Abstract
The present report investigated the correlation between the expression levels of matrix metalloproteinase (MMP)-9 in gastric carcinoma patients and the clinicopathological characteristics. Forty-five samples of gastric carcinoma and distal gastric mucosa tissue, and 10 samples of healthy gastric mucosa tissue were analyzed using semi-quantitative polymerase chain reaction, as well as immunohistochemical and hematoxylin and eosin staining. MMP-9 protein levels in serum samples from the same patients were quantified by enzyme-linked immunosorbent assay. The present report identified that MMP-9 expression was markedly higher in the gastric carcinoma tissue (86.67%) than in the adjacent healthy tissue (10.00%). A positive association was identified between the level of MMP-9 protein expression and the depth of cancer invasion (P<0.05). Furthermore, the preoperative serum levels of the MMP-9 protein in the gastric carcinoma tissue were correlated with the tumor-node-metastasis stage and occurrence of lymph node metastasis (P<0.01). Data from the present report indicates that MMP-9 may be key in gastric carcinoma malignancy, and implies that MMP-9 may serve as a novel biomarker in the diagnosis and prognosis of gastric carcinoma.
Keywords: gastric carcinoma, matrix metalloproteinases-9, semi-quantitative polymerase chain reaction, immunohistochemistry, malignancy
Introduction
Gastric carcinoma is one of the most common malignancies worldwide. Although the early diagnosis and treatment of gastric cancer has improved in recent years, it remains one of the leading causes of cancer-related mortality in countries, such as China and Japan (1). The aggressive behavior of cancer cells is predominantly attributed to their capacity to invade and metastasize. Metastasis is a complex process that involves the spread of a cancer to a site distant from its origin. Reduced cell-cell adhesion and degradation of the extracellular matrix (ECM) are essential mechanisms for the occurrence of cell invasion and metastasis, leading to the dissemination of a cancer via the blood vessels or the lymph nodes (2).
Matrix metalloproteinase (MMP)-9 is the largest member of the MMPs, an extensive family of zinc-dependent proteolytic enzymes. Previous studies have validated that MMP-9 degrades the principal components of the ECM, collagen types IV and V, and gelatin and, thus, is closely associated with tumor cell invasion and metastasis (3–5). Furthermore, it has been reported that MMP-9 serum levels in the peripheral blood can be used for preoperative prognosis (6,7). The MMP-9 expression level is elevated in non-small cell lung cancer and is associated with lymph node metastasis, although, MMP-9 expression levels are not associated with postoperative survival rate (8). MMP-9 is associated with the growth, invasion and metastasis of gastric carcinoma (9), however, there are few reports regarding preoperative serum levels of MMP-9 in gastric carcinoma. In the current study, the expression of MMP-9 in gastric carcinoma samples was analyzed and the correlation between preoperative serum levels of the MMP-9 protein and the clinicopathological characteristics of gastric carcinoma was investigated.
Patients and methods
Patients and sample collection
Forty-five primary gastric carcinoma patients, who had undergone a gastrectomy at The First Affiliated Hospital of Shantou University Medical College (Shantou, China) between June 2005 and January 2006, were involved in the present study. Thirty cases were male and 15 cases were female, with a mean age of 60.4 years. Fourteen patients had a family history of cancer. None of the patients had received chemotherapy or radiotherapy prior to the surgery and no additional malignancies were evident. All patients were informed of the nature of the study and provided written informed consent, and the study was approved by the ethics commitee of the Medical College of Shantou University.
Peripheral venous blood (3 ml) was collected from each patient prior to the surgery and stored at −75°C for the enzyme-linked immunosorbent assay (ELISA; Sino Biological Inc., Bejing, China). Tumor tissue samples were obtained immediately from the resected specimens and distal healthy gastric mucosa tissue was collected from a distance of >5 cm from the tumor. For immunohistochemistry, the tissue samples were fixed with 10% formalin, dehydrated with gradient alcohol, cleared with xylene and embedded in paraffin. In addition, 10 specimens of peripheral serum and gastric mucosa tissue from 10 healthy individuals were randomly selected to serve as controls. Classification of the tumors was conducted according to the tumor-node-metastasis (TNM) staging system issued by the International Union Against Cancer in 1997 (10).
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis of MMP-9 expression
Total RNA was extracted using TRI Reagent® (Molecular Research Center, Inc., Cincinnati, OH, USA) followed by complementary DNA synthesis using the Moloney murine leukemia virus reverse transcriptase (Thermo Fisher Scientific, Pittsburgh, PA, USA). PCR was performed using Taq polymerase (Takara Biotechnology Co., Ltd., Dalian, China) under the following conditions: 94°C for 2 min; 22 cycles (for β-actin) or 32 cycles (for MMP-9) of 94°C for 30 sec, 57°C for 30 sec and 72°C for 40 sec; followed by 72°C for 5 min. β-actin served as an internal control. The PCR product lengths for MMP-9 and β-actin were 462 and 592 bp, respectively and were sequenced by Invitrogen Biotechnology Co., Ltd. (Shanghai, China). The primers for the PCR were as follows: Sense, 5′-CACCCTTGTGCTCTTCCCTG-3′ and anti-sense, 5′-GGATACCCGTCTCCGTGCT-3′ for MMP-9; sense, 5′-CCAAGGCCAACCGCGAGAAGATGAC-3′ and anti-sense, 5′-AGGGTACATGGTGGTGCCGCCAGAC-3′ for β-actin. Each PCR product was examined by agarose gel electrophoresis and images were captured using a digital camera (PowerShot A2400; Canon Inc., Tokyo, Japan). The protein band intensities were analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemical staining of MMP-9
Serial sections were cut from the paraffin-embedded tissues at a thickness of 5 μm, and routine hematoxylin and eosin (H&E) staining was conducted prior to immunohistochemical staining. The staining procedure was performed according to the immunohistochemical EliVision™ two-step method (11) and a tissue section with a known MMP-9 expression level served as the positive control. Staining with phosphate-buffered saline, as a substitute for the primary antibody, served as the negative control. Cells exhibiting a brown-stained cytoplasm, at a higher intensity than the non-specific background staining, were regarded as positively stained and tumors exhibiting positive staining in >10% of the total number of tumor cells were regarded as positive for MMP-9 expression. The human monoclonal anti-MMP-9 and poly-horseradish peroxidase secondary antibodies were purchased from Maxim Biotechnology Development Co., Ltd. (Fuzhou, China).
ELISA of MMP-9
The concentration of the MMP-9 protein in the peripheral serum was measured using a double-antibody sandwich ELISA method (Wuhan Boster Biological Technology, Ltd, Wuhan, China) according to the manufacturer’s instructions. Following coloration with 3,3′-diaminobenzidine, the optical density value was measured at a wavelength of 450 nm and a standard curve was determined to calculate the concentration of MMP-9 protein.
Statistical analysis
Statistical analysis was performed using SPSS software (version 10.0; SPSS, Inc., Chicago, IL, USA). Student’s t-test was applied for data analysis between the two groups, χ2 and Fisher’s exact tests were applied for numeration data, and a Pearson correlation coefficient was used to analyze the correlation between gene expression and the protein level. P<0.05 was considered to indicate a statistically significant difference.
Results
MMP-9 expression levels in tissue samples
Semi-quantitative RT-PCR (Fig. 1) identified MMP-9 expression in 48.9% (22/45) of the gastric carcinoma tissues, 13.3% (6/45) of the distal gastric mucosa tissues (>5 cm from the gastric tumor) and 10% (1/10) of the healthy gastric mucosa control tissues. The mean level of MMP-9 mRNA in the gastric carcinoma tissues (0.42±0.65) was significantly higher than in the distal (0.22±0.76; P<0.05) and control tissues (0.03±0.06; P<0.05; Table I).
Figure 1.
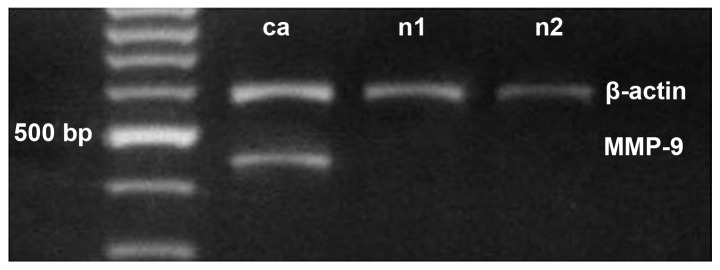
mRNA expression of MMP-9 and β-actin extracted from gastric carcinoma tissue (ca), distal gastric mucosa tissue (n1) and healthy gastric tissue (n2). MMP-9, matrix metalloproteinase-9.
Table I.
Matrix metalloproteinase-9 parameters of the gastric carcinoma, distal tissue and control groups (mean ± standard deviation).
| Group | Cases, n | mRNA value | Positive expression, n (%) | Protein concentration, μg/ml |
|---|---|---|---|---|
| Gastric carcinoma | 45 | 0.42±0.65a | 39 (86.67)a | 0.41±0.26a |
| Distal tissue | 45 | 0.22±0.76 | 12 (26.67)b | |
| Control | 10 | 0.03±0.06 | 1 (10.00)b | 0.15±0.04 |
P<0.01 vs. the control group;
P<0.05 vs. the distal tissue and control group.
H&E (Fig. 2) and immunohistochemical (Fig. 3) staining for MMP-9 was observed in the majority of gastric carcinoma tissues. The expression rate of MMP-9 was 86.67% (39/45) in the gastric carcinoma tissues, 26.67% (12/45) in the distal gastric mucosa tissues and 10% (1/10) in the healthy gastric mucosa tissues (Table I). Correlation between MMP-9 expression levels and the clinicopathological characteristics of the tumor revealed that patients with seromembranous invasion had a significantly higher proportion of MMP-9-positive tumor cells (92.31%; 36/39) than patients without seromembranous invasion (50.00%; 3/6) (P<0.05). However, no significant difference in the levels of MMP-9 expression was identified with respect to the tumor size, family history of tumors, TNM stage, or occurrence of lymph node or distant metastasis (Table II).
Figure 2.
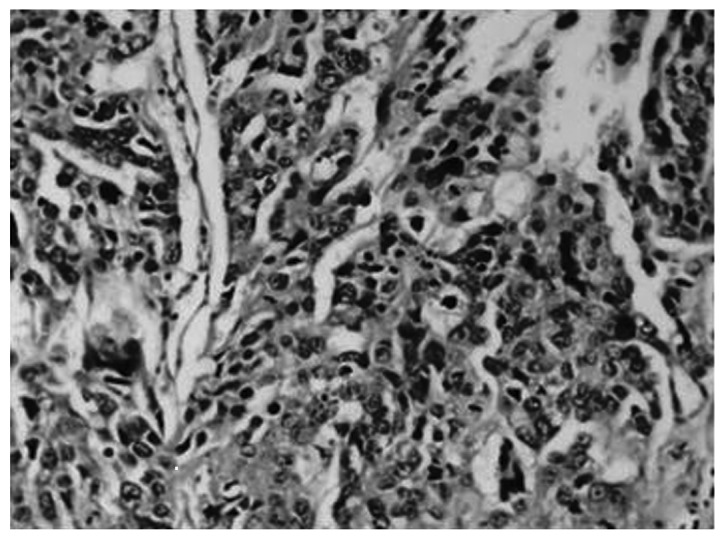
Hematoxylin and eosin staining of matrix metalloproteinase-9 in gastric carcinoma tissue, as shown by light gray grains in the cytoplasm (magnification, ×200).
Figure 3.
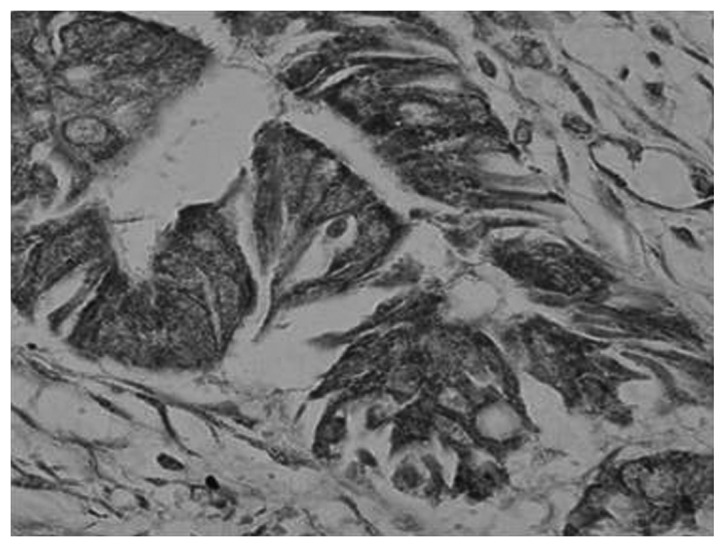
Immunohistochemical staining of matrix metalloproteinase-9 in gastric carcinoma tissue, as shown by dark grains in the cytoplasm (magnification, ×400).
Table II.
Correlation between the MMP-9 protein levels in the peripheral serum and clinicopathological features (mean ± standard deviation).
| Feature | Cases, n | Mean gray value | Positive rate of MMP-9 protein, % (n) | Serum (μg/ml) |
|---|---|---|---|---|
| Tumor size, cm | ||||
| <5 | 22 | 0.47±0.61 | 81.82 (18/22) | 378.14±278.61 |
| ≥5 | 23 | 0.75±0.59 | 91.30 (21/23) | 432.71±238.05 |
| Family history of tumor | ||||
| Negative | 31 | 0.55±0.56 | 83.87 (26/31) | 391.81±283.28 |
| Positive | 14 | 0.75±0.72 | 92.86 (13/14) | 437.52±192.69 |
| Affected region | ||||
| Upper part | 21 | 0.48±0.44 | 85.71 (18/21) | 450.39±289.75 |
| Middle-lower part | 24 | 0.73±0.72 | 87.5 (21/24) | 367.22±223.97 |
| Differentiation | ||||
| Well and moderate | 27 | 0.60±0.62 | 81.48 (22/27) | 380.22±257.50 |
| Poor or none | 18 | 0.79±0.61 | 94.44 (17/18) | 444.75±259.12 |
| Depth of invasion | ||||
| No seromembranous invasion | 6 | 0.25±0.28 | 50.00 (3/6)b | 303.47±202.06 |
| Seromembranous invasion | 39 | 0.67±0.63 | 92.31 (36/39)b | 421.81±250.33 |
| TNM stage | ||||
| I + II | 23 | 0.54±0.60 | 78.26 (18/23) | 305.22±223.99a |
| III + IV | 22 | 0.68±0.63 | 95.45 (21/22) | 511.42±251.52a |
| Lymphatic metastasis | ||||
| Negative | 21 | 0.58±0.61 | 85.71 (18/21) | 300.30±205.62a |
| Positive | 24 | 0.64±0.62 | 87.50 (21/24) | 498.55±265.77a |
| Distant metastasis | ||||
| Absent | 43 | 0.59±0.62 | 86.05 (37/43) | 396.28±254.94 |
| Present | 2 | 1.00±0.11 | 100.00 (2/2) | 615.72±297.53 |
MMP-9, matrix metalloproteinase-9; TNM, tumor-node-metastasis.
P<0.01 and
P<0.05.
Correlation between preoperative serum MMP-9 protein levels and clinicopathological features of gastric carcinoma
The mean preoperative serum MMP-9 protein levels in gastric carcinoma patients (0.41±0.26 μg/ml) was significantly higher than those of the control group (0.15±0.04 μg/ml; P<0.05) (Table I). In addition, the serum concentration of MMP-9 protein in patients with an advanced TNM stage (511.42±251.52), and lymph node metastasis (498.55±265.77) was significantly higher than that in the patients at an early TNM stage (305.22±223.99) and exhibiting no lymph node metastasis (300.30±205.62), respectively (P<0.01). However, there was no significant correlation between the serum MMP-9 protein level and other clinicopathological characteristics, such as the tumor size, a family history of tumors, localization of the tumor, tumor differentiation, degree of invasion or distal metastasis (Table II).
Correlation between MMP-9 expression levels in gastric carcinoma tissues and preoperative serum MMP-9 protein levels
The expression level of MMP-9 in gastric carcinoma tissues and the preoperative serum was higher when compared with the healthy tissues. However, the correlation between MMP-9 mRNA levels in gastric carcinoma tissues and the preoperative serum MMP-9 levels was not identified to be statistically significant (r=0.13; P=0.394).
Discussion
Degradation of the ECM and basement membranes is a critical step in tumor invasion and metastasis. The process of metastasis depends on the activity of various proteolytic enzymes, particularly serine and cysteine proteases, and MMP. MMPs, a family of closely associated enzymes that degrade the ECM, are considered to be important in facilitating tumor invasion and metastasis, and are associated with tumor invasion, lymph node metastasis and survival of gastric carcinoma (12). Previously, studies have identified a significant correlation between the expression of MMP-2, -7 and -9, and the depth of tumor invasion, vessel invasion, lymph node and distant metastasis, TNM stage and microvessel density, indicating that these markers may be significant in cell migration in gastric carcinoma (13–15). Zymographic analysis of the expression levels of MMP-9 and active MMP-2 demonstrated a proportional increase with tumor grade and invasiveness in bladder cancer (16). MMP-9 mRNA expression levels in lymphocytes tended to be higher in malignant pleural effusions of lung cancer when compared with healthy pleural effusions (17). Consistent with the findings of the above-mentioned reports, the present study demonstrated that MMP-9 was highly expressed in gastric carcinoma tissues and preoperative serum when compared with distal and healthy tissue.
Correlation between the MMP-9 expression levels and the clinicopathological parameters of tumors did not identify any significant difference, with the exception of the depth of tumor invasion. The incidence of MMP-9 positive expression was observed to be greater in tumors with seromembranous invasion compared with non-invasive tumors, indicating that MMP-9 may contribute to invasion and tissue infiltration in gastric carcinoma. Furthermore, the present study identified that patients with gastric carcinoma exhibited higher preoperative peripheral serum MMP-9 levels compared with the control groups, and demonstrated that the serum MMP-9 levels correlated with the TNM stage and occurrence of lymph node metastasis. These data indicate that serum protein expression levels may contribute to the malignant tumor phenotype, as reported in previous studies (15,18). MMP-9 mRNA and serum expression were upregulated in gastric carcinoma, however, the present study did not observe a correlation between MMP-9 expression levels in the gastric carcinoma tissues and those in the preoperative serum. This may be due to mechanisms, in addition to the influence of cancerous tissue on serum MMP-9 protein secretion (19), such as the action of macrophages, leucocytes and other inflammatory cells (20,21). In the present study, it was identified that MMP-9 expression tended to be higher in patients with a positive family history of cancer compared with patients without, however, no significant correlation was observed. Additionally, the present study demonstrated that MMP-9 expression levels in distal tissues were significantly higher than those of the healthy control group, indicating that abnormal gene activation may be involved, possibly in a similar manner to MMP-9 overexpression in benign gastric lesions (22).
Previous investigations, regarding how differences between plasma and serum samples influence the diagnostic and prognostic performance of MMP-9, identified that plasma MMP-9 levels were elevated in gastric cancer patients when compared with control subjects, whilst serum MMP-9 levels did not differ between the groups (23–25). By contrast, in non-small cell lung cancer, serum MMP-9 levels were demonstrated to be higher in patients with metastatic cancer (26,27), indicating that detection of the preoperative plasma MMP-9 expression level may serve as an improved marker for tumor progression when compared with serum MMP-9 levels.
In conclusion, the present study demonstrated that MMP-9 protein levels in the preoperative serum and mRNA expression in carcinoma tissue were higher than the healthy controls, and were correlated with specific clinicopathological features of gastric carcinoma. The data indicates that MMP-9 has potential as a diagnostic marker and therapeutic molecular target for management of gastric carcinoma.
Acknowledgements
The present study was supported by the Science and Technology Planning Project of Guangdong Province, China (grant no. 2004B31201012).
References
- 1.Wei F, Huang P, Li S, et al. Enhancement patterns of gastric carcinoma on contrast-enhanced ultrasonography: relationship with clinicopathological features. PLoS One. 2013;8:e73050. doi: 10.1371/journal.pone.0073050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paz H, Pathak N, Yang J. Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene. 2014;33:4193–4202. doi: 10.1038/onc.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iizasa T, Fujisawa T, Suzuki M, et al. Elevated levels of circulating plasma matrix metalloproteinase 9 in non-small cell lung cancer patients. Clin Cancer Res. 1999;5:149–153. [PubMed] [Google Scholar]
- 4.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337–344. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 5.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 6.Tan SY, Wang JY, Shen L, Luo HS, Shen ZX. Relationship between preoperative staging by endoscopic ultrasonography and MMP-9 expression in gastric carcinoma. World J Gastroenterol. 2007;13:2108–2112. doi: 10.3748/wjg.v13.i14.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylisirniö S, Höyhtyä M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases -2, -9 and tissue inhibitors of metalloproteinases -1, -2 in lung cancer - TIMP-1 as a prognostic marker. Anticancer Res. 2000;20:1311–1316. [PubMed] [Google Scholar]
- 8.Czyzewska J, Guzińska-Ustymowicz K, Kemona A, Bandurski R. The expression of matrix metalloproteinase 9 and cathepsin B in gastric carcinoma is associated with lymph node metastasis, but not with postoperative survival. Folia Histochem Cytobiol. 2008;46:57–64. doi: 10.2478/v10042-008-0007-6. [DOI] [PubMed] [Google Scholar]
- 9.Zheng H, Takahashi H, Murai Y, et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–3583. [PubMed] [Google Scholar]
- 10.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(SICI)1097-0142(19971101)80:9<1803::AID-CNCR16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Peng SH, Deng H, Yang JF, et al. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol. 2005;11:6521–6524. doi: 10.3748/wjg.v11.i41.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo K, Maehara Y, Baba H, et al. Elevated levels of serum and plasma metalloproteinases in patients with gastric cancer. Anticancer Res. 1997;17:2253–2258. [PubMed] [Google Scholar]
- 13.Alakus H, Grass G, Hennecken JK, et al. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–923. doi: 10.14670/HH-23.917. [DOI] [PubMed] [Google Scholar]
- 14.Lee KH, Shin SJ, Kim KO, et al. Relationship between E-cadherin, matrix metalloproteinase-7 gene expression and clinicopathological features in gastric carcinoma. Oncol Rep. 2006;16:823–830. [PubMed] [Google Scholar]
- 15.Lee LY, Wu CM, Wang CC, et al. Expression of matrix metalloproteinases MMP-2 and MMP-9 in gastric cancer and their relation to claudin-4 expression. Histol Histopathol. 2008;23:515–521. doi: 10.14670/HH-23.515. [DOI] [PubMed] [Google Scholar]
- 16.Papathoma AS, Petraki C, Grigorakis A, et al. Prognostic significance of matrix metalloproteinases 2 and 9 in bladder cancer. Anticancer Res. 2000;20:2009–2013. [PubMed] [Google Scholar]
- 17.Park KJ, Hwang SC, Sheen SS, Oh YJ, Han JH, Lee KB. Expression of matrix metalloproteinase-9 in pleural effusions of tuberculosis and lung cancer. Respiration. 2005;72:166–175. doi: 10.1159/000084048. [DOI] [PubMed] [Google Scholar]
- 18.Torii A, Kodera Y, Uesaka K, et al. Plasma concentration of matrix metalloproteinase 9 in gastric cancer. Br J Surg. 1997;84:133–136. doi: 10.1002/bjs.1800840147. [DOI] [PubMed] [Google Scholar]
- 19.Unemori EN, Hibbs MS, Amento EP. Constitutive expression of a 92-kD gelatinase (type V collagenase) by rheumatoid synovial fibroblasts and its induction in normal human fibroblasts by inflammatory cytokines. J Clin Invest. 1991;88:1656–1662. doi: 10.1172/JCI115480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 21.Herman MP, Sukhova GK, Libby P, et al. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 22.Bergin PJ, Raghavan S, Svensson H, et al. Gastric gelatinase B/matrix metalloproteinase-9 is rapidly increased in Helicobacter felis-induced gastritis. FEMS Immunol Med Microbiol. 2008;52:88–98. doi: 10.1111/j.1574-695X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu CY, Wu MS, Chiang EP, et al. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin Cancer Res. 2007;13:2054–2060. doi: 10.1158/1078-0432.CCR-06-2299. [DOI] [PubMed] [Google Scholar]
- 24.Dragutinović VV, Radovanović NS, Izrael-Zivković LT, Vrvić MM. Detection of gelatinase B activity in serum of gastric cancer patients. World J Gastroenterol. 2006;12:105–109. doi: 10.3748/wjg.v12.i1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerlach RF, Uzuelli JA, Souza-Tarla CD, Tanus-Santos JE. Effect of anticoagulants on the determination of plasma matrix metalloproteinase (MMP)-2 and MMP-9 activities. Anal Biochem. 2005;344:147–149. doi: 10.1016/j.ab.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Laack E, Scheffler A, Burkholder I, et al. Pretreatment vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP-9) serum levels in patients with metastatic non-small cell lung cancer (NSCLC) Lung Cancer. 2005;50:51–58. doi: 10.1016/j.lungcan.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Shimanuki Y, Takahashi K, Cui R, et al. Role of serum vascular endothelial growth factor in the prediction of angiogenesis and prognosis for non-small cell lung cancer. Lung. 2005;183:29–42. doi: 10.1007/s00408-004-2521-4. [DOI] [PubMed] [Google Scholar]


