Abstract
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are overlapping manifestations on a spectrum of acute drug-induced conditions associated with severe blistering, skin peeling, and multi-organ damage. TEN is an eruption resembling severe scalding, with ≥30% skin detachment. SJS is a mild form of TEN, characterized histologically by epidermal keratinocyte apoptosis with dermo-epidermal separation and extensive small blisters with <10% body surface skin detachment. The syndrome can be induced by numerous medications and typically occurs 1–4 weeks after the initiation of therapy. Granulysin is found in the lesions of patients with SJS/TEN and plays a significant pathogenic role in the condition, but the overall mechanisms linking medications, granulysin, and disease manifestations remain obscure. This paper reviews evidence suggesting that the different medications implicated in SJS/TEN have the common property of interacting and synergizing with endogenous retinoids (vitamin A and its congeners), in many instances causing the latter to accumulate in and damage the liver, the main storage organ for vitamin A. It is hypothesized that liver damage leads to the spillage of toxic retinoid compounds into the circulation, resulting in an endogenous form of hypervitaminosis A and cytotoxicity with widespread apoptosis, mediated by granulysin and recognized as SJS/TEN. Subject to testing, the model suggests that symptom worsening could be arrested at onset by lowering the concentration of circulating retinoids and/or granulysin via phlebotomy or plasmapheresis or by pharmacological measures to limit their expression.
MeSH Keywords: Retinoids, Stevens-Johnson Syndrome
Background
Stevens-Johnson syndrome (SJS), first described in 1922 by the American pediatricians Albert Stevens and Frank Johnson, involves a distinctive macular rash with painful vesicular and bullous lesions, fever, purulent conjunctivitis, and stomatitis [1]. Toxic epidermal necrolysis (TEN), also known as Lyell’s syndrome [2], is a more severe and potentially life-threatening condition. Annual incidence rates of TEN range from 0.4 to 1.2 cases per million people per year, and those of SJS from 2 to 6 cases per million people per year [3]. SJS and TEN are considered variants of the same disorder on a spectrum of severe blistering conditions, characterized histologically by epidermal keratinocyte apoptosis and detachment of the top layers of the skin (epidermal-dermal separation) over large areas of the body [4]. In the early stages they are clinically similar to ordinary drug-induced skin reactions. SJS includes extensive small blisters with <10% body surface skin detachment, whereas TEN resembles scalding of the skin and involves ≥30% skin detachment. Patients with SJS are generally younger than those with TEN (mean age 25–47 years for SJS; 46–63 years for TEN) and have a lower mortality rate (SJS, 1–3%; TEN, 10–50%). The spectrum of acute, drug-induced disease also includes erythema multiforme, a less severe manifestation [5]. Since SJS and TEN are viewed as overlapping conditions on a continuum, they are jointly referred to as SJS/TEN.
The syndrome usually begins 4–14 days after the initiation of drug therapy but may not be seen for 3–6 weeks after ingestion. Malaise, fever, headache, cough, and conjunctivitis develop within 1–3 weeks. Macules first appear on the face, neck, and upper trunk and then elsewhere, coalescing into large flaccid bullae, followed by sloughing over 1–3 days. Nails and eyebrows may also be lost. In severe cases, large sheets of epithelium slide off the body at pressure points (Nikolsky’s sign), exposing weepy, painful, and erythematous skin, accompanied by painful oral crusts, erosions, and keratoconjunctivitis. Sloughing may also occur in the bronchi, causing cough, dyspnea, pneumonia, pulmonary edema, and hypoxemia. Glomerulonephritis and hepatitis may also develop [6].
SJS/TEN can be induced by many types of drugs as well as infections (e.g., Mycoplasma pneumoniae and herpes virus), organ or bone marrow transplants, and certain vaccinations, including smallpox, anthrax, and tetanus [7–13]. The most commonly implicated drugs are anticonvulsants, sulfonamides, other antibiotics, nonsteroidal anti-inflammatory drugs, antifungals, antimalarials, and allopurinol.
An illustrative cases series involved an epidemic of severe, drug-induced disease among a group of Filipino workers in Taiwan who had taken a combination of metronidazole and mebendazole in an effort to avoid a positive stool test for intestinal parasites at the time of examination for employment [14]. SJS/TEN had been previously reported as a sequel to the use of metronidazole, but not to the combined use of metronidazole and mebendazole. Fifty-three individuals were hospitalized with SJS/TEN between February 1996 and January 1997. The most common symptoms were fever (100%), erosion or blistering of mucous membranes (100%), rash (92%), muscle pain (62%), jaundice (53%), vomiting (46%), skin detachment (31%), and liver transaminases over twice the normal level (66%). Five of the 53 patients died. The risk of SJS/TEN was not significantly higher among those who used only mebendazole or metronidazole, but was much higher among those who used both drugs (OR=9.5; 95% CI: 3.9, 23.9; P<.001). Among the patients with SJS/TEN, 76% had been exposed to both drugs compared to 25% among a group of unaffected controls. All of the cases had taken high doses of either metronidazole or mebendazole (>500 mg). A graded increase in the severity of STS/TEN was seen with increasing exposure to metronidazole. No evidence suggested that the outbreak was due to a microorganism and no further cases were reported after the practice was stopped of routinely prescribing antihelmintic drugs for persons traveling abroad. The authors concluded with the recommendation that antihelmintics should only be used for persons testing positive for parasites, and the combined use of metronidazole and mebendazole should be avoided.
Pathogenesis of SJS/TEN
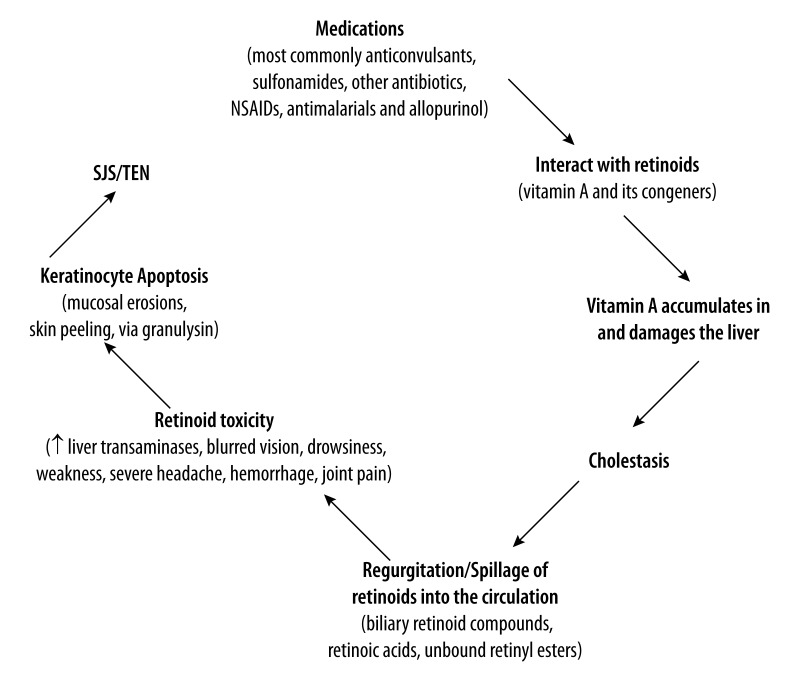
The mechanisms underlying drug-induced SJS/TEN remain obscure and no specific treatment has been proven beneficial. Secretory granulysin, a cationic protein produced by cytotoxic T lymphocytes and natural killer cells, has recently been identified as a major cytolytic molecule responsible for the widespread keratinocyte necrosis in SJS/TEN. However, it remains unclear how numerous drugs of different classes lead to the secretion of granulysin in SJS/TEN, and how cytotoxic T lymphocytes and natural killer cells regulate the secretion of granulysin in SJS/TEN. This paper presents the hypothesis that the different medications implicated in SJS/TEN have the common property of interacting and synergizing with endogenous retinoids (vitamin A and its congeners), causing the latter to accumulate in and damage the liver, inducing cholestatic liver dysfunction; this in turn causes toxic retinoid compounds that are stored in the liver and normally excreted harmlessly via the intestine to be spilled into the circulation, inducing extensive cytotoxicity and apoptosis via granulysin, and the clinical symptoms recognized as SJS/TEN. In this hypothesis, SJS/TEN is an endogenous form of hypervitaminosis A, mediated by granulysin (Figure 1).
Figure 1.
Retinoid toxicity hypothesis of Stevens-Johnsons Syndrome/Toxic Epidermal Necrolysis (SJS/TEN).
Vitamin A and its natural and synthetic congeners (collectively termed retinoids) are mainly dietary-derived fat-soluble signaling molecules that are stored principally in the stellate cells of the liver. In normal physiological concentrations, retinoids are essential for numerous biological functions, including cellular homeostasis, embryonic development, vision, tissue differentiation, growth, and mucus secretion. In higher concentration, retinoids inhibit cell growth and can be cytotoxic, mutagenic, and teratogenic [15,16]. Retinoic acid (RA), the most biologically active metabolite of retinol, is produced from free retinol in a 3-phase process that involves: 1) hydrolysis of retinyl esters in the liver and the release of retinol into the circulation and its delivery to the target tissues bound to retinol-binding protein (RBP); 2) oxidation of retinol to retinal (aldehyde) via the action of an alcohol dehydrogenase; and 3) synthesis from retinaldehyde via an aldehyde dehydrogenase reaction, primarily in the cell microsomes. RA exerts its effects by binding to 2 types of nuclear protein receptors: the retinoic acid receptors (RARs) and retinoid X receptors (rexinoids, RXRs), both of which exist as 3 distinct gene products (alpha, beta, and gamma). These receptors are members of the steroid/thyroid superfamily of ligand dependent nuclear transcription factors that includes the receptors for steroids, thyroid hormone, and vitamin D. Following ligand activation, the receptors function as heterodimeric transcription factors and control expression of numerous target genes by binding to specific DNA sequences termed RA response elements (RAREs) [17,18]. Some retinoids, in addition to activating the RAR/RXR pathway, also bind to aryl hydrocarbon (Ah) receptors, which play a role in controlling the catabolism of retinoic acid via cytochrome P450 [19].
RBP is transported into cells by a membrane protein STRA6 (“stimulated by retinoic acid”), a cell surface signaling receptor activated by the RBP-retinol complex. The association of RBP-retinol with STRA6 triggers tyrosine phosphorylation, which recruits and activates Janus Kinase (JAK) 2 and the transcription factor STAT5. This RBP-retinol/STRA6/JAK2/STAT5 signaling cascade induces the expression of STAT target genes including SOCS3 (“suppression of cytokine signaling 3”), which inhibits insulin signaling and PPAR-gamma, which in turn enhances lipid accumulation. Thus retinol, the vitamin A parent molecule, is a transcriptional regulator in its own right, not only via the RARs and RXRs [20]. Since RBP levels are markedly elevated in obese mice and humans, and high serum RBP levels induce insulin resistance [21], the findings of Berry et al. [20] that RBP-retinol activates STAT5 through a pathway mediated by STRA6 and JAK2 provides a molecular mechanism by which retinoids play a role in insulin resistance.
After a large vitamin A-containing meal, retinyl esters in serum (normally <0.2 μmol/L in the fasting state) increase significantly, following which they are converted to retinol and stored in the liver. Retinyl esters comprise up to 75% of dietary vitamin A in developed countries. Serum retinol concentrations remain stable due to a carefully regulated transport system that ensures the target tissues receive the necessary amounts of retinol despite major fluctuations in dietary intake [22]. Vitamin A toxicity from provitamin A plant carotenoid sources is unusual and almost impossible; on the other hand, the absorption and hepatic storage of preformed vitamin A from animal foods as well as fortified foods and supplements occurs efficiently until a pathologic condition develops [23]. Hypervitaminosis A is thus due mainly to excessive dietary intake or self-medication, when the liver becomes saturated with vitamin A and stored retinyl esters spill over into the circulating blood. Vitamin A toxicity occurs when retinol is presented to cell membranes in a free form, unbound to RBP, with increased fractions of retinyl esters circulating with plasma lipoproteins. Retinyl esters react more randomly with cell membranes than the physiologically-sequestered RBP and hence are a major form of vitamin A toxicity. Fasting retinyl ester concentrations >10% of total circulating vitamin A (retinol plus esters) are considered a biomarker for toxicity. Endogenous and/or administered retinoic acid (and other acidic retinoids), although not forming similar esters, is much more biologically active and hence potentially toxic than retinol itself [23–25].
Retinoid toxicity can also occur endogenously in cholestatic liver conditions, when the secretion of RBP is interrupted and retinoids accumulate in the liver, resulting in the spillage of metabolites of retinol, retinyl acetate, retinal, or retinoic acid into the circulation and the leakage of retinyl esters from damaged hepatocytes [26]. For instance, in hepatitis B virus-associated cholestasis, plasma retinol is decreased in conjunction with high plasma levels of retinyl esters and very high liver levels of vitamin A due to reduced hepatic mobilization. The result is an altered retinoid profile of low plasma retinol concentrations and a high retinyl ester: retinol ratio, inducing a mixed-symptom pattern of hypo- and hypervitaminosis A [27]. It is suggested here that a wide variety of medications interact with endogenous sources of vitamin A to induce SJS/TEN via cholestatic liver dysfunction and retinoid toxicity.
The precise ranges of serum retinoic acid associated with symptomatic acute or chronic vitamin A toxicity are not well defined. Treatment with 13-cis-RA at 30 mg/kg/d raises circulating levels of retinoic acid from a physiological range of about 1–2 ng/ml to >10 ng/ml, and occasionally to as high as 70 ng/ml [24]. Serum retinol concentrations (normally 1–3 μmol/L) do not reflect hepatic vitamin A concentrations over a wide range of liver values, since the secreted RBP is under homeostatic control. Case reports of hypervitaminosis A often show serum retinol concentrations within normal limits, indicating that serum retinol is not a valid measure of vitamin A status during toxicity [25].
Evidence of Retinoid Toxicity in SJS/TEN
Several lines of evidence support the hypothesis that drug-induced SJS/TEN involves cholestatic liver dysfunction and an endogenous form of retinoid toxicity.
Cholestatic liver disease occurs in SJS/TEN
Cholestatic liver disease is associated with SJS/TEN and precedes the skin manifestations. Fever, myalgia, and headache accompanied by high liver transaminases often precede the blistering lesions and papular eruptions [28]. The observation that symptoms can occur up to 6 weeks after drug ingestion supports the notion that progressively worsening liver dysfunction could be implicated in the pathogenesis of SJS/TEN.
SJS/TEN resembles hypervitaminosis A
There are strong similarities between the features of SJS/TEN and those of acute hypervitaminosis A. The major symptoms reported in the Taiwan outbreak, involving 53 cases [14], were fever, erosion or blistering of mucous membranes, rash, muscle pain, jaundice, vomiting, skin detachment, and liver transaminases over twice the normal level. These symptoms are also seen following excessive vitamin A supplement ingestion or therapy with synthetic retinoids (Table 1).
Table 1.
Signs and symptoms of SJS/TEN and acute hypervitaminosis A compared.
| Signs/symptoms | SJS/TEN | Hypervitaminosis A |
|---|---|---|
| Abdominal pain | + | + |
| Anorexia | + | + |
| Blurred vision | + | + |
| Conjunctivitis | + | + |
| Cough | + | + |
| Cutaneus erythema | + | + |
| Drowsiness | + | + |
| Eosinophilia | + | + |
| Epidermolysis | + | + |
| Erosive mucosal lesions | + | + |
| Fever | + | + |
| Glomerulosclerosis | + | ? |
| Headache | + | + |
| Hepatitis | + | + |
| Hypocalcaemia | + | + |
| Irritability | + | + |
| Jaundice | + | + |
| Liver transaminases increased | + | + |
| Malaise | + | + |
| Muscle pain | + | + |
| Nausea, vomiting | + | + |
| Peripheral neuritis | + | + |
| Progressive cutaneous blisters | + | + |
| Pulmonary edema | + | + |
| Skin desquamation | + | + |
| Stomatitis | + | + |
Similarities between SJS/TEN and acute vitamin A intoxication are evident in historical reports on the effects of consumption of vitamin A-rich polar bear, seal, or dog liver. Descriptions of these effects, based on the experiences of early polar explorers, include all of the major signs of SJS/TEN. In their review of these incidents, Rodahl and Moore [29] noted that ingestion of bear and seal liver could cause severe illness. In one incident, several members of an Arctic expedition who ate bear liver subsequently lost skin from head to foot. The first signs of illness occurred 2–4 hours after a meal of polar bear or seal liver and included drowsiness, sluggishness, irritability, severe headache, and vomiting. During the second 24 hours, peeling occurred around the mouth, beginning in spots, and gradually spread over larger areas. In some cases, skin peeling was total. Specimens of polar bear liver examined by Rodahl and Moore were very rich in vitamin A and ranged from 13 000 to 18 000 IU/g of wet material. In biological tests, groups of rats were given the liver oils in daily doses estimated at 2.6 or 10.3 IU. The effects varied according to the size of the rat and the amount and duration of the overdose. Skin lesions ranged from roughening of the hair to alopecia. Drops of concentrate given orally resulted in skin peeling at the corners of the mouth. Enteritis, emaciation, and pneumonia followed. Softening and fracturing of the bones frequently occurred in growing rats; in adult rats, profuse and sudden internal hemorrhage occurred, and occasionally death. Prolonged feeding led to anemia and paralysis of the legs. At autopsy there was profuse internal hemorrhage typical of hypervitaminosis A, under the skin and in the pericardium. A rat that cut its paw on the side of the cage bled to death from the wound. Life-threatening hemorrhage can also occur as a feature of SJS/TEN, resulting from minor complications associated with placement of nasal tubes for enteral nutrition or laryngoscopy for endotracheal intubation [12].
Therapeutic retinoids can induce SJS/TEN
In addition to the noted similarities between the manifestations of SJS/TEN and hypervitaminosis A, therapy with synthetic retinoids has been reported to cause symptoms resembling SJS/TEN. A fatal case of TEN was described following therapy of severe acne with etretinate, a synthetic retinoid [30]. In a second case, etretinate was reported to induce skin blistering followed by ulceration and scarring [31]. Acitretin therapy can induce widespread desquamation similar to that seen in the “peeling skin syndrome”, a rare form of ichthyosis involving superficial, painless, continual, or seasonal cutaneous exfoliation associated with a probable autosomal recessive inheritance that tends to appear at birth or in infancy [32]. In one such case, a man taking low-dose acitretin presented with generalized congenital ichthyosiform dermatosis, severe cheilitis, palmar and plantar hyperkeratosis, and superficial blistering. The skin was moderately hyperkeratotic and the palmar blisters were subcorneal. Electron microscopy revealed splitting within the desmosomal plaque. Further investigations revealed epidermal hypervitaminosis A related to alterations in epidermal retinoic acid metabolism [33]. Severe skin irritation and stratum corneum peeling induced by retinol and its synthetic derivatives is thought to be mediated through monocyte chemoattractant protein-1 (MCP-1) and IL-8, as shown by increased levels of mRNA expression and protein secretion [34], but may also occur through granulysin, as discussed below.
Compounds that induce SJS/TEN affect retinoid metabolism
The drugs most commonly implicated in SJS/TEN are anticonvulsants (phenobarbital, phenytoin, carbamazepine, lamotrigine, valproate), sulfonamides (cotrimoxazole, sulfasalazine), other antibiotics (aminopenicillins, quinolones, cephalosporins), certain nonsteroidal anti-inflammatory drugs (oxicams and Butazone derivatives), antifungals, antimalarials, and allopurinol [7,8,10,11,35,36].
The risk of SJS/TEN and of other hypersensitivity reactions is strongly associated with the short-term (≤8 weeks) therapeutic use of anticonvulsant drugs, including phenytoin, phenobarbital, carbamazepine and lamotrigine [37]. Retinol and its oxidative metabolites all-trans-, 13-cis- and 13-cis-4-oxo-retinoic acid were measured in the plasma of 75 infants and children treated with various antiepileptic drugs for the control of seizures, and in 29 untreated controls of comparable age. Retinol levels increased with age while the concentrations of retinoic acid compounds did not exhibit age-dependency. Valproic acid monotherapy increased retinol levels in the infants and a trend toward increased retinol concentrations was also observed in all other patient groups. The plasma levels of the oxidative metabolites 13-cis- and 13-cis-4-oxo-retinoic acids were strongly decreased in all patient groups treated with phenytoin, phenobarbital, carbamazepine, and ethosuximide, in combination with valproic acid, to levels which were below 1/3rd and 1/10th of corresponding control values, respectively. Few changes were observed with all-trans-retinoic acid, except in 1 patient group treated with valproic acid/ethosuximide cotherapy where increased levels of this retinoid were found. These compounds markedly affected retinoid metabolism, increasing retinol concentrations but reducing plasma levels of the oxidative metabolites 3-cis and 13-cis-4-oxo-retinoic acids below 1/3rd and 1/10th of corresponding control values, respectively [38]. Exfoliative dermatitis, noted 25 days after the start of anticonvulsant use, was associated with increased liver enzymes and biopsy-proven cholestatic hepatotoxicity [39]. These findings show that therapy with antiepileptic agents has a profound effect on liver function and endogenous retinoid metabolism, but the mechanisms linking these changes to SJS/TEN remain uncertain.
Antibiotics
Antibiotics, including sulfonamides, can result in a wide variety of hypersensitivity reactions, as can other sulfonamide-containing medications. HIV-infected individuals in particular are at high risk of hypersensitivity reactions to sulfamethoxazole, but a literature search failed to detect an association between sulfonamides and SJS/TEN [40]. With regard to antibiotics that have been linked to SJS/TEN, the tetracyclines, particularly demethylchlortetracycline and doxycycline, have phototoxic properties [41] and interact with retinoids [42]. Large doses of tetracycline are known to cause hepatic steatosis [40]. Combined use of tetracyclines and retinoids such as acitretin and other systemic retinoids or vitamin A supplements would be expected to increase the risk of known retinoid toxicity reactions, including increased intracranial pressure and its symptoms of papilledema, headache, nausea, vomiting, and visual disturbances. In an early report, penicillin was found to increase retinol levels in blood and liver when added to the diet of chicks [43]. Vancomycin can induce the skin reaction known as “red man syndrome” and may cause immune-mediated skin reactions. For instance, a 76-year-old Caucasian woman with a history of penicillin and sulfa allergies received vancomycin for persistent methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia. On day 4 of treatment, she developed a papular rash with small blisters on her distal upper extremities. The rash worsened and spread to her neck and torso. Skin biopsy confirmed a severe leukocytoclastic, necrotizing small-cell vasculitis that met the criteria for a hypersensitivity vasculitis associated with drug therapy. Discontinuation of vancomycin resolved the vasculitis [44].
Antifungal drugs
Antifungal drugs such as metronidazole, ketoconazole, miconazole, clotrimazole, liarozole, and fluconazole induce SJS/TEN by altering gene expression of cytochrome P450 (CYP) 26 isoforms. CYPs are a superfamily of heme-containing mono-oxygenases associated with the metabolism of drugs and other substrates and are highly expressed in the liver, small intestine, and mitochondrial membranes. Different CYPs are involved at several steps in the biosynthesis and metabolism of retinoic acids. In vivo, all-trans retinoic acid (ATRA) is metabolized into several oxidized metabolites, 4-hydroxylation being the principal route of metabolism [45]. Each ATRA stereoisomer is primarily metabolized by a specific set of human CYPs. The net effect of ketoconazole and related compounds on retinoid metabolism is therefore to increase ATRA levels by delaying plasma clearance. Disruption of embryonal retinoic acid homeostasis has been postulated to represent an etiological factor in fluconazole-induced teratogenesis. Fluconazole exposure is associated with an up-regulation of gene expression of CYP26a1 and CYP26b1 in mouse embryos [46]. Blocking ATRA metabolism by ketoconazole increases the potency of ATRA [47]. Inhibition of retinoic acid 4-hydroxylation activity by ketoconazole also potentiates the activation of RA receptors by ATRA [48]. Recalling the collective outbreak of SJS/TEN following the combined use of metronidazole and mebendazole [14], our model suggests that the pathophysiologic mechanism may have involved P450 enzymatic inhibition of ATRA catabolism, retinoid accumulation in the liver and subsequent inflammation, and the spillage of vitamin A, leading to increased circulating retinoid concentrations and toxicity.
NSAIDS
NSAIDS SJS/TEN is occasionally associated with the use of nonsteroidal anti-inflammatory drugs, particularly oxicam derivatives (<2 cases per million users per week for oxicam derivatives, <1 per million users per week for other NSAIDs, and 6 cases per million person-years for celecoxib) [49]. The primary effect of the NSAIDs is to inhibit cyclooxygenase (prostaglandin synthase), which impairs the transformation of arachidonic acid to prostaglandins, prostacyclin, and thromboxanes. These drugs can produce an unpredictable, idiosyncratic hypersensitivity reaction characterized by the triad of fever, rash, and internal organ involvement that starts 1 day to 12 weeks after the initiation of therapy [49]. NSAID-induced hypersensitivity reactions include asthma and rhinitis and appear to be precipitated by the inhibition of cyclo-oxygenase-1 (COX-1) associated with increased cysteinyl leukotriene levels and increased expression of the cysteine leukotriene receptor (CysLT1) on leukocytes infiltrating the mucosa [50]. Leukotriene C4 (LTC4) is derived from LTC4 synthase, which is present in mast cells, eosinophils, and basophils [51]. The cause of the overproduction of cysteinyl leukotrienes has remained unclear. The cysteinyl leukotrienes (LTC4, LTD4, and LTE4) derive from mast cells and eosinophils and are products of the enzyme 5-lipoxygenase on arachidonic acid, which is released from cell membrane phospholipids by the action of phospholipase A2. The 3 cysteinyl leukotrienes potently contract bronchial smooth muscle, induce submucosal edema and mucus secretion, and recruit eosinophils to the airways [50]. Mast cell involvement and cysteinyl leukotriene overproduction in NSAID-induced hypersensitivity reactions may be due to retinoid overproduction and/or overexpression, since topical application of the synthetic retinoid tretinoin to photoaged hairless mouse skin increases mast cell production, epidermal mast cell growth factor, and associated mast cell hyperplasia [52]. Application of retinoic acid led to a dose- and time-dependent promotion of intercellular adhesion molecule-3 (ICAM-3) expression in the human mast cell line (HMC-1), and retinoid receptor expression (in RAR-alpha, RAR-gamma, RXR beta, and RXR gamma transcripts) was present in all mast cell lines studied [53]. Rat basophilic leukemia-1 cells cultured for 48 hours in the presence of 0.1 μg/ml of all-trans-retinoic acid (RA) also exhibited a 27-fold greater increase in LTC4 synthase activity than control cells, together with up-regulation of LTC4 synthase mRNA [54]. In addition, RA (0.1 μg/ml) significantly potentiated calcium ionophore-stimulated production of LTC4 synthesis, which was inhibited by dexamethasone. RA-induced LTC4 synthase activity was also inhibited by dexamethasone [55].
With regard to the association between the antimalarials and SJS/TEN, the quinolones are one of the most successful yet poorly understood classes of drugs, with important uses in the treatment of malaria, systemic lupus erythematosus, and HIV. The mechanism of action of these drugs, which include chloroquine, primaquine, mefloquine, and quinine, remains controversial. Using a functional proteome approach to exploit the structural similarity between quinolones and the purine ring on ATP to identify quinoline-binding proteins, Graves et al. [56] identified 2 human proteins, aldehyde dehydrogenase 1 (ALDH1) and quinine reductase 2 (QR2), which were selectively inhibited by quinolones. Other known inhibitors of ALDH1 (diethylamineobenzaldehyde) and QR2 (quercetin and chrysin) were lethal to or inhibited the growth of the malaria parasite Plasmodium falciparum in vitro, but they were not as effective in killing the parasites as the quinoline compounds themselves. Chloroquine actively accumulates to millimolar concentrations in the skin and eye when administered at therapeutic levels [57]. A major function of ALDH1 in the eye is to catalyze the conversion of retinaldehyde to retinoic acid (visual pigment). Hence, prolonged therapeutic use of chloroquine or hydroxychloroquine could result in retinopathy and blindness due to ALDH enzymatic inhibition and the accumulation of retinaldehyde in the retina. In the skin, the quinolones may similarly lower the availability of retinoic acid via enzymatic inhibition yet increase the accumulation of retinaldehyde, thereby inducing SJS/TEN in susceptible persons.
Most cases of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) associated with allopurinol use are middle-aged men with hypertension and/or renal failure, receiving the drug for asymptomatic hyperuricemia. The most common findings are cutaneous rash and fever [58]. Allopurinol inhibits xanthine oxidase, the enzyme responsible for converting hypoxanthine to xanthine, and xanthine to uric acid; the latter can also oxidize retinol (vitamin A alcohol) to its more toxic metabolite, retinoic acid [59]. Since xanthine oxidase can result in elevated levels of both uric acid and retinoic acid, the efficacy of allopurinol in treating gout could be due to inhibition of the conversion of retinol to retinoic acid, of xanthine to uric acid, or both reactions [60]. Indeed, the synthesis of retinoic acid is strongly inhibited by allopurinol [61]. It is conjectured that allopurinol-induced SJS/TEN is due to inhibition of the synthesis of retinoic acid by allopurinol and the subsequent accumulation of its precursor retinol to toxic concentrations. This conjecture is supported by the current recommendation that patients with gout should avoid supplementation with vitamin A [62].
The role of corticosteroids in SJS/TEN has been the subject of prolonged debate. Some have argued from uncontrolled studies that the management of SJS/TEN requires corticosteroid therapy, on the basis that SJS/TEN mimics a graft-versus-host reaction (in which the patient rejects skin, mucous membrane, kidney, or liver cells to which the drug has bound) and that corticosteroids suppress inflammation [63]. Other studies with improved designs indicate that corticosteroid use increases the risk of mortality [64] and is itself a cause of SJS/TEN [7]. Corticosteroids reduce liver and lung concentrations of vitamin A by about 50% but increase circulating retinol levels [65,66], which would be expected to increase retinoid expression in cutaneous tissues and thus worsen overall symptomatology of SJS/TEN. The current consensus is that systemic corticosteroids are of unproven benefit in mild forms and clearly deleterious in severe forms of SJS/TEN [67]. The drugs implicated in SJS/TEN and the suggested mechanisms by which they interact with retinoids to induce SJS/TEN are summarized in the Table 2.
Table 2.
Drugs and proposed mechanisms of SJS/TEN involving retinoids (see text for references).
| Drug | Mechanism |
|---|---|
| Anticonvulsants | Alterations in liver function and retinoid metabolism |
| Antibiotics (tetracyclines) | Inhibition of P450-mediated degradation of retinoic acid |
| Antifungals | P450 enzymatic inhibition of ATRA catabolism |
| Nonsteroidal anti-inflammatory drugs | Retinoid overproduction and/or overexpression |
| Antimalarials | Aldehyde dehydrogenase enzymatic inhibition and the accumulation of retinol and retinaldehyde |
| Allopurinol | Inhibition of retinoic acid synthesis and the accumulation of retinol to toxic concentrations |
| Corticosteroids | Reduced liver and lung concentrations but increased circulating retinol concentrations |
Mechanism of SJS/TEN
Biochemical and morphological studies on early epidermal lesions in patients with SJS/TEN have shown that keratinocytes undergo apoptosis. Earlier studies implicated dysregulation of fatty acid synthetase (Fas) expression in the pathophysiology of tissue destruction both in SJS/TEN and graft-host rejection. This was thought to occur through Fas and fas ligand (FasL) interactions or through cytotoxic T-cell release of perforin and granzyme B [12]. At the molecular level, Fas is activated through FasL [68], thereby activating caspases [69] and causing apoptosis. The activation of Fas through FasL was thought to be an important first step leading to diffuse apoptotic cell death of epidermal cells in SJS/TEN [4]. Increased serum levels of soluble FasL are reported in some patients with TEN, which supports this view [70]. The retinoid toxicity model of SJS/TEN is consistent with these observations, since retinoic acid in the skin upregulates the expression of FasL molecules by fibroblasts [68]. On the other hand, recent evidence suggests that the Fas-sFasL, perforin, and granzyme pathways are not specific to SJS/TEN and are upregulated in skin eruptions where massive apoptosis does not occur [71]. While low concentrations of retinoic acid inhibit apoptosis, higher doses (≥1 μM) promote it [72] in many different cell lines [73]. Retinoic acid increases the expression of the tumor suppressor protein p53 and proapoptopic caspases and sensitizes keratinocytes to apoptosis [74]. Natural and synthetic retinoids also induce apoptosis in the BALB/MK mouse keratinocyte cell line [75]. The mechanism by which vitamin A can both enhance and inhibit cell growth, depending on dose or concentration, is due to the alternate activation of 2 different nuclear receptors. Schug et al. [76] have shown that, in addition to functioning through RAR, RA activates the “orphan” nuclear receptor PPAR-beta/delta. Partitioning of RA between the 2 receptors (beta/delta) is regulated by the intracellular lipid binding proteins cellular retinoic acid-binding proteins (CRABP-II) and fatty acid binding protein (FABP5). These proteins specifically deliver RA from the cytosol to nuclear RAR and PPAR-beta/delta, respectively, thereby selectively enhancing the transcriptional activity of their cognate receptors. RA functions through RAR as a proapoptotic agent in cells (leading to phosphorylation) with high CRABP-II/FABP5 ratio, but it signals through PPAR-beta/delta and promotes survival in cells that highly express FABP5.
In sum, the postulated interaction between the drugs implicated in SJS/TEN and retinoids involves activation of the retinoid cascade and increased retinoid accumulation in the liver, leading to cholestasis, the subsequent spillage of stored retinoids and retinoid toxicity, marked by keratinocyte apoptosis and the evolving manifestations of SJS/TEN. Many patients with SJS/TEN are elderly persons with underlying medical conditions that require regular medical treatment [12]. Age-associated liver dysfunction and the tendency for retinoids to accumulate with age [77] could account for the apparent susceptibility of the elderly to SJS/TEN.
Granulysin
Strong evidence implicates granulysin in the pathogenesis of SJS/TEN. Granulysin belongs to a family of saposin-like lipid-binding proteins and is a potent antimicrobial protein. Contained in the granules of CTLs, principally CD8+ T cells and natural killer (NK) cells, it is produced as an intact 15-kDa form and cleaved to yield a 9-kDa form. Granulysin is the most highly expressed cytotoxic molecule in the blisters of patients with SJS/TEN, which are mainly composed of CD8+ CTLs. Higher amounts are found in the blisters of patients with TEN and cause disseminated keratinocyte death. Injection of purified 15-kDa granulysin into mouse skin induces blistering and considerable epidermal and dermal necrosis, with proportionally higher concentrations in TEN compared to SJS lesions. Granulysin (but neither granzyme B, perforin, nor sFasL) was found to be the key molecule responsible for disseminated keratinocyte death. Depleting granulysin from the SJS-TEN blister fluids reduced blister fluid cytotoxicity to a much greater extent than did depleting sFasL, granzyme B, or perforin, providing further evidence that granulysin causes the rapidly developing extensive apoptosis and necrosis in SJS/TEN. Massive amounts of secretory granulysin are produced by the cells, leading to severe apoptosis and tissue damage and the unique presentation of SJS/TEN [71]. Patients with SJS/TEN also have significantly higher serum concentrations of granulysin compared to healthy controls and patients with ordinary drug-induced skin reactions. Samples obtained from 5 patients with SJS/TEN showed that granulysin was increased 2–4 days before skin detachment or the development of mucosal lesions [78]. Granulysin induces in vitro chemotaxis and activation of both human and mouse dendritic cells (DCs) via TLR4/Myd88; it also enhances the proliferation of Th1 (and Th2 to a lesser extent) cytokine production in response to ovalbumin (OVA) and increases OVA-specific antibodies, although this adjuvant effect was observed only in Toll-like receptor 4 (TLR4)-sufficient and not in TLR4-deficient mice [79]. These findings suggest that granulysin released from lymphocytes acts as an alarmin, i.e., a cytokine-like molecule that alerts innate immune effectors, a role previously thought to be confined to neutrophils, phagocytes or epithelial cells [80].
Retinoids and granulysin
The mechanism of retinoid toxicity at the molecular level is not well understood. This paper suggests that retinoic acid is both a risk factor as well as a direct cause of SJS/TEN in some instances, via treatment with synthetic retinoids; furthermore, granulysin is clearly involved in the causation of SJS/TEN. If, as proposed here, SJS/TEN is induced by endogenous alterations and increases in the concentration and expression of retinoic acid, then granulysin may be an effector molecule for retinoid toxicity. The suggested link between retinoic acid and granulysin does not appear to have been recognized or investigated to date. There are, however, similarities between retinoic acid and granulysin, which suggest the existence of such a link. Like retinoic acid, granulysin can activate DCs, it is an antimicrobial protein, it can function as an immune-adjuvant, it is involved in the expansion and proliferation of T regulatory cells [79], and it induces apoptosis in target cells involving caspases and other pathways [81].
Granulysin has a cationic ampholytic structure that has the capacity to lyse bacterial membranes, which generally contain negatively charged lipids, and can induce lysosomal membrane permeabilization [82]. Granulysin can also kill human cells that are attacked by CTL or NK by virtue of their membrane-permeabilizing effects on mitochrondria [83]. Retinoids, including retinol and retinoic acid, can also inhibit cell growth and interact directly with membranes to increase their permeability and fluidity, causing hemolysis of erythrocytes and increased secretion of enzymes from lysosomes [84]. Cells exposed briefly to retinol show marked swelling, while longer exposures cause cell death. Retinoids also arrest the growth of cultured lymphocytes due to a membrane destabilizing effect [85]. Retinol-induced erythrocyte hemolysis results from physical damage to the membrane micelle induced by the penetration of retinol molecules rather than by oxidative disruption of erythrocyte membrane lipids initiated by ROL oxidation [86].
In peripheral blood, the predominant T cell source of granulysin and killing activity is CD8+ T cells. The cytokines IL-21 and IL-15 are strong inducers of granulysin in these cells via JAK/STAT5 [87]. In a further parallel with granulysin, retinoic acid in conjunction with IL-15, a cytokine that is greatly overexpressed in the gut of patients with celiac disease, rapidly activates intestinal dendritic cells to induce JNK (aka MAPK8) phosphorylation and releases the proinflammatory cytokines IL-12p70 and IL-23, impairing the generation of Treg cells. Thus, in a stressed intestinal environment, retinoic acid can combine with IL-15 to act as an adjuvant to promote inflammatory cellular and humoral responses [88]. The combined negative effect of retinoic acid and IL-15 on Treg differentiation occurred in parallel with the induction of the T helper (Th1) response. Thus, in the presence of IL-15, retinoic acid has unforeseen co-adjuvant properties that induce Th1 immunity to fed antigens. Under infectious conditions associated with the induction of IL-15 and IL-6 (instead of IL-12p70) in intestinal mucosa, retinoic acid promotes IL-17 immunity [88]. These findings caution against use of vitamin A or retinoic acid for the treatment of autoimmunity and intestinal disorders such as celiac disease associated with high levels of IL-15 [89]. Isotretinoin is commonly prescribed for the treatment of severe acne. Case reports and clinical studies have suggested that in a subgroup of patients with acne, isotretinoin might serve as a trigger for inflammatory bowel disease (IBD) [90]. A case-control study using a large insurance claims database in which incident cases of IBD were identified and matched to three controls on demographic and other factors showed that ulcerative colitis (UC) was strongly associated with previous isotretinoin exposure (OR 4.36, 95% CI: 1.97–9.66) but there was no association between Crohn’s disease and isotretinoin use. Increasing doses of isotretinoin were associated with elevated risk of UC. Compared with non-users, the risk of UC was highest in those exposed to isotretinoin for over 2 months (OR 5.63, 95% CI: 2.10–15.03) [91]. These data on the association between IL-15 and retinoic acid support the hypothesis that retinoid toxicity may be mediated by granulysin and that retinoid-induced granulysin may be the pathophysiological basis for SJS/TEN.
Conclusions
Adverse drug reactions account for 6–7% of all hospital admissions, of which Stevens-Johnson syndrome and toxic epidermal necrolysis are the most serious and life-threatening, having a 10–15% mortality rate [92]. The present review suggests that SJS/TEN results from drug and endogenous biochemical interactions that can cause liver damage and increase retinoid compounds to toxic concentrations and induce apoptosis in the affected tissues via granulysin. This hypothesis could be tested by comparing retinoid profiles and granulysin concentrations in patients with SJS/TEN versus controls. The model predicts that patients have higher percent retinyl esters to total vitamin A (retinol plus esters) >10% (indicating retinoid toxicity) and increased retinoic acid concentrations compared to controls, and that percent retinyl esters and retinoic acid concentrations correlate significantly with granulysin.
Standard therapy for SJS/TEN currently includes meticulous wound care, fluid replacement, and nutritional support in an intensive care setting. Subject to testing, the present model suggests that progression of SJS/TEN could be arrested by lowering the concentration of circulating retinoids, e.g., by phlebotomy or plasmapheresis, or by administering drugs that inhibit the expression of retinoids. Both phlebotomy and plasmapheresis reduce plasma retinol and RBP [93]. With regard to the latter, the outcomes of 10 patients with TEN who were treated in a burn unit over a 9-year period using conventional support measures were compared with those of 6 patients treated with plasmapheresis. Of the10 patients given standard care, 4 died but all 6 patients treated with plasmapheresis survived. It was concluded that plasmapheresis is a safe intervention in extremely ill patients with TEN and may reduce mortality [94]. Case-control studies and clinical trials of retinoid antagonists are needed to test and refine these proposals.
Footnotes
Source of support: Publication costs were provided by the College of Public Service. Jackson State University
References
- 1.Stevens AM, Johnson FC. A new eruptive fever associated with stomatitis and ophthalmia. Am J Dis Child. 1922;24:526–53. [Google Scholar]
- 2.Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355–61. doi: 10.1111/j.1365-2133.1956.tb12766.x. [DOI] [PubMed] [Google Scholar]
- 3.Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56(2):181–200. doi: 10.1016/j.jaad.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 4.Abe R, Shimizu T, Shibaki A, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble fas ligand. Am J Pathol. 2003;162:1515–20. doi: 10.1016/S0002-9440(10)64284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farthing P, Bagan J, Scully C. Mucosal disease series. Number IV. Erythema multiforme. Oral Dis. 2005;11(5):261–67. doi: 10.1111/j.1601-0825.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 6.Rehmus WE. Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) Merck Manuals. Oct, 2009. http://www.merck.com/mmpe/sec10/ch117/ch117i.html.
- 7.Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–7. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 8.Fritch PO, Sidoroff A. Drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Am J Clin Dermatol. 2000;1:349–60. doi: 10.2165/00128071-200001060-00003. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar S, Mockenhaupt M, Roujeau JC, Townshend A. Interventions for toxic epidermal necrolysis. Cochrane Database Syst Rev. 2002;4:CD001435. doi: 10.1002/14651858.CD001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leone R, Venegoni M, Motola D, et al. Adverse drug reactions related to the use of fluoroquinolone antimicrobials: an analysis of spontaneous reports and fluroquinolone consumption data from three Italian regions. Drug Saf. 2003;26:109–20. doi: 10.2165/00002018-200326020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:53–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 12.Struck MF, Hilbert P, Mockenhaupt M, et al. Severe cutaneous adverse reactions: emergency approach to non-burn epidermolytic syndromes. Intensive Care Med. 2010;36:22–32. doi: 10.1007/s00134-009-1659-1. [DOI] [PubMed] [Google Scholar]
- 13.Chopra A, Drage LA, Hanson EM, Touchet NL. Stevens-Johnson syndrome after immunization with smallpox, anthrax and tetanus vaccines. Mayo Clinic Proc. 2004;79:1193–96. doi: 10.4065/79.9.1193. [DOI] [PubMed] [Google Scholar]
- 14.Chen KT, Two SJ, Chang HJ, Lin RS. Outbreak of Stevens-Johnson syndrome/Toxic epidermal necrolysis associated with mebendazole and metronidazole use among Filipino laborers in Taiwan. Am J Public Health. 2003;93:489–92. doi: 10.2105/ajph.93.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganguly J. The Biochemistry of Vitamin A. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- 16.Hoffman C, Eichele G. Retinoids in development. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. pp. 387–441. [Google Scholar]
- 17.Litwack G, editor. Vitamin A: Vitamins and Hormones. Vol. 75. San Diego, CA: Elsevier Academic Press; 2007. [Google Scholar]
- 18.Theodosiou M, Laudet V, Schubert M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell Mol Life Sci. 2010;67:1423–45. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambone CJ, Hutcheson JM, Gabriel JL, et al. Unique property of some synthetic retinoids: Activation of the aryl hydrocarbon receptor pathway. Mol Pharmacol. 2002;61:334–42. doi: 10.1124/mol.61.2.334. [DOI] [PubMed] [Google Scholar]
- 20.Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Nat Acad Sci USA. 2011;108(11):4340–45. doi: 10.1073/pnas.1011115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 22.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 23.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 24.Miller RK, Hendricks AG, Mills JL, et al. Periconceptional vitamin A use: how much is teratogenic? Reprod Toxicol. 1998;12:75–88. doi: 10.1016/s0890-6238(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 25.Olson JA. Vitamin A. In: Ziegler EE, Filer LJ Jr, editors. Present knowledge in nutrition. 7. Washington, DC: International Life Sciences Institute Press; 2001. pp. 109–19. [Google Scholar]
- 26.Leo MA, Lieber CS. Hypervitaminosis A: A liver lover’s lament. Hepatology. 1988;8:412–17. doi: 10.1002/hep.1840080237. [DOI] [PubMed] [Google Scholar]
- 27.Mezey E. Liver disease and protein needs. Annu Rev Nutr. 1982;2:21–50. doi: 10.1146/annurev.nu.02.070182.000321. [DOI] [PubMed] [Google Scholar]
- 28.Morelli MS, O’Brien FX. Stevens-Johnson Syndrome and cholestatic hepatitis. Dis Dis Sci. 2001;46:2385–88. doi: 10.1023/a:1012351231143. [DOI] [PubMed] [Google Scholar]
- 29.Rodahl K, Moore T. The vitamin A content and toxicity of bear and seal liver. Biochem J. 1943;37:166–68. doi: 10.1042/bj0370166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIvor A. Fatal toxic epidermal necrolysis associated with etretinate. Br Med J. 1992;304:548. doi: 10.1136/bmj.304.6826.548-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay B, Bloxham C, Eldred A, et al. Blistering, erosions and scarring in a patient on etretinate. Br J Dermatol. 1989;121:397–400. doi: 10.1111/j.1365-2133.1989.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 32.Kharfi M, Khaled A, Ammar D, et al. Generalized peeling skin syndrome: Case report and review of the literature. Dermatol Online J. 2010;16(3):1. [PubMed] [Google Scholar]
- 33.Mevorah B, Salomon D, Siegenthaler G, et al. Ichthyosiform dermatosis with superficial blister formation and peeling: evidence for a desmosomal anomaly and altered epidermal vitamin A metabolism. J Am Acad Dermatol. 1996;34:379–85. doi: 10.1016/s0190-9622(07)80013-2. [DOI] [PubMed] [Google Scholar]
- 34.Kim BH, Lee YS, Kang KS. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett. 2003;15:65–73. doi: 10.1016/j.toxlet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Kumar G, Fadel HJ, Beckman TJ. 36-year-old man with productive cough and diffuse rash. Mayo Clinic Proc. 2006;81:945–48. doi: 10.4065/81.7.945. [DOI] [PubMed] [Google Scholar]
- 36.Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature. Am J Health Syst Pharm. 2010;67:206–13. doi: 10.2146/ajhp080603. [DOI] [PubMed] [Google Scholar]
- 37.Rzany B, Correia O, Kelly JP, et al. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: a case-control study. Lancet. 1999;353:2190–94. doi: 10.1016/s0140-6736(98)05418-x. [DOI] [PubMed] [Google Scholar]
- 38.Nau H, Tzimas G, Mondry M, et al. Antiepileptic drugs alter endogenous retinoid concentrations: a possible mechanism of teratogenesis of anticonvulsant therapy. Life Sci. 1995;57:53–60. doi: 10.1016/0024-3205(95)00242-x. [DOI] [PubMed] [Google Scholar]
- 39.Altuntaş Y, Oztürk B, Erdem L, et al. Phenytoin-induced toxic cholestatic hepatitis in a patient with skin lesions: case report. South Med J. 2003;96:201–3. doi: 10.1097/01.SMJ.0000051269.23361.4A. [DOI] [PubMed] [Google Scholar]
- 40.Slatore CG, Tilles SA. Sulfonamide hypersensitivity. Immunol Allergy Clin North Am. 2004;24(3):477–90. doi: 10.1016/j.iac.2004.03.011. vii. [DOI] [PubMed] [Google Scholar]
- 41.Harber LC. Abnormal response to ultraviolet radiation: drug-induced photosensitivity. Cha42. Blue M, Bozdech JM. Drug-induced liver injury. In: Achkar E, Farmer RG, Fleshler B, editors. Clinical Gastroenterology. 2nd edition. Philadelphia: Lea & Febiger; 1992. pp. 574–80. [Google Scholar]
- 42.Hellmann-Regen J, Herzog I, Fischer N, et al. Do tetracyclines and erythromycin exert anti-acne effects by inhibition of P450-mediated degradation of retinoic acid? Exp Dermatol. 2014;23(4):290–93. doi: 10.1111/exd.12358. [DOI] [PubMed] [Google Scholar]
- 43.Moore T. Vitamin A. Cambridge: Cambridge University Press; 1957. [Google Scholar]
- 44.Felix-Getzik E, Sylvia LM. Vancomycin-induced leukocytoclastic vasculitis. Pharmacotherapy. 2009;29:846–51. doi: 10.1592/phco.29.7.846. [DOI] [PubMed] [Google Scholar]
- 45.Marill J, Idres N, Capron CC, et al. Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab. 2003;4:1–10. doi: 10.2174/1389200033336900. [DOI] [PubMed] [Google Scholar]
- 46.Tiboni GM, Marotta F, Carletti E. Fluconazole alters CYP26 gene expression in mouse embryos. Reprod Toxicol. 2009;27(2):199–202. doi: 10.1016/j.reprotox.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Napoli JL. Retinoic acid: its biosynthesis and metabolism. Prog Nucleic Acid Res Molec Biol. 2000;63:139–88. doi: 10.1016/s0079-6603(08)60722-9. [DOI] [PubMed] [Google Scholar]
- 48.Marikar Y, Wang Z, Duell EA, et al. Retinoic acid receptors regulate expression of retinoic acid 4-hydroxylase that specifically inactivates all-trans retinoic acid in human keratinocyte HaCaT cells. J Invest Dermatol. 1998;111:434–39. doi: 10.1046/j.1523-1747.1998.00297.x. [DOI] [PubMed] [Google Scholar]
- 49.Nanau RM, Neuman MG. Ibuprofen-induced hypersensitivity syndrome. Transl Res. 2010;155:275–93. doi: 10.1016/j.trsl.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Drazen JM. Leukotrienes as mediators of airway obstruction. Am J Respir Care Med. 1998;108:47–51. doi: 10.1164/ajrccm.158.supplement_2.13tac180. [DOI] [PubMed] [Google Scholar]
- 51.Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci USA. 1994;91:4275–79. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kligman LH, Murpy GF. Topical tretinoin increases dermal mast cells, induces epidermal mast cell growth factor (c-kit ligand) and modulates its distribution in hairless mice. Arch Dermatol Res. 1996;288:537–42. doi: 10.1007/BF02505251. [DOI] [PubMed] [Google Scholar]
- 53.Babina M, Mammeri K, Henz BM. Retinoic acid up-regulates myeloid ICAM-3 expression and function in a cell-specific fashion – evidence of retinoid signaling pathways in the mast cell lineage. J Leukoc Biol. 2001;69:361–72. [PubMed] [Google Scholar]
- 54.Abe M, Shibata, Saruwater S, et al. cDNA cloning and expression of rat leukotriene C4 synthase: elevated expression in rat basophilic leukemia-1 cells after treatment with retinoic acid. Prostaglandins Leukot Essent Fatty Acids. 2002;67:319–26. doi: 10.1054/plef.2002.0436. [DOI] [PubMed] [Google Scholar]
- 55.Hamasaki Y, Abe M, Matsumoto S, et al. Inhibition by dexamethasone of retinoic acid-induced enhancement of leukotriene C4 synthesis in rat basophilic leukemia-1 cells. Am J Resp Cell Mol Biol. 1994;11:49–56. doi: 10.1165/ajrcmb.11.1.8018338. [DOI] [PubMed] [Google Scholar]
- 56.Graves PR, Kwiek JJ, Fadden P, et al. Discovery of novel targets of quinoline drugs in the human purine binding proteome. Mol Pharmacol. 2002;62:1364–72. doi: 10.1124/mol.62.6.1364. [DOI] [PubMed] [Google Scholar]
- 57.Rynes RI. Antimalarial drugs in the treatment of rheumatological diseases. Br J Rheumatol. 1997;36:799–805. doi: 10.1093/rheumatology/36.7.799. [DOI] [PubMed] [Google Scholar]
- 58.Arellano F, Sacristan JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337–43. doi: 10.1177/106002809302700317. [DOI] [PubMed] [Google Scholar]
- 59.Moffa DJ, Lotspeich FJ, Krause RF. Preparation and properties of retinal-oxidizing enzyme from rat intestinal mucosa. J Biol Chem. 1970;245:439–47. [PubMed] [Google Scholar]
- 60.Mawson AR, Onor GI. Gout and vitamin A intoxication: Is there a connection? Semin Arthritis Rheum. 1991;20:297–304. doi: 10.1016/0049-0172(91)90030-4. [DOI] [PubMed] [Google Scholar]
- 61.Taibi G, Paganini A, Geuli MC, et al. Milk xanthine oxidase catalyzes the synthesis of retinoic acid. J Enzyme Inhib. 2001;16(3):275–85. doi: 10.1080/14756360109162376. [DOI] [PubMed] [Google Scholar]
- 62.Rees F, Hui M, Doherty M. Optimizing current treatment of gout. Nature Rev Rheumatol. 2014;10:271–83. doi: 10.1038/nrrheum.2014.32. [DOI] [PubMed] [Google Scholar]
- 63.Patterson R, Miller M, Kaplan M, et al. Effectiveness of early therapy with corticosteroids in Stevens-Johnson syndrome: experience with 41 cases and a hypothesis regarding pathogenesis. Ann Allergy. 1994;73:27–34. [PubMed] [Google Scholar]
- 64.Keleman JJ, Cioffi WG, McManus WF, et al. Burn center care for patients with toxic epidermal necrolysis. J Am Coll Surg. 1995;180:273–78. [PubMed] [Google Scholar]
- 65.Clark I, Colburn RW. A relationship between vitamin A metabolism and cortisone. Endocrinology. 1955;56:232–38. doi: 10.1210/endo-56-3-232. [DOI] [PubMed] [Google Scholar]
- 66.Georgieff MK, Radmer WJ, Sowell AL, et al. The effects of glucocorticosteroids on serum, liver, and lung vitamin A and retinyl ester concentrations. J Pediatr Gastroenterol Nutr. 1991;13:376–82. doi: 10.1097/00005176-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Ghislain P-D, Roujeau J-C. Treatment of severe drug reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis and hypersensitivity syndrome. Dermatol Online. 2002;8(1):5. [PubMed] [Google Scholar]
- 68.Saitoh A, Kawanabe T, Weidong H, et al. Selective upregulation of fibroblast fas ligand expression, and prolongation of fas/fas ligand-mediated skin allograft survival, by retinoic acid: the skin as a retinoid-inducible immune privileged site. J Invest Derm. 2000;115:154–61. doi: 10.1046/j.1523-1747.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 69.French LE, Tschopp J. Protein-based therapeutic approaches targeting death receptors. Cell Death and Differ. 2003;10:117–23. doi: 10.1038/sj.cdd.4401185. [DOI] [PubMed] [Google Scholar]
- 70.Abe R. Toxic epidermal necrolysis and Stevens-Johnson syndrome: soluble Fas ligand involvement in the pathomechanisms of these diseases. J Dermatol Sci. 2008;52:151–59. doi: 10.1016/j.jdermsci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–50. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 72.Noy N. Between death and survival: retinoic acid in regulation of apoptosis. Annu Rev Nutr. 2010;30:201–17. doi: 10.1146/annurev.nutr.28.061807.155509. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt-Mende J, Gogvadze V, Hellström-Lindberg E, Zhivotovsky B. Early mitochondrial alterations in ATRA-induced cell death. Cell death and differentiation. 2006;13:119–28. doi: 10.1038/sj.cdd.4401715. [DOI] [PubMed] [Google Scholar]
- 74.Mrass P, Rendl M, Mildner M, et al. Retinoic acid increases the expression of p53 and proapoptopic caspases and sensitizes ketatinocytes to apoptosis: a possible explanation for tumor preventive action of retinoids. Cancer Res. 2004;64:6542–48. doi: 10.1158/0008-5472.CAN-04-1129. [DOI] [PubMed] [Google Scholar]
- 75.Islam TC, Skarin T, Sumitran S, Toftgård R. Retinoids induce apoptosis in cultured keratinocytes. Br J Dermatol. 2000;143:709–19. doi: 10.1046/j.1365-2133.2000.03823.x. [DOI] [PubMed] [Google Scholar]
- 76.Schug TT, Berry DC, Shaw NS, et al. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–33. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krasinski SD, Kohn JS, Schaefer EJ, Russell RM. Post prandial plasma retinyl ester response is greater in older subjects compared with younger subjects. J Clin Invest. 1990;85:883–92. doi: 10.1172/JCI114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abe R, Yoshioka N, Murata J, et al. Granulysin as a marker for early diagnosis of the Stevens-Johnson Syndrome. Ann Intern Med. 2009;151:514–15. doi: 10.7326/0003-4819-151-7-200910060-00016. [DOI] [PubMed] [Google Scholar]
- 79.Tewary P, Yang D, de la Rosa G, et al. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010;116(18):3465–74. doi: 10.1182/blood-2010-03-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zitvogel L, Kroemer G. The multifaceted granulysin. Blood. 2010;116(18):3379–80. doi: 10.1182/blood-2010-08-299214. [DOI] [PubMed] [Google Scholar]
- 81.Kaspar AA, Okadas S, Kumar J, et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol. 2001;167:350–56. doi: 10.4049/jimmunol.167.1.350. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H, Zhong C, Shi I, et al. Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochrondria through processing of bid leading to Necroptosis. J Immunol. 2009;182(11):6993–7000. doi: 10.4049/jimmunol.0802502. [DOI] [PubMed] [Google Scholar]
- 83.Pardo J, Perez-Galan P, Gamen S, et al. A role of the mitochondrial apoptosis-inducing factor in granulysin-induced apoptosis. J Immunol. 2001;167(3):1222–29. doi: 10.4049/jimmunol.167.3.1222. [DOI] [PubMed] [Google Scholar]
- 84.Dingle T, Lucy JA. Studies on the mode of action of excess vitamin A. The effect of vitamin A on the stability of the erythrocyte membrane. J Biochem. 1962;84:611–21. doi: 10.1042/bj0840611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gery I. Inhibition of DNA and RNA synthesis in lymphocyte cultures by rod outer segments and its counteraction by vitamin E and other antioxidants. Invest Ophthalmol Vis Sci. 1980;19:751–59. [PubMed] [Google Scholar]
- 86.Urano S, Inomori Y, Sugawara T, et al. Vitamin E: inhibition of retinol-induced hemolysis and membrane-stabilizing behavior. J Biol Chem. 1992;267:18365–70. [PubMed] [Google Scholar]
- 87.Hogg AE, Bowick GC, Herzog NK, et al. Induction of granulysin in CD8+ T cells by IL-21 and IL-15 is suppressed by human immunodeficiency virus-1. J Leukoc Biol. 2009;86(5):1191–203. doi: 10.1189/jlb.0409222. [DOI] [PubMed] [Google Scholar]
- 88.DePaolo RW, Abadie V, Tang F, et al. Co-adjuvant effects of retinoic acid and IL-15 promote inflammatory immune responses to dietary antigens. Nature. 2011;471:220–24. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arranz E, Garrote JA. IL-15 modulates the effect of retinoic acid, promoting inflammation rather than oral tolerance to dietary antigens. Expert Rev Gastroenterol Hepatol. 2011;5(3):315–17. doi: 10.1586/egh.11.36. [DOI] [PubMed] [Google Scholar]
- 90.Reddy D, Siegel CA, Sands BE, Kane S. Possible association between isotretinoin and inflammatory bowel disease. Am J Gastroenterol. 2006;101(7):1569–73. doi: 10.1111/j.1572-0241.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 91.Crockett SD, Porter CQ, Martin CF, et al. Isotretinoin use and the risk of inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2010;105(9):1986–93. doi: 10.1038/ajg.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Clin Immunol. 2005;5:309–16. doi: 10.1097/01.all.0000173785.81024.33. [DOI] [PubMed] [Google Scholar]
- 93.Ihara H, Shino Y, Hashizume N, et al. Decline in plasma retinol in unconjugated hyperbilirubinemia treated with bilirubin adsorption using an anion-exchange resin. J Nutr Sci Vitaminol (Tokyo) 1998;44:329–36. doi: 10.3177/jnsv.44.329. [DOI] [PubMed] [Google Scholar]
- 94.Egan CA, Grant WJ, Morris SE, et al. Plasmapheresis as an adjunct treatment in toxic epidermal necrolysis. J Am Acad Dermatol. 1999;40:458–61. doi: 10.1016/s0190-9622(99)70497-4. [DOI] [PubMed] [Google Scholar]