Abstract
Objectives
Individuals with anorexia nervosa (AN) and bulimia nervosa (BN) tend to have disordered thinking and eating behaviours in regards to fat containing foods. This is the first study to investigate neuronal pathways that may contribute to altered fat consumption in eating disordered patients.
Methods
We used functional magnetic resonance imaging (fMRI) to compare responses to a high-fat cream stimulus, water, and a non-caloric viscous stimulus (CMC) to control for response to viscosity in individuals recovered from AN (N = 15), BN (N = 14) and a healthy control sample (CW, N = 18).
Results
An interaction analysis (ANOVAR) comparing the three groups (AN, BN, CW) and the three conditions (cream, CMC, water) revealed significant differences in the left anterior ventral striatum (AVS). A post hoc analysis displayed a higher magnitude of response for the contrast cream/water in BN compared to AN or CW and for the contrast CMC/water in BN compared to AN.
Conclusions
BN showed an exaggerated AVS response for the cream/water contrast in comparison to AN or CW. Moreover, BN showed an exaggerated AVS response for the CMC/water contrast in comparison to AN. These findings support the possibility that BN have an altered hedonic and/or motivational drive to consume fats.
Keywords: Anorexia nervosa, bulimia nervosa, fMRI, striatum, fat
Introduction
Anorexia nervosa (AN) and bulimia nervosa (BN) are disorders of multidimensional aetiology that tend to affect young women (APA 2000). They are characterized by extreme eating behaviour and distorted body image, and have high rates of chronicity, morbidity, and mortality. AN is also characterized by severe emaciation. Two types of eating behaviours are seen in AN: in restricting-type anorexia, weight loss is accomplished purely via dietary restriction without incidence of binge-eating/purging, whereas in binge-eating/purging-type anorexia, individuals restrict their food intake to lose weight, but also experience periodic disinhibition of restraint and engage in binge eating and/or purging. Individuals with bulimia nervosa do not become emaciated and are able to maintain an average body weight (ABW) above 85%. The term EDNOS is used for patients, who meet some, but not all DSM-IV criteria needed for AN or BN. Lifetime-prevalence estimates for AN and BN were reported at approximately 0.9 and 1.5%, respectively (Hudson et al. 2007). Despite the behavioural categorizations of AN and BN, little is known about the strong urges and food choices made by people suffering from these diseases. However, it is well known, that individuals with AN dislike high-fat or high-sweet food and tend to “fear fat” (Fernstrom et al. 1994). Individuals with BN tend to binge on sweet, high-fat foods (Weltzin et al. 1991) but avoid fats when not bingeing. These behaviours are in contrast to healthy people who tend to experience high-fat and sweet food as pleasant (Drewnowski and Greenwood 1983).
In the past decade, a series of studies have used functional imaging to investigate the neuronal response to pictures of food in ill as well as recovered AN and BN subjects. These studies show altered activation of brain regions implicated in feeding behaviour such as the insula, frontal, mesial/temporal, parietal and anterior cingulate cortex (Ellison et al. 1998; Naruo et al. 2000; Gordon et al. 2001; Uher et al. 2003, 2004; Santel et al. 2006; Schienle et al. 2008; Gizewski et al. 2010; Brooks et al. 2011; Joos et al. 2011). An early study differentiated between AN and BN by finding group differences in hypo- and hyperactivity of cerebral blood flow in frontal and parietal areas prior to meal ingestion (Nozoe et al. 1993). More recently, individuals with AN were found to have aberrant gustatory responses to tastes of sucrose, relative to controls, in AN in pathways thought to modulate the sensory-reward response to sugar (Wagner et al. 2008). A different study assessing reward association learning in BN using a comparison of conditioned visual and unconditioned sweet taste stimuli also showed altered activation of the insula, orbital frontal cortex and ventral putamen (Frank et al. 2011).
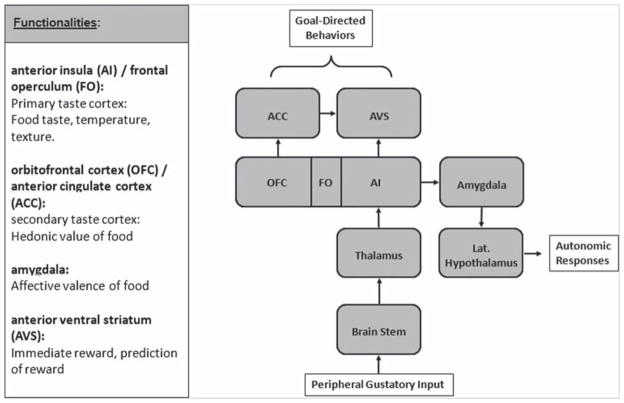
To our knowledge, there have been no imaging studies that have explored the gustatory response to dietary fat in AN and BN. In humans, our understanding of the higher-order neuronal pathways that contribute to the sensory, hedonic, and incentive motivational aspects of fat consumption is more limited than our respective understanding of sugars (Mitzushige et al. 2007). In the case of sucrose, a pathway has been specified in which taste receptors in the tongue (Kitagawa et al. 2001), which recognize a sweet taste, send signals via cranial nerves through the brain stem to thalamic afferents, which then project to the primary gustatory cortex, that in humans comprises the frontal operculum and the anterior insula (FO/AI) (Ogawa 1994; Schoenfeld et al. 2004). Projections from the FO/AI reach the amygdala, and from there, extend to the lateral hypothalamus and midbrain dopaminergic regions (Simon et al. 2006). The primary taste cortex also projects to the striatum (Fudge et al. 2005). The AI is contiguous with the posterior orbitofrontal cortex (OFC) at the operculum. This region is reciprocally connected with the medial prefrontal cortex which includes the anterior cingulate cortex (ACC). The anterior ventral striatum (AVS) receives input from the FO/AI and ACC (Fudge et al. 2005). The primary taste cortex provides representations of taste, temperature and texture of food independent of the reward value (Rolls 2005). The OFC and ACC are linked to pleasant properties of food (Rolls et al. 2003) while other studies suggest overlapping representations of sensory and hedonic processing of taste in the AI and OFC (Small et al. 2001). The AVS is thought to translate sensory-interoceptive-hedonic aspects of feeding into motivated motor behaviour (approach or avoidance) of highly palatable foods (Kelley et al. 2002). Animal studies indicate that dopamine in the striatum and putamen corresponds to motivational aspects of stimuli (Montague et al. 2004). The amygdala is associated with affective valence of food (Rolls 2005) (Figure 1).
Figure 1.
Simplified model of functional segregation of gustatory processing. ACC, anterior cingulated cortex; AI, anterior insula; AVS, anterior ventral striatum; FO, frontal operculum; OFC, orbitofrontal cortex.
Unlike for the classical tastes such as sweet, sour, salty, bitter the mechanism underlying the network between the oral cavity, gastrointestinal tract including hormonal responses (i.e. grehlin), and brain that processes fat is poorly understood (Mitzushige et al. 2007; Bello et al. 2010). Still, there appears to be a substantial overlap of pathways in the central processing of fat and sucrose: studies show (De Araujo and Rolls 2004) that fat taste activates regions, such as the AI, OFC, ACC and AVS, that are also activated by sucrose. We decided to use these brain regions as a basis for our regions of interest (ROI) based approach.
Importantly, differences of central processing between fat and classical tastes need to be considered: It is not clear (De Araujo and Rolls 2004; Grabenhorst et al. 2009) whether the brain responds to the caloric content of fat or senses the texture and/ or viscosity of fat molecules. In other words, the taste and non-taste AI (posterior primary taste cortex) in humans is activated by oral sensing of viscosity. In order to disentangle the taste of fat with caloric content compared to non-caloric viscous properties, this study compared the neuronal response to whipping cream, a high-fat stimulus, to carboxymethyl cellulose mix (CMC), a fluid as viscous as cream. In addition, we included water as a neutral stimulus comparison. We also performed a whole brain analysis to confirm that regions of the gustatory pathways were activated by our paradigm. Anxiety (to gain weight) and pleasantness ratings were collected for each of the taste stimuli in order to better understand the subjective perception of the taste solutions.
In order to avoid the confounding effects of altered nutritional state caused by acute illness, we studied recovered AN and BN subjects. It is important to emphasize that core temperament and personality traits persist after recovery from both AN and BN and are similar to the symptoms described premorbidly in childhood (Wagner et al. 2006). There is evidence, that aberrant neuronal responses to oral stimuli similarly persist after recovery (Uher et al. 2003 #1912; Cowdrey et al. 2011) even though the extent of activation might differ depending on the state (recovery or acute illness) (Uher et al. 2003). These psychophysiological disturbances are likely to be trait-related, but even if they are “scars” they are still likely to help understand the processes contributing to these disorders.
Material and methods
Sample collection
We examined 15 formerly anorexic and 14 formerly bulimic women, who were recovered for at least one year. All patients were diagnosed and previously treated in the eating disorder (ED) program at the Department of Child and Adolescent Psychiatry of the J.W. Goethe University in Frankfurt. All AN individuals met previously DSM IV criteria for the restricting type, four of the BN patients had an antecedence of AN. Recovery was defined as having maintained a normal body mass index, having had regular menstrual cycles and not having engaged in pathological eating behaviour (no restrictive eating patterns, no binge eating or purging, no excessive exercise). Participants had not used psychoactive medications or met criteria for drug or alcohol abuse or dependence, major depressive disorder, psychotic symptoms or severe anxiety disorder within 3 months prior to the study.
Eighteen healthy normal weight control women (CW) were recruited through local advertisement. The CW had no history of an eating disorder or any psychiatric, serious medical or neurological illness. They had no first-degree relatives with an eating disorder. They had normal menstrual cycles and had been within normal weight range since menarche. CW were not on medication.
The MR imaging was performed during the first 10 days of the follicular phase for all subjects to standardize phase of cycle. The MR study was done at 11:00 h. All subjects had the same standardized breakfast (bread, butter, marmalade, milk/juice; no coffee) in the morning (07:00 h) of the study. This design was chosen to standardize the subjects’ state of satiety as “mildly hungry”.
This study was conducted according to the institutional review board regulations of the J.W. Goethe-University of Frankfurt. Written informed consent was obtained from all participants prior to scanning.
Assessments
Axis I psychiatric diagnoses were assessed with the Structured Clinical Interview for DSM-IV Axis I disorders (First et al. 1996). A comprehensive description of the battery is described elsewhere (Wagner et al. 2006). In brief, current symptoms were assessed by the Beck Depression Inventory (BDI) (Beck et al. 1961), Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (Goodman et al. 1989), State Trait Anxiety Inventory (STAI-Y) (Spielberger et al. 1970), Temperament and Character Inventory (TCI) (Cloninger et al. 1994) and the Eating Disorder Inventory (EDI-2) (Garner et al. 1983).
To test the overall ability to perceive different fat intensities, subjects were asked to line up commercially available products by rising concentrations of fat (0.1, 3, 10 and 30%).
After scanning, subjects were asked to provide subjective pleasantness ratings for each individual taste (using a scale from +3 = very pleasant, 0 = neutral, through to −3 = very unpleasant). In addition, they were asked how anxious they got (+3 = very anxious, 0 = no effect, −3 = calm) and how much they felt like they were gaining weight or becoming fat (+3 = very fat, 0 = no effect, −3 = very thin).
Experimental paradigm
In the present paradigm three different taste solutions were used as stimuli. The high-fat stimulus was whipping cream (Ja™-Sahne, Hochwald Nahrungsmittel-Werke, Hungen, Germany; 30% wt/ wt fat, 3% wt/wt sugar), a commercial product, which subjects are familiar with from everyday life. A comparison viscosity solution was produced with CMC (Wolff Cellulosics, Walsrode, Germany), a tasteless thickening agent commonly found in commercially produced food. CMC was dissolved in water (Evian™, Danone Waters, Wiesbaden, Germany) and sweetened adequately to be comparable to the whipping cream (0.6% wt/wt CMC and a 3% wt/wt sugar level). In order to use the same solvent, tasteless water was used as a control solution.
Stimuli were delivered into the subject’s mouth via separate Tefl on™ tubes (Braun AG, Melsungen, Germany). The tubes were connected to one common mouth piece and placed in the buccal region.
Before scanning sessions, the tubes were filled with the respective stimuli by means of syringes. These remained connected to the tubing via three-way valves and non-return valves, enabling replenishment of the syringes and blockage as well as controlled delivery (Hummel et al. 2007). Each trial was defined by the following sequence: cue (4 s), application of fluid (2 s), scanning (22 s), rinsing (4 s), break (16 s). Each taste stimulus consisted of 2 ml volume of liquid given as a bolus; in between stimulations, the subject’s mouth was rinsed by 5 ml of water in order to prevent smearing effects and enhance distinction of the taste stimuli. Subjects were instructed to swallow only during the “rinse” condition. During one block, each stimulus was presented five times in a pseudorandomized order. As four runs were presented, each condition was assessed by 20 scans.
Image procedures
Whole-brain magnetic resonance imaging (MRI) data were collected on a TRIO Magnetom 3.0 T scanner (Siemens, Erlangen, Germany). Echo-planar imaging data for functional MRI (fMRI) were acquired using standard parameters (z-shim-sequence, angulation fixed at 30°; field of view 200 mm; matrix 64 × 64; 1 volume = 30 axial slices, 2 mm slice thickness; in-plane resolution 3.1 mm; repetition time (TR) 2250 ms; echo time (TE) 4.38 ms; flip angle 80°; 363 volumes per run).
Stimulus presentation was synchronized with the fMRI sequence at the beginning of each trial by using Presentation™ (Neurobehavioral Systems Inc., Albany, Canada). In order to reduce possible effects of T1-saturation, five dummy volumes were obtained before each run. To minimize head motion, fixed head pads were used. A T1 weighted 3D anatomical scan (1 × 1 × 1 mm3 resolution) was obtained for each participant.
Data preprocessing and analysis
The fMRI data were preprocessed and analyzed for the regions of interest analysis and the whole brain analysis using BrainVoyager™ QX (Brain Innovation, Maastricht, The Netherlands, Version 1.10.4.1250). The following pre-processing steps were applied: slice-time correction, motion correction, linear trend removal, highpass temporal filtering with two cycles in time course and spatial smoothing using a Gaussian kernel of 7 mm full-width at half-maximum. 3D anatomical scans were transformed into Talairach space (Talairach and Tournoux 1988) and the parameters for this transformation were subsequently used to transform the coregistered functional data. The 3D functional data set was then re-sampled to a voxel size of 3 × 3 × 3 mm3.
We found that cerebral response to a gustatory stimulus is longer (Wagner et al. 2008) than to visual stimuli. Thus we defined the predictors as a trial duration of 12 s. The baseline was defined as a 10 second time-interval with a maximum delay to oral stimuli or oral rinsing. We defined two predictors of no interest: time of the rinsing-procedure and all remaining volumes. We convolved all predictors with a 2-gamma hemodynamic response function.
The primary a priori ROI analysis focused on brain regions, selected from a literature review in fMRI studies on healthy subjects using a fat food stimulus as well as on ED subjects using a sweet taste stimulus or reward paradigm that matched the pathway hypothesis (Table I). We extracted ROI beta-values of the underlying GLM in a 4 voxel radius centred at the Talairach coordinates for each subject during each condition.
Table I.
Regions of interests (ROI).
| ROI | Talairach coordinate | Literature |
|---|---|---|
| Anterior insula | −41/5/5 | Wagner et al. 2008 |
| −36/18/4 | De Araujo and Rolls 2004 | |
| −28/14/−6 | De Araujo and Rolls 2004 | |
| Orbitalfrontal cortex | 32/34/−14 | Grabenhorst et al. 2009 |
| 28/30/−10 | De Araujo and Rolls 2004 | |
| Anterior cingulate cortex | −6/44/−2 | De Araujo and Rolls 2004 |
| −10/48/−2 | De Araujo and Rolls 2004 | |
| 2/30/14 | Grabenhorst et al. 2009 | |
| Anterior ventral striatum | −8/16/−2 | De Araujo and Rolls 2004 |
| −20/8/0 | Wagner et al. 2008 | |
| −8/20/16 | Grabenhorst et al. 2009 | |
| −12/22/−16 | Grabenhorst et al. 2009 | |
| −10/6/−5 | Wagner et al. 2007 | |
| 10/6/−5 | Wagner et al. 2007 | |
| Caudate nucleus | −15/18/12 | Stice et al. 2008 |
| −12/3/27 | Stice et al. 2008 | |
| 11/16/7 | Wagner et al. 2007 | |
| −12/15/7 | Wagner et al. 2007 | |
| Amygdala | 24/0/−12 | Grabenhorst et al. 2009 |
Repeated measures analysis of variance (ANOVAR) examined main effects (group, condition) and group by condition interactions across the 19 ROIs. Probability levels were adjusted to an overall family-wise error rate of 0.05. Covariate analysis (ANCOVA) controlled for clinical variables of age, BMI, age of onset, length of recovery, BDI rating, anxiety ratings (STAI-Y) for the significant ROIs.
Post hoc analyses examined three contrast deltas computed for cream/water, cream/CMC and CMC/ water. The contrast delta was defined as the difference between two conditions representing a magnitude of change among stimuli. One-way ANOVAs compared the mean contrast between groups. Post hoc pairwise comparisons were done using the Tukey–Kramer Multiple Comparison Test.
Probability levels were adjusted to an overall family-wise error rate of 0.05 using the Hochberg procedure, a “step-up” modified Bonferroni method for multiple comparisons (Hochberg 1988). Data analyses were performed using SAS™ 9.2 (Cary, NC).
A secondary whole brain voxelwise analysis was also performed with BrainVoyager™ QX. Correction for multiple comparisons was achieved by cluster-level thresholding using the monte carlo simulation tool implemented in Brain Voyager™ (Goebel et al. 2006), which is based on a 3D extension of the randomization procedure described by Forman et al. (1995). The minimum cluster size threshold that yielded a cluster-level false-positive rate of P = 0.001 was applied to the statistical maps.
First, main effects were analyzed comparing each stimulus versus baseline. Secondly, a two-way ANOVA with group as a between subjects factor (two levels) and with stimuli as a within subject factor (three levels) was performed. A post hoc comparison was calculated for each contrast.
Clinical behavioural data analyses were conducted using PASW™ version 18.0 (IBM, Chicago, IL).
Results
Demographic variables and clinical behavioural assessments
All recovered subjects and CW were of similar age and BMI (Table II). AN had an earlier age of onset in comparison to BN (13.4 ± 1.4 vs. 15.1 ± 2.0 years), but were longer recovered (72.4 ± 31.3 vs. 41.1 ± 28.0 months). Compared to CW, AN and BN individuals had significantly higher values for harm avoidance, depression, trait anxiety and overall eating disorder-related symptoms (EDI). Lifetime history (but not current) for the AN included 14 with major depressive disorder (seven for BN), four with obsessive compulsive disorder, three with panic disorder and four with social phobia. In both recovered groups one subject had a lifetime history of alcohol abuse/dependency, five (in AN) and two (in BN) of drug abuse/dependency, respectively.
Table II.
Demographic and eating related variables. Group comparisons by one-way analysis of variance (ANOVA) are presented.
| CW (n = 18)
|
AN (n = 15)
|
BN (n = 14)
|
F | P | Group differences | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||
| Study age (years) | 24.7 | 3.1 | 25.2 | 4.0 | 24.5 | 2.4 | 0.188 | 0.830 | |
| Current BMI (kg/m 2) | 21.5 | 1.8 | 21.0 | 2.4 | 20.6 | 2.1 | 0.819 | 0.447 | |
| Highest past BMI (kg/m 2) | 22.4 | 2.1 | 22.5 | 2.6 | 22.9 | 2.1 | 0.255 | 0.776 | |
| Lowest past BMI (kg/m 2) | 20.0 | 1.2 | 14.5 | 1.8 | 18.0 | 2.0 | 48.40 | <0.001 | 2, 3 < 1, 2 < 3 |
| Age of onset (years) | 13.4 | 1.4 | 15.1 | 2.0 | 7.488 | 0.011 | 2 < 3 | ||
| Length of recovery (months) | 72.4 | 31.3 | 41.1 | 28.0 | 7.983 | 0.009 | 2 > 3 | ||
| BDI (Total) | 1.8 | 2.3 | 7 | 7.3 | 7.3 | 6.3 | 5.218 | 0.009 | 2, 3 > 1 |
| Novelty Seeking/TCI | 40.6 | 13.3 | 40.6 | 12.3 | 41.6 | 12.8 | 0.03 | 0.971 | |
| Harm Avoidance/TCI | 27.1 | 10.2 | 37.4 | 12.8 | 34.5 | 15.8 | 2.829 | 0.07 | |
| Spielberger State Anxiety | 32.2 | 6.3 | 41.5 | 11.4 | 39 | 10.0 | 4.50 | 0.017 | 1 < 2 |
| Y–BOCS (Total) | 0.4 | 1.3 | 7.6 | 9.2 | 0.9 | 1.0 | 8.942 | 0.001 | 1, 3 < 2 |
| EDI (Total) | 12.8 | 8.5 | 36.2 | 27.6 | 40.4 | 22.9 | 8.60 | 0.001 | 2,3 > 1 |
CW (1), healthy control women; AN (2), women recovered from anorexia nervosa, restricting type; BN (3), women recovered from bulimia nervosa; BDI, Beck Depression Inventory; TCI, Temperament and Character Inventory; Y-BOCS, Yale Brown Obsessive Compulsive Scale; EDI, Eating Disorder Inventory: df 2,44. P-values <.05 are marked in bold font weight.
The women recovered from AN found the cream solution significantly less pleasant compared to CW women (t = 3.407, P = 0.002) and felt more anxious in response to the cream solution compared to CW (t = −3.319, P = 0.002) (Table II and supplementary material available online).
fMRI results: Regions-of-interest image analysis
Interaction analysis comparing three groups and three conditions
An interaction analysis comparing the three groups (AN, BN, CW) and the three conditions (cream, CMC, water) revealed significant differences in the left AVS −10, 6, −5 (F = 4.92, df = 4,88, P = 0.001), the left AVS −8, 16, −2 (F = 2.89, df = 4,88, P = 0.026), and the ACC 2, 30, 14 (F = 2.48, df = 4,88, P = 0.049). But only the left AVS −10, 6, −5 (P = 0.0019) remained significant after adjusting for multiple statistical testing. Interaction ANOVAR results of the left AVS had 95% power for detecting a difference in the three groups and three condition paradigm at α = 0.05 level.
Further analyses for the left AVS −10, 6, −5
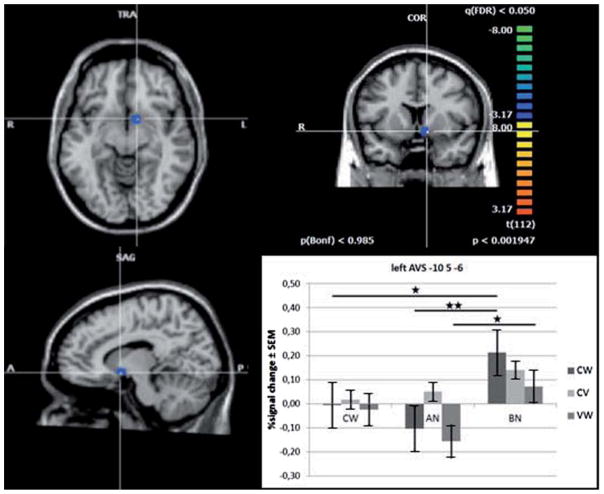
A contrast analysis comparing the stimuli deltas between the 3 groups revealed significant differences for the contrast cream/water (F = 8.04, df = 2,44, P = 0.001) and the contrast CMC/water (F = 5.22, df = 2,44, P = 0.009). A post hoc analysis displayed a higher magnitude of response for the contrast cream/water in BN compared to AN or CW (df = 44, P = 0.001/ P = 0.019) and for the contrast CMC/water in BN compared to AN (df = 44, P = 0.007) (Figure 2).
Figure 2.
Sagittal, coronal and axial view of the left anterior ventral striatum (AVS) ROI (x = −10, y = 6, z = −5) for the contrast AN versus BN and Cream versus Water as result of a significant FDR corrected ANOVA based on a random-effects corrected GLM over all ROIs including all predictors (within-factor: cream and water; between-factor: AN and BN; plot: double star). Bold lines indicate significant group differences for relevant condition contrasts (CW = control women, AN = women recovered from anorexia nervosa restricting-type, BN = women recovered from bulimia nervosa; CW = Cream-Water, CV = Cream-CMC, VW = CMC-Water).
For the left AVS region post hoc within group analyses showed a significantly greater response to cream than water for BN (t = 3.86, P = 0.006). We did not find any differences between the stimuli for AN or CW. In contrast, a within condition comparison showed a significantly lower response to water in BN compared to AN or CW (t = 2.86, P = 0.006; t = 2.91, P = 0.005), but did not reveal any differences for cream or CMC (Figure 2). The AN-CW contrast did not reach significance level for either stimulus. However, while the mean percent signal changes to all fluids in CW seem very similar, the means for the high viscous fluids (more pronounced for cream) compared to water was higher in BN but lower in AN.
The findings were not related to age, BMI, age of onset, length of recovery, depression, anxiety, eating disorder related symptoms or Axis I diagnoses. We also asked the subjects to rate their degree of anxious feelings during the taste procedure. However, the signal activity of any ROI was not related to anxious feelings in any group.
fMRI results: Voxelwise whole brain analysis
In a main effects analysis, significant clusters (when each taste stimulus was compared with baseline) reflected the neuronal circuitry of taste processing, including the insula, OFC, ACC, amygdala and striatum (Supplementary Table 1a–c available online).
As a second step, an ANOVA was calculated. A post-hoc comparison, consistent with the ROI analysis, displayed significant results in the anterior ventral striatum for the contrast cream versus water when AN were compared to BN (t = −3.15, df = 56, P < 0.005) and BN were compared to CW (t = 3.09, df = 62, P < 0.005) (Supplementary Figure 1 available online).
Discussion
This study provides evidence that individuals recovered from BN have disturbances of gustatory processing of a high-fat challenge compared to water in the AVS. That is, BN showed an exaggerated AVS response for the cream/water contrast in comparison to AN or CW. Moreover, BN showed an exaggerated AVS response for the CMC/water contrast in comparison to AN. Together, these findings indicate that caloric and non-caloric viscous stimuli seem to elicit a similar exaggerated AVS response, when comparing BN to AN. Due to the use of recovered patients, these results cannot be attributed to acute illness and starvation. Results might be driven by underlying traits, or scars, that persist after recovery.
Does altered AVS response play a role in pathological eating behaviours in BN? It should be noted that another fMRI study, using a response to monetary reward, supports the likelihood that BN have altered AVS function (Wagner et al. 2007). The AVS encompasses the nucleus accumbens (NA) which is part of the brain reward system. However, the NA is a complex structure, with specific functions that cannot be discriminated by the limited resolution of fMRI imaging. For example, the core of the NA surrounding the anterior commissure, may play a role in guiding behaviour towards a specific goal (Pavlovian conditioning), while the shell, enclosing the core, seems to be crucial for unconditioned reward-seeking behaviours (Goto and Grace 2008). From another perspective, it has been postulated that appetitive reward contains separate functional components, modulated by opioids and dopamine. Both systems are part of the neural circuitry of the NA (Berridge et al. 2010). The dopamine system is thought to mediate “wanting” (incentive motivation) for food reward (Berridge 1996), while “liking” (pleasure) seems to be mediated by opioids and cannabinoids (Kelley et al. 2002). Animal models show that AVS related dopamine and opioid systems play a role in the ingestion of palatable foods, like sugar and fat (Kelley et al. 2002; Kelley et al. 2003; Boggiano et al. 2005; Avena et al. 2009; Mathes et al. 2009). In humans, there is emerging evidence that altered dopamine and opioid function is involved in the pathomechanism of binging behaviour in BN and BED subjects (Drewnowski et al. 1995; Bencherif et al. 2005; Frank et al. 2011).
BN individuals are particularly drawn to consuming sweet, high-fat, palatable foods during binges (Weltzin et al. 1991). When binging they often describe an automatic, dissociated type of state where they feel powerless to exert self-control and stop eating (Moreno et al. 2009). It is possible that the “approach” of food during bingeing is, in part, a dopamine mediated compulsive reward/food-seeking behaviour, which leads to motivated food consumption that is no longer hedonically driven (Berridge et al. 2010). Such a mechanism is thought to play a key role in motivated behaviour in addictive disorders (DiChiara et al. 2004). “Food addiction” might be a plausible hypothesis to explain the core symptom bingeing behaviour in BN. Alternatively, or in addition, BN could have an exaggerated hedonic response to palatable foods.
The present study replicated the activation of the AI, OFC, ACC, amygdala and striatal regions by a fatty or viscous fluid (De Araujo and Rolls 2004; Stice et al. 2008; Grabenhorst et al. 2009). Araujo observed in healthy individuals that the fat activation of ACC and AVS is viscosity independent and showed that there is greater activation by CMC than fat in the AI. We did not replicate the latter, and due to different methods, we did not control for whether the activation was viscosity independent, but instead, we tested for cream/CMC differences which were negative (De Araujo and Rolls 2004). The present data suggest that the BN AVS response is related to the viscosity of fat, rather than to sugar in the cream solution. This is in accordance to previous studies which were carried out on different samples of patients with AN and BN. In these studies, no differences in AVS response to sucrose were found (Frank et al. 2006; Wagner et al. 2008).
When we designed this study, we had concerns that an anxious response to the taste of cream might contribute to an altered response, at least in AN. In a recent study that assessed the neural response to chocolate milk, ill AN displayed significant activations in the right amygdala relative to controls during hunger state, whereas no differences were found in the satiety state (Vocks et al. 2011). Although anxiety ratings did not explain the mean differences of our results, there was an unadjusted negative relationship between neuronal activity in response to cream in the amygdala and state anxiety (STAI-Y) in BN, but no relationship for AN (Supplementary Figure 2 and Table 3 available online). Potential contributions of emotional valance should be explored in future studies.
Before scanning we asked the subjects which stimulus they would prefer. Thirteen out of 18 CW preferred the cream, while all AN and 10 out of 14 BN preferred water. Again, after scanning, CW rated cream and AN and BN rated water as the most pleasant solutions. We did not find any correlations to the pleasantness ratings for the left AVS or any ROI areas in either healthy subjects or ED subjects. This corresponds with the findings of Araujo and colleagues who reported the lack of correlations in one of their AVS regions (De Araujo and Rolls 2004) for healthy subjects, and with our previous study (Wagner et al. 2008). In a recent study that assessed the neural response to aversive versus rewarding sweet stimuli in recovered AN, significant differences in neuronal response were found without a correlation to pleasantness ratings (Cowdrey et al. 2011). Given this, it seems implausible that the presented results are due to differences in conscious processing of the different stimuli, rather, it is more likely, that these findings indicate dysfunctional food attributions. Still, the lack of correlation stays uncertain.
A circuit involving AI, the OFC, ACC, and striatum appears to be involved in higher-order sensory-interoceptive-hedonic-incentive response to palatable foods (Volkow et al. 2011). Our main effects analysis supports this previous finding. In general, our studies suggest that BN show an exaggerated, and AN show a diminished response of this circuit when tasting palatable foods. BN displayed a lower response to a neutral water stimulus compared to AN and CW. While this finding probably contributes to the contrast effects, it also might underscore the sensitivity of the reward system in BN to palatable foods. However, it is not clear whether responses to sucrose and fat/ viscous substances engage exactly the same regions. That is, AN showed a diminished AI response to sucrose (Wagner et al. 2008), and while the AVS response to sucrose was normal, we found diminished response in other striatal regions, such as the ventral putamen (Wagner et al. 2008). The present study did not observe any significant differences between fat and water in AN. BN showed an exaggerated AVS response to the fat/water and CMC/water contrast. Taken together, there might be disorder-specific brain regions and functional differences in response to various macronutrients. For example, in BN aberrant sucrose response might be related to altered sensory-interoceptive-hedonic function in the AI, whereas the fat response is related to altered AVS processes. Whether there are truly circuit-specific differences, or whether a lack of power, or method differences between studies confounds results remains to be determined.
Limitations
The study was not designed to distinguish between consummatory food reward and anticipatory food reward. Anticipated reward from food intake seems to be a stronger determinant of caloric intake than the reward experienced when the food is actually consumed (Epstein et al. 2007).
We chose a hypothesis driven ROI-based analysis, so there was the risk of not identifying other interesting findings. However, a whole brain analysis approach did not reveal different results.
The findings are based on a circumscribed sample size and limited amount of trials. Other potential regional differences might be due to a lack of power.
There are a limited number of studies that explore the processing of fat. Thus, it is not certain whether responses to sucrose and fat/viscous substances correspond with the same regions.
The cream and CMC solution contained a minimal amount (approximately 3%) of sugar which could be barely tasted, but nonetheless could result in a potential sucrose/fat confound. Future studies need to investigate fat, sweet-fat and sweet stimuli responses in parallel to determine the contributions of fat and sugar on neuronal activation.
The study was not designed to distinguish between symptom-specific differences (i.e. binging behaviour) and pathology-specific differences. In future work, it might be valuable to examine a non BN group with binging behaviour, like BED.
Summary
This is the first study investigating neuronal activity patterns in response to high-fat food in contrast to a high viscous and neutral solution in subjects recovered from either AN or BN.
The results might imply that motivational response to a high-fat stimulus might contribute to different eating behaviours in BN. It supports the possibility that altered hedonic and/or motivational drives contribute to a stereotypic, automatic food consuming behaviour, individuals with BN struggle to control.
Supplementary Material
Acknowledgments
The authors would like to thank the J.W. Goethe University BIC Laboratory staff for their invaluable contribution to this study. The authors are indebted to the participating subjects for their contribution of time and effort in support of this study.
Footnotes
Data were presented at the Meeting of the Society of Biological Psychiatry, New Orleans, 20–22 May 2010.
Statement of Interest
Dr Wagner has received research support from the NIMH. Dr Kaye has received research funding/ support from the NIMH; Research funding for an investigator initiated treatment study from Astra-Zeneca and consulting fees from Lundbeck and Merck. In addition, there are honoraria for presentations from academic institutions and meetings, and compensation for grant review activities from the National Institutes of Health. Dr Poustka has received funding (consulting fees/treatment study) from Lilly, Janssen-Cilag, Lundbeck, Medice and Astra-Zeneca.
References
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA; 2000. DSM:VI-TR. [Google Scholar]
- Avena N, Rada P, Hoebel B. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–638. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward M, Mendelson M, Mock J, Erbaugh J. An Inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bello N, Coughlin J, Redgrave G, Moran T, Guarda A. Oral sensory and cephalic hormonal responses to fat and non-fat liquids in bulimia nervosa. Physiol Behav. 2010;99:611–617. doi: 10.1016/j.physbeh.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif B, Guarda AS, Colatuoni C, Ravert H, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–1351. [PubMed] [Google Scholar]
- Berridge K, Ho C, Richard J, Deifeliceantonio A. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Boggiano M, Chandler P, Viana J, Oswald K, Maldonado C, Wauford P. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- Brooks S, O’Daly O, Uher R, Frederich H-C, Giampietro V, Brammer M, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. Public Library Sci One. 2011;6:1–8. doi: 10.1371/journal.pone.0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): A guide to its development and use. St. Louis, MO: Center for Psychobiology of Personality, Washington University; 1994. pp. 19–28. [Google Scholar]
- Cowdrey F, Park R, Harmer C, McCabe C. Increased nural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatr. 2011;70:736–743. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- De Araujo I, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara G, Bassareo V, Fenu S, De Luca M, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Greenwood M. Cream and sugar: human preferences for high-fat foods. Physiol Behav. 1983;30:629–633. doi: 10.1016/0031-9384(83)90232-9. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr. 1995;61:1206–1212. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- Ellison Z, Foong J, Howard R, Bullmore E, Williams S, Treasure J. Functional anatomy of calorie fear in anorexia nervosa. Lancet. 1998;352:1192. doi: 10.1016/S0140-6736(05)60529-6. [DOI] [PubMed] [Google Scholar]
- Epstein L, Temple J, Neaderhiser B, Salis R, Erbe R, Leddy J, et al. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. erratum in Behav Neurosci 2008;122(871):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom MH, Weltzin TE, Neuberger S, Srinivasagam N, Kaye WH. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biol Psychiatr. 1994;36:696–702. doi: 10.1016/0006-3223(94)91179-7. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Users guide for the structured clinical interview for DSM-IV Axis I disorders – research version (SCID-I, version 2.0, February 1996) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Forman SC, JD, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frank G, Wagner A, Brooks-Achenbach S, McConaha C, Skovira K, Aizenstein H, et al. Altered brain activity in women recovered from bulimic type eating disorders after a glucose challenge. A pilot study. Int J Eat Disord. 2006;39:76–79. doi: 10.1002/eat.20210. [DOI] [PubMed] [Google Scholar]
- Frank G, Reynolds J, Shott M, O’Reilly R. Altered temporal difference learning in bulimia nervosa. Biol Psychiatr. 2011;70:728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge J, Breitbart M, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490:101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia and bulimia nervosa. Int J Eat Disord. 1983;2:15–34. [Google Scholar]
- Gizewski E, Rosenberger C, de Greiff A, Moll A, senf W, Wanke I, et al. Influence of satiety and subjective valence rating on cerebral activation patterns in response to visual stimulation with high-calorie stimuli among restrictive anorectic and control women. Neuropsychobiology. 2010;62:182–192. doi: 10.1159/000319360. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;33:636–647. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Dougherty DD, Fischman AJ, Emans SJ, Grace E, Lamm R, et al. Neural substrates of anorexia nervosa: a behavioral challenge study with positron emission tomography. J Pediatr. 2001;139:51–57. doi: 10.1067/mpd.2001.114768. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace A. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31:552–558. doi: 10.1016/j.tins.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls E, Parris B, D’Sousa A. How the brain represents the reward value of fat in the mouth. Cereb Cortex. 2009;20:1082–1091. doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Hudson J, Hiripi E, Pope H, Kessler R. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatr. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel C, Gerber J, Hummel T. Cerebral processing of gustatory stimuli in patients with taste loss. Behav Brain Res. 2007;185:59–64. doi: 10.1016/j.bbr.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Joos A, Saum B, van Elst L, Perlov E, Glauche V, Hartmann A, et al. Amygdala hypereactivity in restrictive anorexia nervosa. Psychiatry Res. 2011;191:189–195. doi: 10.1016/j.pscychresns.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Kelley A, Bakshi P, Haber S, Steininger T, Will M, Zhang M. Opioid modulation of taste hedonics within ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley A, Will M, Steininger T, Zhang M, Haber S. Restricted daily consumption of a highly palatable food (chocolate Ensure®) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283:236–242. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- Mathes W, Brownley K, Mo X, Bulik C. The biology of binge eating. Appetite. 2009;52:545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzushige T, Inoue K, Fushiki T. Why is fat so tasty? Chemical reception of fatty acid on the tongue. J Nutr Sci Vitaminol (Tokyo) 2007;53:1–4. doi: 10.3177/jnsv.53.1. [DOI] [PubMed] [Google Scholar]
- Montague R, Hyman S, Cohen J. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Moreno S, Warren C, Rodriguez S, Fernandez M, Cepeda-Benita A. Food cravings discriminate between anorexia and bulimia nervosa. Implications for “success” versus “failure” in dietary restriction. Appetite. 2009;52:588–594. doi: 10.1016/j.appet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Naruo T, Nakabeppu Y, Sagiyama K, Munemoto T, Homan N, Deguchi D, et al. Characteristic regional cerebral blood flow patterns in anorexia nervosa patients with binge/purge behavior. Am J Psychiatry. 2000;157:1520–1522. doi: 10.1176/appi.ajp.157.9.1520. [DOI] [PubMed] [Google Scholar]
- Nozoe S, Naruo T, Nakabeppu Y, Soejima Y, Nakajo M, Tanaka H. Changes in regional cerebral blood flow in patients with anorexia nervosa detected through single photon emission tomography imaging. Biol Psychiatr. 1993;34:578–580. doi: 10.1016/0006-3223(93)90205-r. [DOI] [PubMed] [Google Scholar]
- Ogawa H. Gustatory cortex of primates: anatomy and physiology. Neurosci Res. 1994;20:1–13. doi: 10.1016/0168-0102(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O’Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Santel S, Baving L, Krauel K, Munte T, Rotte M. Hunger and satiety in anorexia nervosa: fMRI during cognitive processing of food pictures. Brain Res. 2006;1114:138–148. doi: 10.1016/j.brainres.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: Reward sensitivity and brain action to images of food. Biol Psych. 2008;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Schoenfeld M, Neuer G, Tempelmann C, Schussler K, Noesselt T, Hopf J, et al. Functional magnetic resonance tomography correlates of taste perception in the human primary taste cortex. Neuroscience. 2004;127:347–353. doi: 10.1016/j.neuroscience.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Simon S, De Araujo I, Gutierrez R, Nicolelis M. The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- Small D, Zatorre R, Dagher A, Evans A, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Stice E, Spoor S, Bohon C, Small D. Relation between obesity and blunted striatal response to foods is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Uher R, Brammer M, Murphy T, Campbell I, Ng V, Williams S, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol Psychiatr. 2003;54:934–942. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- Uher R, Murphy T, Brammer M, Dalgleish T, Phillips M, Ng V, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- Vocks S, Herpertz S, Rosenberger C, Senf W, Gizewski E. Effects of gustatory stimulation on brain activity during hunger and satiety in females with restricting-type anorexia nervosa: An fMRI study. J Psychiatr Res. 2011;45:395–403. doi: 10.1016/j.jpsychires.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Baler R. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Barbarich N, Frank G, Bailer U, Weissfeld L, Henry S, et al. Personality traits after recovery from eating disorders: Do subtypes differ? Int J Eat Disord. 2006;39:276–284. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman M, Fudge J, May J, Mazurkewicz L, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Frank GK, Figurski J, May JC, Putnam K, et al. Altered insula response to a taste stimulus in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–523. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- Weltzin TE, Hsu LK, Pollice C, Kaye WH. Feeding patterns in bulimia nervosa. Biol Psychiatr. 1991;30:1093–1110. doi: 10.1016/0006-3223(91)90180-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.