Abstract
We used functional magnetic resonance imaging (fMRI) to identify brain regions involved in the process of mapping coherent discourse onto a developing mental representation. We manipulated discourse coherence by presenting sentences with definite articles (which lead to more coherent discourse) or indefinite articles (which lead to less coherent discourse). Comprehending connected discourse, compared with reading unrelated sentences, produced more neural activity in the right than left hemisphere of the frontal lobe. Thus, the right hemisphere of the frontal lobe is involved in some of the processes underlying mapping. In contrast, left-hemisphere structures were associated with lower-level processes in reading (such as word recognition and syntactic processing). Our results demonstrate the utility of using fMRI to investigate the neural substrates of higher-level cognitive processes such as discourse comprehension.
A hallmark of coherent discourse is the recurrence and interrelations of key concepts. To build a similarly coherent mental representation, readers and listeners must identify those recurring concepts and have a means for mentally interrelating them; we call this cognitive process mapping (Gernsbacher, 1990). In the experiment reported here, we used functional magnetic resonance imaging (fMRI) to identify brain regions underlying this putative cognitive process of mapping.
We isolated the cognitive process of mapping during discourse comprehension from lower-level sentence-comprehension processes (e.g., letter recognition, word identification, syntactic parsing) by manipulating a subtle marker of discourse coherence: the definite article the. In languages that employ an article system, the definite article signals repeated reference. Consider the two series of sentences in Table 1. The series on the left contains only indefinite articles (a, an, and some), whereas the series on the right contains only the definite article, the. The sentences on the left seem less related to one another, more independent; the sentences on the right seem more coherent and interrselated.
Table 1.
Example sentence sets containing indefinite and definite articles
| Sentences containing indefinite articles | Sentences containing the definite article |
|---|---|
| A grandmother sat at a table. | The grandmother sat at the table. |
| A child played in a backyard. | The child played in the backyard. |
| A mother talked on a telephone. | The mother talked on the telephone. |
| A husband drove a tractor. | The husband drove the tractor. |
| A grandchild walked up to a door. | The grandchild walked up to the door. |
| A little boy pouted and acted bored. | The little boy pouted and acted bored. |
| A grandmother promised to bake cookies. | The grandmother promised to bake cookies. |
| A wife looked out at a field. | The wife looked out at the field. |
| Some dark clouds were rapidly accumulating. | The dark clouds were rapidly accumulating. |
| A mother worried about a harvest. | The mother worried about the harvest. |
| A grandfather opened a door. | The grandfather opened the door. |
| Some rain began to pour down. | The rain began to pour down. |
| A day’s work ended early. | The day’s work ended early. |
| A grandmother tried to lighten a mood. | The grandmother tried to lighten the mood. |
| An elderly woman led some others outside. | The elderly woman led the others outside. |
| A family ran through a wet field. | The family ran through the wet field. |
Behavioral data confirm these intuitions. The same sentences are read more rapidly (Haviland & Clark, 1974), recalled in a more integrative fashion (Gernsbacher & Robertson, in press), and rated as more coherent (de Villiers, 1974) when their articles are definite rather than indefinite. Moreover, sentences with definite articles produce a priming-in-item-recognition phenomenon. After several series of sentences with definite articles have been read, recognition memory for a sentence is facilitated if it is preceded by another sentence from the same series. This priming, which is not evident if the sentences contain only indefinite articles, suggests that a more interrelated and coherent mental representation is fostered by the definite article (Gernsbacher & Robertson, in press). We have suggested that the definite article the is a cue to discourse coherence, which serves as the basis for the cognitive process of mapping (Gernsbacher, 1997; Gernsbacher & Robertson, in press). When readers encounter the definite article, it cues them to map a representation of the current information onto a representation of previous information.
The general cognitive process of mapping most likely comprises several discourse-level structure-building operations (e.g., co-reference, alignment, integration), and discourse coherence can certainly be cued by devices other than the article system. We chose to manipulate the article system to assay a general cognitive process of mapping because the manipulation involves altering only one word.
Participants read series of sentences in which all the articles were definite (the), thus signaling the recurrence and interrelation of concepts (i.e., connected discourse) and enabling the cognitive process of mapping, or all the articles were indefinite (a, an, some). The participants also alternated between reading series of sentences and viewing series of nonletter character strings (e.g., @#$)\&@/$%% @==} \~=/ ‘$/). We used fMRI to identify regions of neural activity associated with comprehending connected discourse (sentences containing the definite article) versus comprehending unconnected discourse (sentences containing only indefinite articles). During periods of increased neural activity in the brain, the local ratio of oxygenated to deoxygenated hemoglobin increases (Malonek et al., 1997), resulting in an increase in the MR signal (Ogawa et al., 1992). Regions of increased neural activity are determined by statistical analysis.
METHOD
Participants
Eight neurologically normal participants (4 female) participated in exchange for payment. All participants answered “right-hand” to every question on the Chapman and Chapman (1987) handedness questionnaire. Two participants contributed data to only the first two blocks.
Stimuli and Design
We constructed numerous sets of sentences, based on the one set presented by de Villiers (1974) and the sets presented in our earlier work (Gernsbacher & Robertson, in press). (Two example sets are shown in Table 1, and all the stimuli can be seen on the World Wide Web at http://psych.wisc.edu/lang/material.html.) Each set comprised 16 sentences and totaled 140 (±2) syllables. The experiment presented three blocks; during each block, the participant viewed 11 sets of sentences and nonletter character strings. During the first block, sets of sentences containing only indefinite articles were alternated with sets of nonletter strings. During the second block, sets of sentences containing only the definite article were alternated with sets of sentences containing only indefinite articles; during the third block, sets of sentences containing only the definite article were alternated with sets of nonletter strings. The nonletter strings were derived from the sentences by replacing all letters with nonletter characters, retaining interword spacing, and equating for length. For the block that alternated reading sets of sentences with indefinite versus definite articles, two versions of each set of sentences were constructed—one version with only the definite article and one with indefinite articles; each participant was presented with only one version of each set, and the versions were counterbalanced across participants. Block order was held constant for all participants to minimize possible carryover effects (e.g., interpreting sentences that contained indefinite articles as more “storylike” after experiencing sets of the storylike sentences containing the definite article).
Procedure
Prior to scanning, participants were acclimated to the environment and procedures in a mock MR scanner. Stimuli were displayed with fiber-optic goggles (Avotec, Inc., Jensen Beach, Florida). Sentences were displayed one whole sentence at a time. Display time per syllable was equalized. Each set lasted for 48 s (i.e., an average rate of 0.34 s/syllable). Head movements were restricted by use of a padded head coil and a dental impression bite-bar. Estimated head movements were less than 1 mm within a block and less than 2 mm over the whole scan session. Participants were instructed to read the sentences; no mention was made of the sentences potentially composing narratives. For the nonletter character strings, participants were instructed to visually scan the lines. After each block, participants performed a recognition test, judging whether test sentences were “old” or “new”; no image data were collected during the recognition tests.1
Scanning Protocol
Functional images were collected in the coronal plane using a gradient-echo, echo-planar imaging sequence sufficient to cover the whole brain (echo time/repetition time = 50/3,000 ms, 64 × 64 matrix, field of view = 240 mm, slice/gap = 7/1 mm, flip angle = 90°, 23 interleaved slices). A total of 191 images was collected for each slice in each block. The first 5 non-steady-state images were excluded from analysis to allow for signal stabilization. Additional high-resolution, T1-weighted spin-echo images in the coronal plane, directly corresponding to the functional images, and a three-dimensional image volume (256 × 256 × 124, Spoiled Gradient Recalled) were collected prior to the functional scans.
Data Processing
The data were analyzed with Statistical Parametric Mapping (SPM96) software (Wellcome Department of Cognitive Neurology, London, United Kingdom) implemented in Matlab (Mathworks, Inc., Sherborn, Massachusetts). SPM96 combines the general linear model (to create the statistical map, or SPM) and the theory of Gaussian fields to make statistical inferences about regional effects while controlling for multiple comparisons (Friston, Worsley, Frackowiak, Mazziotta, & Evans, 1994; Friston et al., 1995; Worsley, Evans, Marrett, & Neelin, 1992). Data were realigned using the first scan of the experiment as a reference, spatially normalized to a standard stereo-tactic space approximating the Talairach and Tournoux (1988) atlas, and smoothed (spatially using an isotropic Gaussian kernel, 5-mm full width at half maximum [FWHM], and temporally using a 2.8-s FWHM kernel). Analyses were conducted using a 6-s delayed boxcar corresponding to the task paradigm, using proportional global scaling, treating subjects as fixed effects.
To test the hemispheric asymmetry of neural activity for the block comparing sentences containing the definite article with sentences containing indefinite articles, we calculated activation maps using a three-parameter least squares fitting procedure (cf. Sorenson & Wang, 1996). Anatomical regions of interest were selected using T1-weighted high-resolution images as an underlay to the activation maps, and were defined for the frontal lobe as the seven most anterior coronal slices. To avoid regions of the temporal lobe that showed signal loss due to susceptibility artifact, we considered only the first three slices of the temporal lobe. No statistically reliable hemispheric differences were detected in the temporal regions.
We computed an activation index by counting the number of voxels with signal change exceeding a threshold (t ≥ 2, p < .05, uncorrected), excluding the two columns of voxels adjacent to the longitudinal and Sylvian fissures, and deriving the mean t-statistic value of these voxels. This value was then divided by the total number of voxels in the volume. Activation indices were statistically compared using region, hemisphere, and sex as predictors (for similar approaches, see Bavelier et al., 1997; Pugh et al., 1996). We did not detect any effect of or interactions with sex.
RESULTS
Functional Neuroanatomy of Sentence Reading
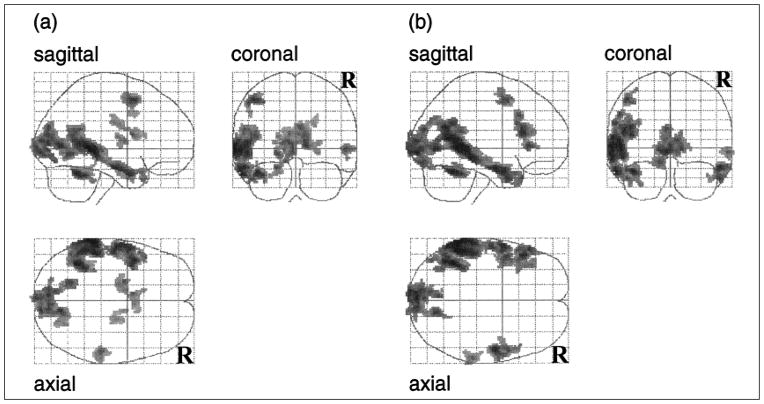
Analyses of the two blocks that alternated reading sentences with viewing nonletter strings allowed us to identify neural regions involved in reading sentences, while equating approximately for visual stimulation. As shown in Figure 1 and Table 2, these comparisons produced robust regions of activation in the left hemisphere, extending from the angular gyrus rostrally to the left anterior temporal pole along the middle temporal gyrus. A smaller region of activation was also observed in the right hemisphere. These results corroborate other brain-imaging studies of sentence reading (Bavelier et al., 1997; Helenius, Salmelin, Service, & Connolly, 1998; Just, Carpenter, Keller, Eddy, & Thulborn, 1996), and are suggestive of a language-processing circuit primarily localized to the left hemisphere.
Fig. 1.
Glass brain projections of the statistical parametric maps (SPMs) showing regions of activation for (a) sentences with indefinite articles versus nonletter character strings and (b) sentences with the definite article versus nonletter character strings. Each SPM is displayed in a standard format as a maximum-intensity projection viewed from the back, the right-hand side, and the top of the brain. The SPM has been extent-thresholded at p < .05, corrected for multiple comparisons.
Table 2.
Table of regional differences for the blocks that alternated sentences with nonletter strings
| Location | Hemisphere | Volume (cm3) | Intensity (Z) | Coordinates (mm)
|
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Sentences with indefinite articles versus nonletter character strings | ||||||
| Positive activations | ||||||
| Middle temporal gyrus, BA 21 | Left | 15.50 | 8.44 | −66 | −38 | 0 |
| Cerebellum | Left | 2.51 | 7.50 | −40 | −52 | −26 |
| Lingual gyrus, BA 19 | 12.08 | 6.94 | 12 | −72 | 4 | |
| Middle frontal gyrus, BA 6, 8 | Left | 2.25 | 6.83 | −40 | 6 | 52 |
| Inferior frontal gyrus, BA 45 | Left | 0.52 | 6.48 | −56 | 26 | 0 |
| Middle temporal gyrus, BA 21 | Right | 1.54 | 5.83 | 54 | −28 | 0 |
| Parahippocampal gyrus | Left | 0.62 | 5.64 | −20 | −2 | −20 |
| Anterior cingulate, BA 24 | 1.24 | 5.35 | −6 | 6 | 16 | |
| Negative activations | ||||||
| Superior parietal lobule, BA 7, 19 | Right | 4.70 | −7.85 | 22 | −74 | 52 |
| Cerebellum/fusiform gyrus | Right | 5.76 | −7.29 | 30 | −52 | −16 |
| Precuneus, BA 7 | 6.68 | −7.11 | −6 | −74 | 56 | |
| Lateral and superior occipital gyrus, BA 18 | Right | 2.58 | −7.09 | 34 | −88 | 12 |
| Cerebellum/fusiform gyrus | Left | 0.96 | −6.76 | −26 | −50 | −20 |
|
| ||||||
| Sentences with definite article versus nonletter character strings | ||||||
| Positive activations | ||||||
| Middle temporal gyrus, BA 22 | Left | 18.99 | 8.02 | −62 | −42 | 4 |
| Cerebellum | Left | 2.47 | 7.59 | −40 | −54 | −24 |
| Middle temporal gyrus, BA 21 | Right | 3.07 | 7.36 | 54 | −8 | −20 |
| Inferior/mid frontal gyrus, BA 8, 9, 10 | Left | 7.38 | 7.02 | −48 | 22 | 20 |
| Lingual gyrus, BA 17, 18 | 12.38 | 6.92 | −4 | −92 | −4 | |
| Cerebellum/fusiform gyrus | Right | 0.47 | 6.06 | 38 | −46 | −26 |
| Superior temporal gyrus, BA 22 | Right | 0.26 | 5.85 | 52 | −44 | 14 |
| Superior frontal gyrus, BA 6 | Left | 0.34 | 5.81 | −36 | 12 | 60 |
| Negative activations | ||||||
| Superior parietal lobule, BA 7, 19 | Right | 4.03 | −7.10 | 16 | −76 | 54 |
| Superior parietal lobule, BA 7, 40 | Left | 1.17 | −6.07 | −38 | −48 | 48 |
| Medial frontal gyrus, BA 9 | Left | 0.64 | −6.04 | −34 | 38 | 40 |
| Supramarginal gyrus, BA 7, 40 | Right | 1.21 | −5.70 | 46 | −38 | 60 |
| Anterior cingulate gyrus, BA 32 | 0.70 | −5.46 | −2 | 36 | 22 | |
Note. Coordinates are estimated locations of the primary maxima in stereotactic space. All regions are statistically reliable based on peak height of 3.09 (p < .001, uncorrected) and spatial extent (p < .05, corrected). The eight clusters with the greatest primary maxima for positive activations and the five greatest deactivations are reported for both blocks. For the comparison of sentences with indefinite articles versus nonletter character strings, N = 8, df = 612, smoothness full width at half maximum = 6.3, 8.8, 6.4 mm. For the comparison of sentences with the definite article versus nonletter character strings, N = 6, df = 459, smoothness full width at half maximum = 7.2, 9.5, 7.2 mm. BA = Brodmann’s Area.
Functional Neuroanatomy of the Cognitive Process of Mapping
Analyses of the block that alternated reading sentences containing the definite article with reading sentences containing indefinite articles allowed us to identify neural regions involved in comprehending connected discourse. This manipulation isolated the cognitive process of mapping from basic sentence-reading processes. Indeed, the comparison of reading sentences with definite articles versus indefinite articles revealed virtually no differences in activation in the left-hemisphere regions that are typically thought to underlie sentence processing and that we identified in the comparisons of sentence versus nonletter-string blocks. Instead, differential activation was observed in frontal regions, particularly in the right superior and medial frontal gyri. Table 3 shows that the two most prominent clusters of activation for sentences with definite articles were in the right hemisphere of the frontal lobe, whereas the two most prominent clusters of activation for sentences with indefinite articles were in the left hemisphere.
Table 3.
Two most prominent activations and deactivations from the block that alternated sentences that contained the definite article with sentences that contained only indefinite articles
| Location | Volume (mm3) | Intensity (Z) | Coordinates (mm)
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| Positive activations | |||||
| Right inferior frontal sulcus | 232 | 4.70 | 38 | 14 | 16 |
| Right inferior frontal gyrus | 192 | 4.30 | 46 | 12 | 4 |
| Negative activations | |||||
| Left inferior frontal gyrus | 120 | −3.98 | −34 | 22 | 0 |
| Left anterior cingulate gyrus | 144 | −3.61 | −10 | 20 | 36 |
Note. The table presents the results of an analysis using SPM96, using a minimum peak threshold of p < .001, uncorrected for spatial extent. N = 8; df = 611; smoothness = 6.3, 8.6, 6.2 mm.
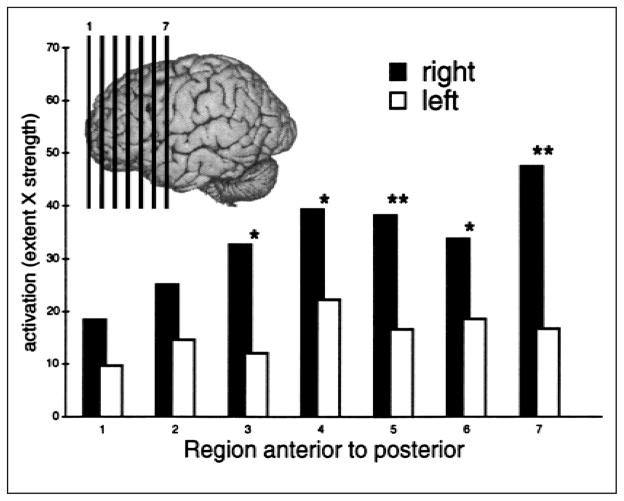
To statistically assess the hemispheric asymmetry, we computed an activation index for each hemisphere in seven homotopic regions of the frontal lobe based on activation maps calculated for each participant while reading sentences with definite articles and while reading sentences with indefinite articles. These regional activation-index values were analyzed in a hemisphere-by-region repeated measures analysis of variance, which revealed greater activation in the right than the left frontal lobe during the reading of sentences containing the definite article,2 as indicated in Figure 2. Note that whereas there was marginally greater right-hemisphere activation for sentences with the definite article at all locations, the laterality difference was statistically reliable only in the more caudal portions of the frontal lobe: F(1, 7) = 1.76, p = .19; F(1, 7) = 2.53, p = .13; F(1, 7) = 9.72, p < .02; F(1, 7) = 6.63, p < .04; F(1, 7) = 10.62, p < .01; F(1, 7) = 5.30, p < .05; and F(1, 7) = 21.73, p < .01, for each region, listed anterior to posterior.
Fig. 2.
Activation for sentences with the definite article compared with sentences with indefinite articles. Activations are shown for seven regions in the left and right hemispheres separately. The lines on the inlay are approximate centers of the regions analyzed. Units are the mean proportion of voxels in each hemisphere that were activated, multiplied by the mean t-statistic value. *p < .05, **p < .01 (using Greenhouse-Geisser adjustment).
DISCUSSION
We observed that the cognitive process of mapping during discourse comprehension was accompanied by more neural activity in the right than the left hemisphere. This observation challenges conventional beliefs about language lateralization. All early theories of brain organization emphasized left-hemisphere dominance for language, most likely because most aphasias are associated with left-hemisphere lesions. Recent neuroimaging studies have buttressed the long-held belief about left-hemisphere dominance for language by reporting greater left-hemisphere activation during language tasks (Bavelier et al., 1997; Helenius et al., 1998; Just et al., 1996; Price, 1997; Pugh et al., 1996).
However, people with right-hemisphere lesions experience difficulty processing more complex language, particularly the pragmatic (intentional), prosodic (intonational), figurative, and idiomatic aspects of discourse (Brownell, Carroll, Rehak, & Wingfield, 1992; van Lancker & Kempler, 1987; Winner & Gardner, 1977; Zaidel, Zaidel, Oxbury, & Oxbury, 1995). Further, increased right-hemisphere activity has been reported during discourse tasks such as judging the aptness of metaphors (Bottini et al., 1994) or evaluating each sentence’s fit in an ongoing narrative (Robertson, Gernsbacher, & Guidotti, 1999), compared with tasks requiring only simple sentence judgments.
Based on the neuropsychological and psycholinguistic literatures, we did not expect to identify a single brain location per se underlying the cognitive process of mapping during discourse comprehension.3 We did expect to find frontal lobe involvement because frontal lobe damage is often associated with a reduced ability to generate mental representations of situations, and the right frontal lobe is hypothesized to be dominant for allocating internal attention (Knight & Grabowecky, 1995). Allocating internal attention must be an important subcomponent of the process of mapping (e.g., interpreting the definite article as a discourse cue to direct attention to previous information). Thus, our finding of right-frontal dominance for the cognitive process of mapping is consistent with the literature, despite its apparent contradiction of traditional accounts of left-hemisphere dominance for language processing.
Although many psychologists are skeptical that knowing the answer to “where?” will illuminate the answer to “how?” we feel optimistic that studying functional neuroanatomy will help us investigate cognitive processes. For example, our finding of right-hemisphere frontal dominance for our putative process of mapping suggests that mapping definite reference is more related to episodic memory retrieval than episodic encoding or semantic retrieval, according to neuroimaging studies (Gabrieli et al., 1996; Nyberg, Cabeza, & Tulving, 1996; Tulving, Kapur, Craik, Moscovitch, & Houle, 1994). Such speculations, of course, await further behavioral and neuroanatomical investigations.
Our results demonstrate that altering a single word in the language input can result in qualitatively different activity in the brain, provided that single word carries an important cue for comprehension processes. Thus, our results demonstrate the efficacy of using neuroimaging techniques to test psychological hypotheses about higher-order cognition.
Acknowledgments
We thank Mark Beeman and Alex Shackman for comments on the manuscript; Michael Anderle, Richard Davidson, Dietmar Cordes, Melissa Rosenkranz, and James Sorenson for assistance in the design and execution of the research; and two anonymous reviewers for helpful comments on the manuscript. This research was supported by grants from the National Institutes of Health (RO1 NS 29926) and the Army Research Institute (DASW0194-K-0004, DASW0196-K-0013, and DAAG55-97-1-0224), and by a University of Wisconsin Faculty Development Award to Morton Ann Gernsbacher, a National Institute of Mental Health (T32-MH18931) predoctoral traineeship to David Robertson, and a National Science Foundation graduate research fellowship to William Irwin.
Footnotes
Average correct response was 83%, with no statistically reliable differences. We did not predict a difference on this gross measure of recognition memory because in another study (Gernsbacher & Robertson, in press) we found no differences in the quantity of sentences recalled by participants who read them with definite versus indefinite articles; we found striking differences in the forms of the sentences recalled (reading sentences with definite articles led participants to recall more integrative sentences, more synonym substitutions, and more insertions of pronominal anaphora, all of which are discourse markers of greater coherence).
We interpreted increases in MR signal during the reading of sentences with definite articles compared with the reading of sentences with indefinite articles as reflecting increased right-frontal neural activity reflecting the mapping process. However, according to the strict statistical threshold presented in Table 3, no significant right-frontal activity was observed in the blocks that alternated between sentences and nonletter strings, so it could be that reading sentences with definite articles does not result in increased activity relative to a low-level baseline. In another neuroimaging study, we observed increased activity in this region for reading sentences that promote mapping relative to a low-level baseline task (Robertson, Gernsbacher, & Guidotti, 1999). The data from this study are equivocal in that respect; as with any observed difference between two experimental treatments (either physiological or behavioral measurements), one cannot tell if the difference is an increase for one treatment or a decrease for the other. The interaction between article and hemisphere, rather than main effect, is of primary concern for this report.
One region implicated in the mapping process for most of our participants’ single-subject analyses is Brodmann’s Area 8, which has been reported as activated during the comprehension of stories (Mazoyer et al., 1993), tasks that require judgments about characters’ mental states (Fletcher et al., 1995), and narrative comprehension (Robertson et al., 1999).
References
- Bavelier D, Corina D, Jezzard P, Padmanabhan S, Clark VP, Karni A, Prinster A, Braun A, Lalwani A, Rauschecker JP, Turner R, Neville H. Sentence reading: A functional MRI study at 4 Tesla. Journal of Cognitive Neuroscience. 1997;9:664–686. doi: 10.1162/jocn.1997.9.5.664. [DOI] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RSJ, Frith CD. The role of the right hemisphere in the interpretation of figurative aspects of language. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Brownell HH, Carroll JJ, Rehak A, Wingfield A. The use of pronoun anaphora and speaker mood in the interpretation of conversational utterances by right hemisphere brain-damaged patients. Brain & Language. 1992;43:121–147. doi: 10.1016/0093-934x(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain & Cognition. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- de Villiers PA. Imagery and theme in recall of connected discourse. Journal of Experimental Psychology. 1974;103:263–268. [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, Frith CD. Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychological Science. 1996;7:278–283. [Google Scholar]
- Gernsbacher MA. Language comprehension as structure building. Hillsdale, NJ: Erlbaum; 1990. [Google Scholar]
- Gernsbacher MA. Two decades of structure building. Discourse Processes. 1997;23:265–304. doi: 10.1080/01638539709544994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernsbacher MA, Robertson RRW. The definite article the as a cue to map thematic information. In: van Peer W, Louwerse MM, editors. Thematics: Interdisciplinary studies. Philadelphia: John Benjamins; in press. [Google Scholar]
- Haviland SE, Clark HH. What’s new? Acquiring new information as a process in comprehension. Journal of Verbal Learning and Verbal Behavior. 1974;13:512–521. [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Distinct time courses of word and context comprehension in the left temporal cortex. Brain. 1998;121:1133–1142. doi: 10.1093/brain/121.6.1133. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Knight RT, Grabowecky M. Escape from linear time: Prefrontal cortex and conscious experience. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 1995. pp. 1357–1371. [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: Relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proceedings of the National Academy of Sciences, USA. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J. The cortical representation of speech. Journal of Cognitive Neuroscience. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Cabeza R, Tulving E. PET studies of encoding and retrieval: The HERA model. Psychonomic Bulletin & Review. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences, USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. Functional anatomy of reading. In: Frackowiak RSJ, Friston KJ, Dolan RJ, Mazziotta JC, editors. Human brain function. San Diego: Academic Press; 1997. pp. 301–328. [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Robertson DA, Gernsbacher MA, Guidotti SJ. FMRI investigation of the comprehension of written versus picture narratives. Paper presented at the annual meeting of the Cognitive Neuroscience Society; Washington, DC. 1999. Apr, [Google Scholar]
- Sorenson JA, Wang X. ROC methods for evaluation of fMRI techniques. Magnetic Resonance in Medicine. 1996;36:737–744. doi: 10.1002/mrm.1910360512. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proceedings of the National Academy of Sciences, USA. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lancker DR, Kempler D. Comprehension of familiar phrases by left-but not by right-hemisphere damaged patients. Brain & Language. 1987;32:265–277. doi: 10.1016/0093-934x(87)90128-3. [DOI] [PubMed] [Google Scholar]
- Winner E, Gardner H. The comprehension of metaphor in brain-damaged patients. Brain. 1977;100:717–729. doi: 10.1093/brain/100.4.717. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for rCBF activation studies in the human brain. Journal of Cerebral Blood Flow Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zaidel DW, Zaidel E, Oxbury SM, Oxbury JM. The interpretation of sentence ambiguity in patients with unilateral focal brain surgery. Brain & Language. 1995;51:458–468. doi: 10.1006/brln.1995.1071. [DOI] [PubMed] [Google Scholar]