCV(+) subjects. There was a trend towards higher HOMA-IR for HCV(+) than HCV(−) subjects during the first year post-LT when evaluated in univariate analysis.
Figure 4.
Lower BMI among HCV(+) subjects. There was a lower BMI in HCV(+) than HCV(−) subjects during the first yr post-LT (P < 0.01).
TABLE 4.
Predictors of Increased Insulin Resistance (HOMA-IR) During the First Year Post-LT
| Covariate | Effect on ln (IR) | SE | P value |
|---|---|---|---|
| +HCV | 0.57 (HCV associated with 77% increase in IR) | 0.18 | 0.035 |
| BMI | 0.07 | 0.02 | 0.002 |
| High HDL | 0.22 | 0.19 | 0.25 |
Abbreviations: SE, standard error, HDL, high-density lipoprotein.
Higher HCV RNA Levels Correlate With More Rapid Development of Elevated IR
Subjects with higher mean integrated HCV RNA levels (defined as mean HCV RNAs from month 1 post-LT to the time at which high HOMA-IR was attained) reached high HOMA levels significantly earlier than subjects with lower HCV RNA levels. We found a hazard ratio of 5.2 (P = 0.03) when evaluating integrated mean HCV RNA levels as a function of time. In other words, subjects with high integrated HCV RNA levels (>500,000 IU/mL) reached high HOMA-IR 5.2 times earlier than those with medium integrated HCV RNA levels (>600 and <500,000 IU/mL), and these in turn reached high HOMA IR 5.2 times earlier than those with low integrated HCV RNA levels (>60 and <600 IU/mL). This result remained statistically significant after adjusting for BMI (P = 0.04) (Table 5). The hazard ratio using HOMA2-IR was 4.8 (P = 0.037).
TABLE 5.
Time to Development of High HOMA-IR as a Function of HCV RNA Level
| Covariate | Hazard ratio | 95% CI for hazard ratio | P value |
|---|---|---|---|
| Integrated HCV RNA (low, medium, high)* | 5.2 | 1.2−22.7 | 0.03 |
Abbreviation: CI, confidence interval.
HCV RNA values were categorized as low (>60 IU/mL and <600 IU/mL, qualitatively +), medium (≥600 IU/mL and ≤500,000 IU/mL), or high (>500,000 IU/mL), and integrated as a mean of HCV RNAs from 1 month post-LT until the time that high HOMA was recorded.
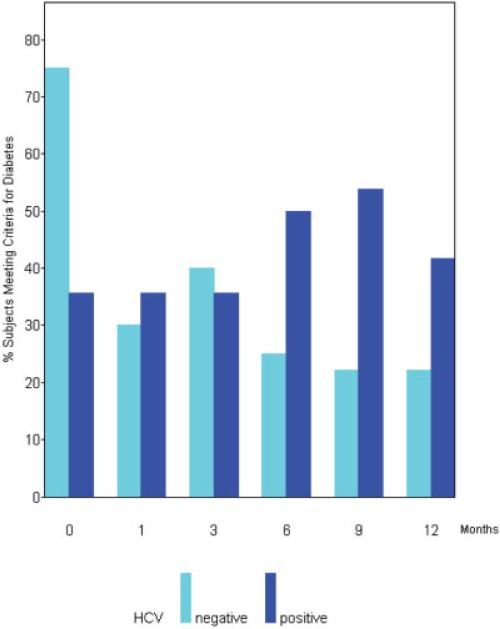
Development of Diabetes During the First Yr Post-LT
The prevalence of diabetes in our cohort, over time, is depicted in Figure 5. Prior to transplantation, there was no statistically significant difference in the prevalence of diabetes between the two groups (Table 1). Immediately following transplantation (at month 0), there was a greater prevalence of diabetes in the HCV negative as compared to the HCV positive group (P = 0.03). However, following the first month posttransplantation, the prevalence of diabetes became greater for the HCV(+) than the HCV(−) group. HCV(+) subjects were 4 times more likely to become diabetic when compared to HCV(−) controls (P < 0.01) after the first month post-transplantation, controlling for diabetic status at month 0. This correlation was even stronger when the analysis was restricted to the second 6 months of the study (months 6, 9, and 12), when HCV(+) patients were 5 times more likely to become diabetic relative to HCV(−) controls (P = 0.01). A total of 2 HCV(−) (10%) and 2 HCV(+) (14%) subjects died during the course of the study (during the first year posttransplantation). All subjects who died met criteria for diabetes prior to their death.
Figure 5.
Higher incidence of diabetes among HCV(+) subjects posttransplantation. HCV(+) subjects are 4 times more likely to become diabetic when compared to HCV(−) controls (P < 0.01) after the first month posttransplantation, controlling for diabetic status at month 0, and 5 times more likely when the analysis is restricted to the last 6 months (P = 0.01). Month 0 represents time of LT.
DISCUSSION
Recent reports of increased IR among HCV-infected adults relative to control groups suggest that IR underlies the development of diabetes in HCV infection. However, the cross sectional nature of previously reported investigations has precluded establishment of causality. Furthermore, the confounding element of fibrosis has represented a significant impediment in understanding the independent relationship between HCV and IR. In this study, we investigated a cohort of LT recipients during the initial year posttransplantation. We chose this cohort as a model of de novo HCV liver disease in which the confounding effects of fibrosis on IR would be minimized.
In this longitudinal study of post-LT patients we found that IR was higher in the HCV(+) than the HCV(−) group during the first yr posttransplantation, despite comparable baseline insulin levels. HCV infection was associated with a 77% increase in IR during the first year posttransplantation. All of our analyses were repeated using HOMA2-IR as the main outcome in view of the observation that this model may be a more accurate predictor of IR, especially among individuals with elevated glucose and insulin levels.30 Using HOMA2-IR, all of our results remained unchanged, including the significant association between HCV infection and increased IR. This could not be explained by differences in age, BMI, immunosuppression used, family history of diabetes, or total lifetime alcohol consumption. BMI was actually significantly lower in the HCV(+) group.
Use of potentially diabetogenic medications throughout the study period was carefully evaluated, but as expected, use of medications such as prednisone was minimized in the HCV(+) as compared to the HCV (−) group as per our institutional policy. At the time the study subjects were transplanted at our center, Massachusetts General Hospital had implemented a preemptive antiviral strategy for HCV-infected transplant recipients. A total of 12 of the 14 recipients underwent preemptive treatment with pegylated interferon and ribavirin at some point during the first yr posttransplantation. The remaining 2 patients were not treated because of contraindications. There was no correlation between use of or dose of interferon or ribavirin (either as categorical or numerical variables) and HOMA. Since HCV RNA levels were used to analyze the relationship between HCV status and HOMA, any effect of treatment of HCV was taken into account by evaluation of HCV RNA values.
Biopsies were performed for clinical indications, specifically elevated alanine aminotransferase with HCV RNA positivity. In the 5 patients who underwent allograft biopsy within the first year, stage 0 or 1 fibrosis was noted in all but 1 of the patients. We made the assumption that these biopsies reflect the group at highest risk of HCV progression and that subjects who did not undergo a liver biopsy were even less likely to have meaningful fibrosis that could confound our findings.
Although our initial findings strongly confirmed the independent association between HCV infection and IR, we attempted to more strictly evaluate the question of causality using a different approach. In order to assess whether IR is induced by the HCV infection, we analyzed whether HCV RNA levels are an independent predictor of time to development of elevated HOMA. We found that subjects with higher HCV RNA levels reached elevated HOMA earlier than those with lower HCV RNA levels. This finding strongly implies that the HCV is not merely associated with but that it induces IR. Characterization of the mechanism by which HCV induces IR is beyond the scope of this investigation. However, we speculate that IR may result from a combination of direct effects of HCV and indirect effects mediated by adipokines peripherally.
Finally, we evaluated the development of diabetes during the study period. The prevalence of diabetes immediately posttransplantation was higher in the HCV(−) group. This was likely the result of high doses of induction of corticosteroids among HCV(−) patients. However, during the subsequent months, HCV(+) subjects were 4 to 5 times more likely to meet criteria for diabetes than HCV(−) controls. Once again, this could not be explained by the previously cited confounders, including BMI, medications, or fibrosis. Our findings corroborate those of Saliba et al.,31 who found, in a cross-sectional and retrospective study of LT recipients, that HCV infection is a risk factor for the development of post-LT diabetes.
In summary, we have demonstrated in this longitudinal model of de novo HCV that HCV-infected persons develop increased IR and are significantly more likely to develop diabetes than HCV(−) controls during the first year after LT. Furthermore, the development of IR occurs earlier in HCV(+) patients with high level viremia, strongly suggesting that HCV infection directly induces IR in a dose-dependent manner. Additional investigations with larger number of subjects will be important to corroborate our findings, and further study is necessary to establish the mechanism of induction of IR by HCV. Our findings have important implications for the care of the LT recipient and for the HCV-infected patient in general. In view of the high long-term morbidity and mortality of diabetes and IR, we recommend routine early monitoring of fasting glucose and insulin levels in the care of patients with HCV infection, particularly in the setting of LT.
Acknowledgments
Supported by grants from the National Institutes of Health (NIH) (K08 DK070022 to A.D.B.; R01 DK57857 to R.T.C.), and the Robert Wood Johnson Foundation.
Abbreviations
- HCV
hepatitis C virus
- IR
insulin resistance
- LT
liver transplant(ation)
- HOMA
homeostasis model assessment
- HOMA2
HOMA model that more accurately reflects IR
- BMI
body mass index
- RNA
ribonucleic acid
REFERENCES
- 1.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, et al. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77:703–710. doi: 10.1097/01.tp.0000114283.04840.3a. [DOI] [PubMed] [Google Scholar]
- 3.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, et al. Insulin resistance is associated with chronic hepatitis C and virus infection fibrosis progression. Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Baron AD. Impaired glucose tolerance as a disease. Am J Cardiol. 2001;88:16H–19H. doi: 10.1016/s0002-9149(01)01832-x. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin TL, Reaven GM. Beyond type 2 diabetes: the need for a clinically useful way to identify insulin resistance. Am J Med. 2003;114:501–502. doi: 10.1016/s0002-9343(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 6.Zavaroni I, Bonini L, Gasparini P, Barilli AL, Zuccarelli A, Dall'Aglio E, et al. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: the Barilla factory revisited. Metabolism. 1999;48:989–994. doi: 10.1016/s0026-0495(99)90195-6. [DOI] [PubMed] [Google Scholar]
- 7.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 8.Lakka HM, Lakka TA, Tuomilehto J, Sivenius J, Salonen JT. Hyperinsulinemia and the risk of cardiovascular death and acute coronary and cerebrovascular events in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Arch Intern Med. 2000;160:1160–1168. doi: 10.1001/archinte.160.8.1160. [DOI] [PubMed] [Google Scholar]
- 9.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 10.Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM, Jonsson JR. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. J Hepatol. 2003;39:1042–1048. doi: 10.1016/s0168-8278(03)00463-x. [DOI] [PubMed] [Google Scholar]
- 11.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 12.Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 13.Patel K, Muir A, McHutchison JG, Patton HM. A link between leptin and steatosis in chronic hepatitis C? Time to weigh up the fats. Am J Gastroenterol. 2003;98:952–955. doi: 10.1111/j.1572-0241.2003.07422.x. [DOI] [PubMed] [Google Scholar]
- 14.Garg A, Misra A. Hepatic steatosis, insulin resistance, and adipose tissue disorders. J Clin Endocrinol Metab. 2002;87:3019–3022. doi: 10.1210/jcem.87.7.8736. [DOI] [PubMed] [Google Scholar]
- 15.Heathcote J. Weighty issues in hepatitis C. Gut. 2002;51:7–8. doi: 10.1136/gut.51.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monto A. Hepatitis C and steatosis. Semin Gastrointest Dis. 2002;13:40–46. [PubMed] [Google Scholar]
- 17.Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstal R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837–842. doi: 10.1016/s0168-8278(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 18.Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729–736. doi: 10.1053/jhep.2002.35064. [DOI] [PubMed] [Google Scholar]
- 19.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 20.Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A, Powell EE. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999;29:1215–1219. doi: 10.1002/hep.510290401. [DOI] [PubMed] [Google Scholar]
- 22.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- 23.Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology. 1998;27:1730–1735. doi: 10.1002/hep.510270637. [DOI] [PubMed] [Google Scholar]
- 24.Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, et al. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066–1072. doi: 10.1097/00007890-200109270-00015. [DOI] [PubMed] [Google Scholar]
- 25.Rostaing L, Izopet J, Sandres K, Cisterne JM, Puel J, Durand D. Changes in hepatitis C virus RNA viremia concentrations in long-term renal transplant patients after introduction of mycophenolate mofetil. Transplantation. 2000;69:991–994. doi: 10.1097/00007890-200003150-00055. [DOI] [PubMed] [Google Scholar]
- 26.Nelson DR, Soldevila-Pico C, Reed A, Abdelmalek MF, Hemming AW, Van der Werf WJ, et al. Anti-interleukin-2 receptor therapy in combination with mycophenolate mofetil is associated with more severe hepatitis C recurrence after liver transplantation. Liver Transpl. 2001;7:1064–1070. doi: 10.1053/jlts.2001.29414. [DOI] [PubMed] [Google Scholar]
- 27.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 28.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 31.Saliba F, Lakehal M, Pageaux GP, Roche B, Vanlemmens C, Duvoux C, et al. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis c infection: An observational multicenter study. Liver Transpl. 2007;13:136–144. doi: 10.1002/lt.21010. [DOI] [PubMed] [Google Scholar]