Abstract
Representation of reward value involves a distributed network including cortical and subcortical structures. Because neurodegenerative illnesses target specific anatomic networks that partially overlap with the reward circuit they would be predicted to have distinct impairments in reward processing. This review presents the existing evidence of reward processing changes in neurodegenerative diseases including mild cognitive impairment, Alzheimer's disease, frontotemporal dementia, amyotrophic lateral sclerosis, Parkinson's disease, and Huntington's disease, as well as in healthy aging. Carefully distinguishing the different aspects of reward processing (primary rewards, secondary rewards, reward-based learning, and reward-based decision-making) and using tasks that differentiate the stages of processing reward will lead to improved understanding of this fundamental process and clarify a contributing cause of behavioral change in these illnesses.
Keywords: reward, neurodegenerative disease, dementia
Introduction
Processing of rewards is a daily process, as individuals weigh the relative value of food, money, or social choices. The role of reward processing has been extensively studied with animal models and healthy adults providing much of the data. The neurodegenerative diseases provide an additional resource for understanding these behaviors because each has characteristic selective vulnerability of different brain regions. When these predictably affected regions overlap with those implicated in reward processing, there is an opportunity to further understanding of this fundamental process. This review briefly summarizes the extensive literature on the anatomy of reward processing as well as the literature on reward in individual neurodegenerative diseases, discusses how the study of each informs the other, and identifies needed developments for the advancement of the field of study.
Reward Concepts and Components
The concept of a reward includes anything that an organism will pursue or work for. Though various definitions exist, reward broadly encompasses all “objects or events that generate approach and consummatory behavior, produce learning of such behavior, represent positive outcomes of economic decisions and engage positive emotions and hedonic feelings” (Schultz, 2010). Primary rewards including food, drink, and sex produce pursuit behaviors in and of themselves. Secondary rewards such as money motivate pursuit because they can be used to obtain primary rewards. Opposite rewards are punishments, or stimuli an individual will work to avoid.
The process of evaluating rewards involves multiple steps. Anticipation of an upcoming reward, action selection to seek or avoid the stimulus, the actual experience of receiving the reward, and then re-evaluating or learning from the experience are separate components that may have different anatomic correlates.
Anatomy of Reward Processing
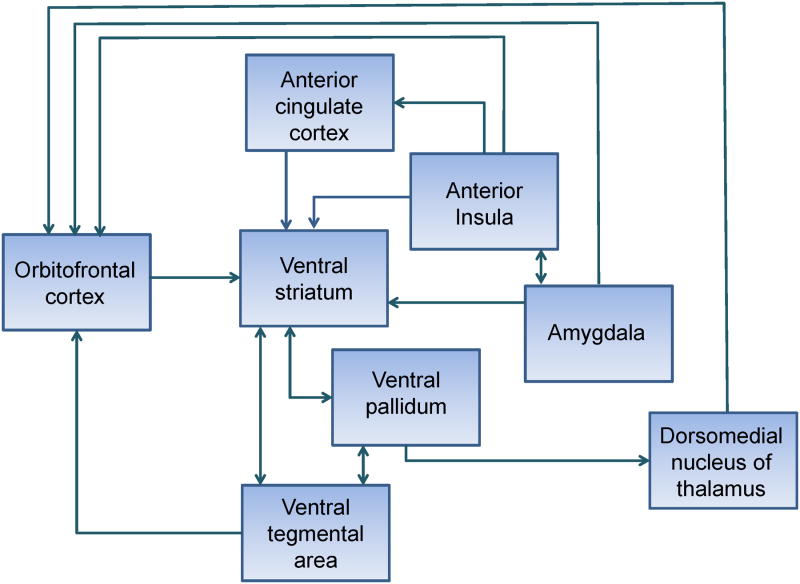
Multiple brain regions have been implicated in assigning reward value (Figure 1). Though a brief description of their known roles in reward processing is given in this section, animal and human studies continue to clarify the complexity of reward circuit anatomy (Haber & Knutson, 2010) and the process of determining reward value (Berridge, 2012; Schultz, 2010; Sescousse, Caldú, Segura, & Dreher, 2013). The orbitofrontal cortex (OFC) shows increased activation to pleasant, presumably rewarding, stimuli in multiple sensory modalities (olfaction (E. T. Rolls, Kringelbach, & De Araujo, 2003), gustation (O'Doherty, Rolls, Francis, Bowtell, & McGlone, 2001), vision (O'Doherty et al., 2003), hearing (Blood, Zatorre, Bermudez, & Evans, 1999), and somatosensation (E. T. Rolls et al., 2003)). In addition to representing primary rewards, more abstract concepts such as economic value are also represented in OFC (Padoa-Schioppa & Assad, 2006). There is also decreasing activation when a rewarding stimulus is consumed to satiety as its reward value decreases (Kringelbach, O'Doherty, Rolls, & Andrews, 2003). It has been suggested that lateral orbitofrontal cortex may be more involved in processing negative consequences and medial orbitofrontal in reward (Anderson et al., 2003; O'Doherty et al., 2001; E. T. Rolls et al., 2003). The amygdala also encodes stimulus reward value, including the valence (Morrison & Salzman, 2010), magnitude (Bermudez & Schultz, 2010), and intensity of a rewarding stimulus (Anderson et al., 2003).
Figure 1. Key reward circuit structures and pathways that can be affected in neurodegenerative disease.
The ventral striatum (nucleus accumbens, olfactory tubercle, and portions of ventral caudate and putamen) is a key reward region that activates with anticipation of reward. Some studies have shown a distinction between activation of these areas in reward anticipation compared to activation of orbitofrontal areas with receipt of reward (Knutson, Fong, Adams, Varner, & Hommer, 2001). This may only be the case after conditioning has taken place. As with orbitofrontal cortex, there may be anatomic distinction between areas of the ventral striatum that are responsive to reward versus punishment, with one study suggesting more anterior BOLD signal on fMRI in reward and more posterior ventral striatal activity in punishment (Seymour, Daw, Dayan, Singer, & Dolan, 2007).
The mechanism of learning to predict reward value may be related to the phasic activity of dopaminergic neurons from the ventral tegmental area (Schultz, Dayan, & Montague, 1997). Neuronal recordings of the caudate and putamen of monkeys have demonstrated that reward based learning also takes place at the level of the striatum (Samejima, Ueda, Doya, & Kimura, 2005). The dopaminergic neurons of the ventral tegmental area project via the mesolimbic pathway to the nucleus accumbens, which also receives excitatory input from the amygdala and hippocampus. The ventral striatum inhibits the ventral pallidum, which has inhibitory connections with the dorsomedial nucleus of the thalamus, which then projects to prefrontal cortex. In addition to this ventral pathway, the dorsal striatum and dorsolateral prefrontal cortex play a role in processing of reward. For example, the dorsal striatum may be involved in selecting actions based on anticipation of reward (Haruno et al., 2004).
The anterior cingulate cortex (ACC) is also implicated in reward processing. It is directly involved in representation of reward value, possibly because projections to its pregenual and dorsal anterior regions from the OFC (Grabenhorst & Rolls, 2011). It has also been proposed to mediate choice between risky alternatives, requiring weighing of potential rewards or punishments (M. Ernst et al., 2004; R. D. Rogers et al., 2004). It is involved in conflict monitoring (R. D. Rogers et al., 1999) and part of the process may involve integrating cognitive processing with autonomic information associated with anticipation (Critchley, Mathias, & Dolan, 2001).
The anterior insula also receives input from the body that conveys sensation and information about the physiological state, which is important in judging how salient a reward might be at a given time (Craig, 2003). The anterior insula, along with lateral orbitofrontal cortex, may be more involved in representing negative consequences, or punishment (Seymour, Singer, & Dolan, 2007). This has been proposed to be due to the role of this area in processing bodily sensations of unease (Paulus & Stein, 2006). Those with lesions in this area do not adjust their bets on gambling tasks in accordance with risk (Clark et al., 2008). This area activates before making a risky decision (Kuhnen & Knutson, 2005) and in anticipation of loss (Samanez-Larkin, Hollon, Carstensen, & Knutson, 2008).
This anatomy reflects areas involved in anticipating reward and responding to receiving rewarding stimuli. The anatomy involved in intentional delay of receiving reward may be different. In the “marshmallow study” of Walter Mischel, individuals who as preschoolers were able to delay the gratification of receiving a marshmallow in order to receive two later were more successful in later life in terms of SAT scores, social and emotional adjustment (Mischel et al.,). This ability to deny immediate reward in favor of delayed or more long-term reward has been proposed to involve intact cognitive control, or executive function through dorsolateral prefrontal-striatal networks (Hare, Camerer, & Rangel, 2009; Hariri et al., 2006).
Methods of Reward Processing Assessment
A variety of techniques have been used to measure reward processing. Some of these involve passive experience of stimuli, such as primary or secondary rewards. Subjects are presented with pleasant or unpleasant smells, tastes, sounds, or visual stimuli. Sometimes the task includes an anticipatory phase where the subject is aware of the possible upcoming reward or punishment. In the Monetary Incentive Delay (MID) task (Knutson et al., 2001) cues are given about upcoming small or large rewards or punishments and subjects are told these are conditional upon rapidly pressing a button. The positive or negative consequences then occur at a fixed frequency and fMRI signal reveals activation in response to anticipation and receiving rewards or punishments of varying magnitudes.
Reward learning tasks include conditioning paradigms where a neutral conditioned stimulus is paired with a rewarding or punishing unconditioned stimulus. Some of these paradigms then employ reversal learning, where the association between stimuli changes, and previously rewarded choices no longer yield positive results. Probabilistic reversal learning is a variant of this task in which stimulus choices are usually, but not always, paired with an outcome (positive or negative) and sometimes are associated with the opposite result.
The assessment of reward-related decision making includes several gambling tasks. In the Iowa Gambling Task (IGT) the subject can select cards from four decks, two of which yield small gains and losses but trend toward monetary gain, and the other two decks give large potential gains, but larger and more frequent losses that lead to net loss of money (Bechara, Damasio, Damasio, & Anderson, 1994). This task is considered a measure of decision-making under uncertainty as the subjects do not know the exact risk associated with each choice at the start. Though it was originally proposed as a measure of OFC integrity, intact performance on the IGT requires widespread prefrontal function (M. Ernst et al., 2002; Manes et al., 2002). In the Cambridge Gamble Task (CGT) (Rahman, Sahakian, Hodges, Rogers, & Robbins, 1999) subjects are presented with 10 tiles colored red or blue and are asked to place a wager on the odds of a token being hidden under a red or a blue tile. The proportion of red versus blue tiles changes with each trial, making the CGT a task of decision-making under risk since the odds are known by the subject. Temporal discounting is another decision-making task in which a choice is presented between smaller immediate gains and larger delayed rewards. These decision-making tasks are tied to the emerging field of neuroeconomics (Lee, Seo, & Jung, 2012; Loewenstein, Rick, & Cohen, 2008), which bring together economic theories of decision making, psychology, and the neuroscience of determining value in order to provide a framework for better understanding behavior in health, aging (Brown & Ridderinkhof, 2009), or illness.
Reward in Neurodegenerative Diseases
Nervous system diseases provide an opportunity beyond animal models and healthy adults to understand the working of reward behavior when it is not functioning appropriately. Some of this work has been done in brain lesion studies, but the anatomy of areas affected by brain lesions is variable and unpredictable. An advantage in studying behavior and cognition in neurodegenerative diseases is that each tends to target areas of selective vulnerability and specific networks are implicated (W. W. Seeley, Crawford, Zhou, Miller, & Greicius, 2009). The reasons for this selective vulnerability are unclear, but the fact that known networks are targeted allows for more predictable study of the role of these networks in a particular behavior of interest.
The discussion of reward in each illness will be framed in terms of evidence regarding processing of primary rewards, secondary rewards, reward-based learning, and reward-based decision-making (Table).
Table. Reward processing deficits by disease.
| Primary reward processing | Secondary reward processing | Reward-based learning | Reward-related decision making | |
|---|---|---|---|---|
| Healthy Aging | Decline in primary sensory function | Positivity bias (stronger anticipation of monetary gain than loss) | Positivity bias with possible negativity bias in “older old” | --- |
| Mild cognitive impairment | --- | --- | Improved learning when faced with negative stimuli or possible loss | --- |
| Alzheimer's Disease | Loss of appetite Poor odor discrimination and identification |
--- | --- | Impairment on tasks of decision-making under uncertainty and risk correlating with cognitive deficits |
| Behavioral variant frontotemporal dementia | Overeating and sweet preference Odor identification deficit due to semantic impairment with intact odor discrimination Decreased pain sensitivity Decreased fear conditioning to loud noise Hyposexuality |
--- | --- | Risky choices on tasks of decision-making under uncertainty and risk |
| Semantic variant primary progressive aphasia | Food fads and restrictive dieting Odor and flavor identification deficit due to loss of semantic knowledge |
--- | --- | --- |
| Amyotrophic lateral sclerosis | --- | --- | Intact probabilistic reversal learning | --- |
| Parkinson's Disease | Olfactory impairment with preservation of gustation | Decreased physiological anticipation of monetary gain with intact response to receipt of monetary reward | Enhanced learning from negative outcomes off dopaminergic medication. Enhanced learning from positive outcomes and decreased learning from negative outcomes on dopaminergic medication |
Mixed results on Decision-making tasks reflecting differences in disease stage and medication |
| Huntington's Disease | --- | Decreased anticipation of monetary reward or punishment | --- | Intact gambling task performance early in illness. Decision-making task deficits later correlate with cognitive impairment. |
Healthy Aging
As patients with neurodegenerative disease tend to be older it is important to understand the natural course of reward processing in healthy aging. Some literature on normal aging suggests a positivity bias, meaning that there is a higher ratio of positive to negative affect and stronger memory and attention for positive valenced information than negative. It has been suggested that this bias is a result of a greater focus on emotional goals (Mather & Carstensen, 2005). Structural changes in aging include generalized volume loss with frontal and parietal lobes particularly affected (Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003). The cognitive effects of aging have been extensively studies and are thought to include executive function impairment, problems with set shifting and working memory (Marschner et al., 2005).
Primary reward processing
Older adults showed increased activity in a wide range of gustatory and reward processing areas on an fMRI task evaluating gustatory processing in hunger, possibly suggesting the recruitment of additional regions in age as primary sensation declines (Jacobson, Green, & Murphy, 2010).
Secondary reward processing
On the MID task healthy older adults showed the same degree of fMRI activation in anticipation of money gain as younger adults, but had less activation in anticipation of loss (Samanez-Larkin et al., 2007), consistent with a positivity bias.
Reward-based learning
The results of reward-based learning in aging have not always been consistent with a positivity bias. Some have found a negativity bias in “older old” adults (age >70) compared “younger old” adults and have suggested that this is the result of a decline in dopamine levels in aging (M. J. Frank & Kong, 2008). A more recent study found that as a group, older adults showed no bias toward positive or negative feedback, but when each individual was looked at, there were more individuals with a positivity or negativity bias in the older group than the younger group, and that those with a negativity bias were slightly older than the positive bias group (Simon, Howard, & Howard, 2010). This may substantiate the hypothesis that negativity bias correlates with decreasing dopamine in late life.
Older subjects also collect fewer points on a probabilistic object reversal task than younger subjects, even when results are corrected for age-related differences on other tests of executive function (Mell et al., 2005). This may suggest a separate deficit in reward learning independent of dorsolateral prefrontal cortex mediated functions.
Mild cognitive impairment
The positivity bias in healthy aging contrasts with what is seen in mild cognitive impairment (MCI). MCI is a syndrome characterized by a cognitive complaint that is supported by impaired memory testing with intact activities of daily living (Petersen et al., 1999). The MCI category includes patients who will develop dementia and be diagnosed with Alzheimer's disease, but also includes patients who will not progress and also those who ultimately be diagnosed with a non-Alzheimer dementia. This suggests that MCI literature may be less specific in terms of the involved neural networks.
Reward-based learning
A study of patients with amnestic mild cognitive impairment compared to healthy aging controls compared the effect of monetary reward or loss on performance of a task of spatial attention. The results indicated that MCI patients had improved performance compared to neutral conditions only during tasks when monetary loss was threatened and this seemed to correlate with posterior cingulate cortex activity on fMRI, whereas the healthy aging controls improved performance more in conditions when monetary gain was possible, and this correlated more with orbitofrontal cortex fMRI activation (Bagurdes, Mesulam, Gitelman, Weintraub, & Small, 2008). In a separate study amnestic MCI patients also showed a bias toward improved working memory for pictures with negative emotional content compared to neutral or positive pictures compared to healthy aging controls who had no significant alteration in performance with emotional targets (Döhnel et al., 2008). The healthy aging subjects showed a decrease in left precuneus activation with viewing positive targets and the MCI patients had increased right precuneus activation with negative targets.
Alzheimer's disease
Alzheimer's disease (AD) is the most common cause of dementia in the elderly and in addition to memory impairment is accompanied by a variable degree of executive function impairment and visuospatial impairment. The areas of atrophy in Alzheimer's disease particularly involve the medial temporal lobe, posterior cingulate, precuneus, and lateral temporoparietal region. Resting state functional connectivity mapping shows that these areas are part of the “default mode network” (W. W. Seeley et al., 2009). Though these areas are not frequently included in the reward circuit, some recent literature implicates the posterior cingulate in representation of reward value (Kable & Glimcher, 2007).
Patients with Alzheimer's disease (AD) have decreased pursuit of rewarding behaviors, but this is often secondary to apathy. Apathy in dementia has been associated with atrophy in the anterior cingulate and medial prefrontal cortex (Rosen et al., 2005). One SPECT study in AD correlated apathy with hypoperfusion in the left anterior cingulate and right orbitofrontal cortex (Lanctôt et al., 2007). The findings in OFC could implicate diminished reward system activity with apathy. Stimulants have been given to patients with Alzheimer's disease for treatment of apathy. These drugs have effects on other neurotransmitters beyond dopamine including norephinephrine, but there is evidence that the dopaminergic activity of these medications on ventral striatum and occupancy of D2 receptors is responsible for their rewarding effects (Drevets et al., 2001)(Volkow et al., 1999). In a dextroamphetamine challenge, AD patients without apathy responded to the rewarding effects of the drug while the apathetic patients did not. Blood pressure response was no different between the groups (Lanctot et al., 2008). One study of methylphenidate found an improvement of apathy in AD patients (though there was also a higher rate of limiting side effects) which the authors attributed to increased activation of the dopamine-mediated brain reward system (Herrmann et al., 2008).
Primary reward processing
Loss of appetite is also observed in Alzheimer's disease. This has been hypothesized to be secondary to apathy or from decreased reward value of food. Loss of appetite has been correlated on functional imaging with left anterior cingulate and left orbitofrontal cortex hypoperfusion and relative sparing of perfusion to right anterior cingulate, right orbitofrontal, and left middle mesial temporal cortices (Ismail et al., 2008). Impairment in primary olfaction or gustation also influences the reward value of food. Poor odor discrimination and identification has been shown in AD (Luzzi et al., 2007).
Reward-related decision-making
Tests of reward-related decision-making have been given to Alzheimer's disease patients but the impairments they have demonstrated likely are not due to impaired reward processing. AD patients show impaired performance on the IGT (Sinz, Zamarian, Benke, Wenning, & Delazer, 2008; Torralva, Dorrego, Sabe, Chemerinski, & Starkstein, 2000) and lack of an advantageous strategy on a test of decision under risk (Game of Dice Task) (Delazer, Sinz, Zamarian, & Benke, 2007) but in these studies the poor performance correlated with impairment in memory, inhibitory control, and set-shifting, respectively.
Frontotemporal dementia
The term frontotemporal dementia encompasses a heterogeneous group of clinical syndromes that can be caused by a variety of different pathologies. The characteristic symptoms of behavioral variant frontotemporal dementia (bvFTD) involve a progressive change in personality and behavior including disinhibition, apathy, eating changes, repetitive or compulsive behaviors, and loss of empathy. The areas that are vulnerable early in this disorder include the anterior insula, orbitofrontal cortex, and anterior cingulate with right frontal areas being more affected early in disease (W. W. Seeley et al., 2008). These areas are part of an intrinsic connectivity network identified by resting state fMRI (W. W. Seeley et al., 2007). This network, called the “salience network” because of its activation with emotionally significant stimuli, has reduced activity in bvFTD, but not in AD (Zhou et al., 2010). These vulnerable regions and functional networks include structures in common with the known anatomy of the reward system, suggesting that patients with bvFTD may have abnormalities in reward processing underlying some of their change in behavior. The changes could also be neurochemical as well as structural, but the evidence of dopamine deficiency in bvFTD has been mixed, with some finding deficiency (Frisoni et al., 1994; Rinne et al., 2002) and others not (Francis et al., 1993; Sjogren, Wikkelso, Ostling, Wallin, & Blennow, 2002).
Deficiencies in cognitive control have also been observed in bvFTD and these may explain impaired performance on some tasks that have been attributed to reward processing. On the flanker task, where patients are asked to select the direction of a centrally presented arrow which is flanked either by arrows pointing in the same or the opposite direction, bvFTD patients perform worse than controls during the incongruent condition (flanking arrows pointing in the opposite direction) (Krueger et al., 2009). Ventrolateral prefrontal/orbitofrontal cortex (specifically BA 47) was found to be particularly active in an fMRI study of this task in healthy individuals during the incongruent condition (Luks, Simpson, Dale, & Hough, 2007).
Primary reward processing
BvFTD patients often have changes in eating habits with a preference for sweet, carbohydrate rich foods and overeating. When 32 patients with neurodegenerative diseases were given all the sandwiches they chose to eat, 6 overate. All of these had bvFTD (of a total of 13 bvFTD patients in the study) and they showed a preference for sweet jelly sandwiches. Five of the six reported being full but continued to eat. A voxel-based morphometry (VBM) analysis of the overeaters correlated the behavior with atrophy of the right ventral insula, including the anterior portion, right rostral orbitofrontal cortex, and right striatum (Woolley et al., 2007). In a separate VBM paper, the sweet preference was associated with atrophy of the bilateral posterolateral orbitofrontal cortex (BA 12/47) and right anterior insula while over-eating was associated with gray matter loss in bilateral anterolateral OFC (BA 11) (Whitwell et al., 2007).
The overeating may result from a change in sensory processing, be a stimulus-bound behavior, or it may represent a change in the reward or punishment value of the food stimulus. Though bvFTD patients have been demonstrated to have a deficit in identifying odors (Luzzi et al., 2007; Rami, Loy, Hailstone, & Warren, 2007), this is more likely due to a deficit in semantic knowledge rather than sensation since they display normal discrimination of odors (Rami et al., 2007).
The fact of continuing to eat past satiety suggests that either the reward value was not decreasing despite lack of hunger, or impairment in the negative (punishment) signal associated with fullness. As has been discussed above, the orbitofrontal cortex has been shown to activate in response to rewarding taste and smell stimuli and then show decreasing activation with consumption to satiety. Atrophy of the OFC alone could therefore hypothetically lead to a diminishment in reward value, rather than an increase. Alternatively, since the OFC not only encodes reward value, but also provides a more detailed representation of reward features than other reward processing regions(E. T. Rolls & Grabenhorst, 2008), its degeneration could lead to less distinction between rewards, less ability to compare the relative value of rewards in different sensory modalities, or insensitivity to contextual cues, such as satiety. Other observations that may suggest decreased sensitivity to punishment is an increased pain threshold and tolerance in bvFTD (Carlino et al., 2010) and decreased fear conditioning to an aversive loud noise (Hoefer et al., 2008).
Patients with bvFTD often have reduced sex drive (54% compared to 8% with increased sex drive (Miller, Darby, Swartz, Yener, & Mena, 1995)) indicating reduced pursuit of another behavior associated with reward. This may suggest the lack of negative consequences play a stronger role in overeating rather than an excess in reward-seeking.
Reward-based learning
Though reward-based learning has not been extensively tested in bvFTD, humans and monkeys with OFC lesions show perseveration on reversal learning (E. T. Rolls, Hornak, Wade, & McGrath, 1994) which was not seen in dorsolateral prefrontal cortex lesions (Fellows & Farah, 2003).
Reward-related decision-making
Financial risk taking in bvFTD has been evaluated through two different tasks. The Iowa Gambling Task has been administered to 20 bvFTD patients compared to matched controls. The groups initially had comparable performance, but over the course of the task the controls learned to choose the less risky alternatives which lead to accumulation of money, while the bvFTD patients developed a preference for the risky options, which ultimately lead to loss of money (Torralva et al., 2007). This result has been reproduced by the same group (Torralva, Roca, Gleichgerrcht, Bekinschtein, & Manes, 2009) and is consistent with studies of patients with OFC lesions. These patients choose risky options on the IGT and they have also been shown to not have the normal autonomic change in skin conductance in anticipation of reward or loss on this task, though they have the appropriate response to receiving the gain or loss (Bechara, Damasio, Tranel, & Damasio, 1997). On the CGT early bvFTD patients with intact measures of executive function had slower decision times and increased risk taking compared to normal controls (Rahman et al., 1999). As previously discussed, the explanation for deficiency on these gambling tasks includes not only impaired assessment of reward value, but also more widespread frontal impairment.
As with Alzheimer's disease, stimulants have been given in bvFTD. One trial of 8 patients with a single methylphenidate challenge resulted in reduced risk-taking behavior on the Cambridge Gamble Task with no change in other aspects of cognitive function (Rahman et al., 2006). As with the treatment of AD, the authors hypothesize that this improvement is secondary to dopaminergic activity either at the OFC or at the striatum.
Semantic-variant primary progressive aphasia
Semantic-variant primary progressive aphasia (svPPA) is another frontotemporal dementia syndrome characterized by progressive language impairment with loss of object knowledge. It is characterized by degeneration of the anterior temporal lobe, usually in asymmetric fashion, though the atrophy involves extratemporal areas as well, including the ventral striatum. In contrast to bvFTD, dietary changes in svPPA include food fads and restrictive dieting. Like bvFTD, svPPA patients have difficulty on odor and flavor tasks that involve semantic knowledge of the stimuli, but perform normally on tasks that assess more basic sensation (Luzzi et al., 2007; Piwnica-Worms, Omar, Hailstone, & Warren, 2010).
Amyotrophic Lateral Sclerosis
There is increasing consensus of a relationship between the diseases of frontotemporal lobar degeneration and motor neuron disease with recognition that many ALS patients have cognitive impairment (Lomen-Hoerth et al., 2003). Reward processing in ALS has yet to be extensively studied but one study has demonstrated impairment on various frontal lobe mediated tasks with intact probabilistic reversal learning (Meier, Charleston, & Tippett, 2010).
Parkinson's disease
The hallmark motor features of Parkinson's disease (PD) include rest tremor, rigidity, bradykinesia, and postural instability. The pathologic feature is abnormal accumulation of the protein alpha-synuclein. While there is no signature atrophy pattern of Parkinson's disease, specific functional circuits are known to be involved. The motor features are due to pathology involving the loss of dopaminergic neurons in the substantia nigra. The substantia nigra pars compacta acts on the striatum through the nigrostriatal pathway via D1 and D2 receptors. Decreased D1 activity leads to decreased direct pathway activity and less activation of D2 receptors leads to increased activity of the indirect pathway. Both of these result in bradykinesia. Parkinson's disease involves multiple non-motor symptoms as well, and cognitive symptoms are described including executive dysfunction (Zgaljardic, Borod, Foldi, & Mattis, 2003).
The ventral tegmental area is also a primarily dopaminergic structure in the midbrain, is adjacent to the substantia nigra, and there is evidence of decreased mesolimbic pathway activity from the VTA in PD as well. Patients with PD have decreased concentrations of dopamine in the nucleus accumbens, with deficiency being of the same magnitude as in the caudate (Farley, Price, & Hornykiewicz, 1977), though the putamen seems most heavily affected (Kish, Shannak, & Hornykiewicz, 1988).
Because of the hypothesized role of dopamine in pleasure processing, it has been suggested that PD patients might experience anhedonia. Though one study showed decreased response to the rewarding effects of methylphenidate in PD (Persico, Reich, Henningfield, Kuhar, & Uhl, 1998), another study showed normal hedonic tone in PD on a pleasure scale (Pluck & Brown, 2002). The latter study also showed apathy in patients with PD, and that apathy correlated with their executive dysfunction.
A variety of impulse control disorders (ICDs) have been described in Parkinson's patients. Dopamine replacement, particularly dopamine agonists are a risk factor for these disorders, which include hypersexuality, pathologic gambling, compulsive shopping, and binge eating. Other disorders associated with dopamine therapy include punding, purposeless stereotypical behaviors initially described in psychostimulant abusers (Evans et al., 2004), and dopamine dysregulation syndrome. This syndrome involves the compulsive use of dopamine medication beyond that needed for control of motor symptoms in spite of negative motor and behavioral consequences (Giovannoni, O'Sullivan, Turner, Manson, & Lees, 2000). This increase in impulsive behavior and insensitivity to negative consequences is thought to be a result of dopamine mediated activation of the reward pathway and is treated by reduction in medication doses. This model is supported by the finding that PD patients with compulsive gambling show increased resting activation on SPECT of right hemisphere orbitofrontal cortex, hippocampus, amygdala, insula, and ventral pallidum (Cilia et al., 2008).
The placebo effect has been hypothesized as a type of reward process mediated by dopamine release. When PD patients were given placebo and told that they had a certain percent chance of receiving actual levodopa their raclopride PET scan indicated significant dopamine release occurred when they were told the probability of receiving active medication was 75% but not at other probabilities. Prior response to levodopa correlated with dopamine release in the dorsal striatum but expectation of improvement was required for dopamine release in the ventral striatum (Lidstone et al., 2010).
Primary Reward Processing
Processing of primary rewards in PD could be affected by deficits in sensation. Though olfactory impairment in PD is well-established, gustation is preserved, including perception of the pleasantness of taste (Sienkiewicz-Jarosz et al., 2005).
Secondary Reward Processing
As discussed above, it has been hypothesized that there is an anatomic distinction between anticipation of reward and receipt of reward. Parkinson's disease patients have been shown to have impaired reward anticipation, particularly on high incentive tasks by an evoked potential (stimulus preceding negativity) (Mattox, Valle-Inclan, & Hackley, 2006). In a monetary incentive delay task with fMRI, both elderly controls and PD patients had an absent reward predictive response in midbrain and ventral striatum but had activation with reward feedback, showing that processing of the reward prediction error was intact. The PD patients only showed increased anterior cingulate cortex activation during reward feedback and decreased functional connectivity of midbrain and ventral striatum (Schott et al., 2007). This pattern of decreased striatal activation with anticipation and increased cortical activation, in particular medial prefrontal and anterior cingulate cortex, with reward receipt has been replicated in other studies as well (Keitz et al., 2008; Rowe et al., 2008). This decrease in response to expectation of a reward may contribute to apathy in PD, with less motivation to pursue an activity, even if the circuitry remains intact to allow receiving the reward.
Reward-based learning
Both the disease state in PD and treatment with dopamine replacement have been shown to affect reward-based learning. On a trial-and-error procedural learning task PD patients off of dopamine medications show improved learning in response to negative outcomes relative to positive, meaning they rapidly learned to avoid choices with negative results. Those on medications show more learning based on positive outcomes than negative (M. J. Frank, Seeberger, & O'Reilly, 2004). In a probabilistic classification task, young unmedicated PD patients showed a deficit in reward processing and novelty seeking that was improved with dopamine agonist treatment, though this led to disrupted responsiveness to punishment (Bodi et al., 2009). Similarly, PD patients on medication had impaired reversal learning with an unexpected punishment, but performed as well as patients off medication or controls in response to an unexpected reward. This was particularly true for patients on pramipexole, a D3 agonist (Cools, Altamirano, & D'Esposito, 2006). D3 receptors are found primarily in the ventral striatum. The authors hypothesize that while dopamine replacement is helpful for areas with dopamine deficiency in the dorsal striatum, it may overdose dopamine in the ventral striatum. When there is an excess of available dopamine there would be strong phasic dopamine-mediated learning based on reward, but not punishment. Learning from neutral or negative consequences requires lower “dips” in dopamine level that could not occur with dopamine replacement, but may be heightened off medication. The selective effect of dopamine on learning from positive outcomes was confirmed in a reinforcement learning task with PD and healthy controls subjects on and off medications, and the PD patients were also shown to make perseverative choices that resolved with dopamine replacement (Rutledge et al., 2009). This hypothesis has also been supported by findings of impaired deactivation of OFC during negative reinforcement in a probabilistic learning task while on pramipexole (van Eimeren et al., 2009). These deficits in reward learning can occur independent of motor symptoms as shown in a group of alpha-synuclein gene duplication carriers without motor findings who displayed impaired reward but not punishment learning (Keri, Moustafa, Myers, Benedek, & Gluck, 2010).
Reward-related decision-making
The Iowa Gambling Task has been given to PD patients many times with mixed results. In several studies the PD patients displayed increased risk taking (Delazer et al., 2009; Ibarretxe-Bilbao et al., 2009; Kobayakawa, Koyama, Mimura, & Kawamura, 2008; Pagonabarraga et al., 2007; Perretta, Pari, & Beninger, 2005). In other studies they did not (Czernecki et al., 2002; Euteneuer et al., 2009; Mimura, Oeda, & Kawamura, 2006; Poletti et al., 2010; Stout, Rodawalt, & Siemers, 2001; Thiel et al., 2003). PD patients have also shown impairment on the Game of Dice Task (Brand et al., 2004; Euteneuer et al., 2009). This discrepancy in performance may reflect differences in stage of disease, differences mediated by medication usage, and that the task involves the use of additional anatomy involved in executive function. A modified IGT has been given to PD patients as well, where the advantageous deck yields immediate large losses and delayed larger rewards and disadvantageous decks yield immediate small losses and delayed smaller rewards. The intent of this task is to determine if the deficit is in sensitivity to reward or punishment, or insensitivity to delayed consequences in favor of immediate ones. PD patients performed as well on this task as controls, suggesting that their impairment on the IGT either is from increased sensitivity of reward, decreased sensitivity to punishment, or responding to reward over punishment whether immediate or delayed (Kobayakawa, Tsuruya, & Kawamura, 2010).
Huntington's disease
Huntington's disease (HD) is an autosomal dominant inherited disorder characterized by degeneration of striatal medium spiny neurons and imaging reveals caudate atrophy. The disease progresses in the caudate from dorsal to ventral (Vonsattel et al., 1985), indicating that early deficits may involve more dorsolateral prefrontal processes than orbitofrontal ones. Performance on reward tasks in HD may help elucidate the role of the striatum in reward processing.
Secondary reward processing
Even early in HD, however, there may be reward processing abnormalities indicative of ventral striatal dysfunction. A recent study using the MID task in early HD patients (without motor manifestations) showed abnormal decreased activation of ventral striatum compared to controls in anticipating monetary reward or punishment (Enzi et al.,).
Reward-related decision-making
A deficit in planning (on the Tower of London task) with intact decision-making on a task of gambling based on probability in early HD patients is consistent with the known anatomy (Watkins et al., 2000). The tasks mediated by dorsal prefrontal and dorsal striatum are affected earlier. Patients with HD show fewer advantageous selections on the Iowa Gambling Task than controls or Parkinson's patients and this correlates with measures of memory and conceptualization (Stout et al., 2001). The same group also showed that during this task HD patients have decreased skin conduction responses to loss compared to controls (Campbell, Stout, & Finn, 2004).
Conclusions
There is limited information on reward processing in many of the neurodegenerative diseases. A positivity bias occurs in healthy aging and a negativity bias in MCI. Patients with Alzheimer's disease are largely only affected through apathy. Patients with bvFTD have overeating that may represent increased reward representation or decreased sensitivity to punishment. The early degeneration of multiple reward circuit structures suggests that further study may reveal impairment in reward processing. More careful analysis of patients with motor neuron disease may also reveal deficits in reward but this has not been established. Studies of Parkinson's disease largely vary depending on the medications the patient is taking, with dopaminergic agents leading to increased sensitivity to learning through positive outcomes and impulse control disorders. The dopaminergic deficiency of the disease itself leads to increased sensitivity to negative or punishing outcomes. Huntington's disease provides a model for the role of the striatum in reward processing, with early deficits in executive function, and there may be later impairment in processing of reward.
An accurate understanding of reward processing changes in these illnesses will require careful clarification of terms, as abnormalities may occur in processing primary rewards (which can also be related to primary sensation), secondary rewards (or a change in the relative value of primary and secondary rewards, e.g., food may be more valued than money or social gain in bvFTD), reward-based learning, or reward-based decision-making. To further define the impairments in reward in these illnesses it will also be necessary to use instruments that more directly assess the separate components of reward processing. Measures of reward-based learning and decision-making such as the gambling tasks often require a broad range of intact functions and have yielded highly variable results in degenerative illness, as has particularly been illustrated in Parkinson's disease. By testing simple sensory rewards, abstract rewards, anticipation of reward, and sensitivity to the timing of reward as well as corresponding punishments, the understanding of behavior change in each illness and the function of each component of the reward circuit will increase. This will inform the approach to addressing abnormal reward processing behaviors in neurodegenerative disease and the application of this knowledge to other illnesses with aberrant sensitivity to reward or punishment, including gambling, drug and alcohol addiction.
References
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, et al. Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Bagurdes LA, Mesulam MM, Gitelman DR, Weintraub S, Small DM. Modulation of the spatial attention network by incentives in healthy aging and mild cognitive impairment. Neuropsychologia. 2008;46(12):2943–2948. doi: 10.1016/j.neuropsychologia.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science (New York, N Y) 1997;275(5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bermudez MA, Schultz W. Reward magnitude coding in primate amygdala neurons. Journal of Neurophysiology. 2010;104(6):3424–3432. doi: 10.1152/jn.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: Mesolimbic computation of reward motivation. The European Journal of Neuroscience. 2012;35(7):1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. doi:10.1111/j.1460-9568.2012.07990.x; 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nature Neuroscience. 1999;2(4):382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Bodi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N, et al. Gluck MA. Reward-learning and the novelty-seeking personality: A between- and within-subjects study of the effects of dopamine agonists on young parkinson's patients. Brain : A Journal of Neurology. 2009;132(Pt 9):2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Labudda K, Kalbe E, Hilker R, Emmans D, Fuchs G, et al. Markowitsch HJ. Decision-making impairments in patients with parkinson's disease. Behavioural Neurology. 2004;15(3):77–85. doi: 10.1155/2004/578354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SB, Ridderinkhof KR. Aging and the neuroeconomics of decision making: A review. Cognitive, Affective & Behavioral Neuroscience. 2009;9(4):365–379. doi: 10.3758/CABN.9.4.365. doi:10.3758/CABN.9.4.365; 10.3758/CABN.9.4.365. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Stout JC, Finn PR. Reduced autonomic responsiveness to gambling task losses in huntington's disease. Journal of the International Neuropsychological Society : JINS. 2004;10(2):239–245. doi: 10.1017/S1355617704102105. [DOI] [PubMed] [Google Scholar]
- Carlino E, Benedetti F, Rainero I, Asteggiano G, Cappa G, Tarenzi L, et al. Pollo A. Pain perception and tolerance in patients with frontotemporal dementia. Pain. 2010;151(3):783–789. doi: 10.1016/j.pain.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Cilia R, Siri C, Marotta G, Isaias IU, De Gaspari D, Canesi M, et al. Antonini A. Functional abnormalities underlying pathological gambling in parkinson disease. Archives of Neurology. 2008;65(12):1604–1611. doi: 10.1001/archneur.65.12.1604. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain : A Journal of Neurology. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Altamirano L, D'Esposito M. Reversal learning in parkinson's disease depends on medication status and outcome valence. Neuropsychologia. 2006;44(10):1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and parkinson's disease: Influence of dopatherapy. Neuropsychologia. 2002;40(13):2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Delazer M, Sinz H, Zamarian L, Benke T. Decision-making with explicit and stable rules in mild alzheimer's disease. Neuropsychologia. 2007;45(8):1632–1641. doi: 10.1016/j.neuropsychologia.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Delazer M, Sinz H, Zamarian L, Stockner H, Seppi K, Wenning GK, et al. Poewe W. Decision making under risk and under ambiguity in parkinson's disease. Neuropsychologia. 2009;47(8-9):1901–1908. doi: 10.1016/j.neuropsychologia.2009.02.034. [DOI] [PubMed] [Google Scholar]
- Döhnel K, Sommer M, Ibach B, Rothmayr C, Meinhardt J, Hajak G. Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia. 2008;46(1):37–48. doi: 10.1016/j.neuropsychologia.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Enzi B, Edel M, Lissek S, Peters S, Hoffmann R, Nicolas V, et al. Saft C. Altered ventral striatal activation during reward and punishment processing in premanifest huntington's disease: A functional magnetic resonance study. Experimental Neurology. doi: 10.1016/j.expneurol.2012.02.003. 0. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Pine DS. Choice selection and reward anticipation: An fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, et al. London ED. Decision-making in a risk-taking task: A PET study. Neuropsychopharmacology. 2002;26(5):682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Euteneuer F, Schaefer F, Stuermer R, Boucsein W, Timmermann L, Barbe MT, et al. Kalbe E. Dissociation of decision-making under ambiguity and decision-making under risk in patients with parkinson's disease: A neuropsychological and psychophysiological study. Neuropsychologia. 2009;47(13):2882–2890. doi: 10.1016/j.neuropsychologia.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Evans AH, Katzenschlager R, Paviour D, O'Sullivan JD, Appel S, Lawrence AD, Lees AJ. Punding in parkinson's disease: Its relation to the dopamine dysregulation syndrome. Movement Disorders. 2004;19(4):397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- Farley IJ, Price KS, Hornykiewicz O. Dopamine in thelimbic regions of the human brain: Normal and abnormal. Advances in Biochemical Psychopharmacology. 1977;16:57–64. [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from a reversal learning paradigm. Brain : A Journal of Neurology. 2003;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Francis PT, Holmes C, Webster MT, Stratmann GC, Procter AW, Bowen DM. Preliminary neurochemical findings in non-alzheimer dementia due to lobar atrophy. Dementia (Basel, Switzerland) 1993;4(3-4):172–177. doi: 10.1159/000107319. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Kong L. Learning to avoid in older age. Psychology and Aging. 2008;23(2):392–398. doi: 10.1037/0882-7974.23.2.392. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O'Reilly RC. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Pizzolato G, Bianchetti A, Chierichetti F, Ferlin G, Battistin L, Trabucchi M. Single photon emission computed tomography with [99Tc]-HM-PAO and [123I]-IBZM in alzheimer's disease and dementia of frontal type: Preliminary results. Acta Neurologica Scandinavica. 1994;89(3):199–203. doi: 10.1111/j.1600-0404.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, O'Sullivan JD, Turner K, Manson AJ, Lees AJL. Hedonistic homeostatic dysregulation in patients with parkinson's disease on dopamine replacement therapies. Journal of Neurology, Neurosurgery & Psychiatry. 2000;68(4):423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. doi:http://dx.doi.org/10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science (New York, N Y) 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2006;26(51):13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, et al. Kawato M. A neural correlate of reward-based behavioral learning in caudate nucleus: A functional magnetic resonance imaging study of a stochastic decision task. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24(7):1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, Lanctot KL. Methylphenidate for the treatment of apathy in alzheimer disease: Prediction of response using dextroamphetamine challenge. Journal of Clinical Psychopharmacology. 2008;28(3):296–301. doi: 10.1097/JCP.0b013e318172b479. [DOI] [PubMed] [Google Scholar]
- Hoefer M, Allison SC, Schauer GF, Neuhaus JM, Hall J, Dang JN, et al. Rosen HJ. Fear conditioning in frontotemporal lobar degeneration and alzheimer's disease. Brain : A Journal of Neurology. 2008;131(Pt 6):1646–1657. doi: 10.1093/brain/awn082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Junque C, Tolosa E, Marti MJ, Valldeoriola F, Bargallo N, Zarei M. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early parkinson's disease. The European Journal of Neuroscience. 2009;30(6):1162–1171. doi: 10.1111/j.1460-9568.2009.06892.x. [DOI] [PubMed] [Google Scholar]
- Ismail Z, Herrmann N, Rothenburg LS, Cotter A, Leibovitch FS, Rafi-Tari S, et al. Lanctot KL. A functional neuroimaging study of appetite loss in alzheimer's disease. Journal of the Neurological Sciences. 2008;271(1-2):97–103. doi: 10.1016/j.jns.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Jacobson A, Green E, Murphy C. Age-related functional changes in gustatory and reward processing regions: An fMRI study. NeuroImage. 2010;53(2):602–610. doi: 10.1016/j.neuroimage.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitz M, Koerts J, Kortekaas R, Renken R, de Jong BM, Leenders KL. Prefrontal cortex and striatal activation by feedback in parkinson's disease. Brain Research. 2008;1236:225–233. doi: 10.1016/j.brainres.2008.07.110. [DOI] [PubMed] [Google Scholar]
- Keri S, Moustafa AA, Myers CE, Benedek G, Gluck MA. {Alpha}-synuclein gene duplication impairs reward learning. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15992–15994. doi: 10.1073/pnas.1006068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic parkinson's disease. New England Journal of Medicine. 1988;318(14):876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kobayakawa M, Koyama S, Mimura M, Kawamura M. Decision making in parkinson's disease: Analysis of behavioral and physiological patterns in the iowa gambling task. Movement Disorders : Official Journal of the Movement Disorder Society. 2008;23(4):547–552. doi: 10.1002/mds.21865. [DOI] [PubMed] [Google Scholar]
- Kobayakawa M, Tsuruya N, Kawamura M. Sensitivity to reward and punishment in parkinson's disease: An analysis of behavioral patterns using a modified version of the iowa gambling task. Parkinsonism & Related Disorders. 2010;16(7):453–457. doi: 10.1016/j.parkreldis.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex (New York, N Y: 1991) 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Krueger CE, Bird AC, Growdon ME, Jang JY, Miller BL, Kramer JH. Conflict monitoring in early frontotemporal dementia. Neurology. 2009;73(5):349–355. doi: 10.1212/WNL.0b013e3181b04b24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lanctôt KL, Moosa S, Herrmann N, Leibovitch FS, Rothenburg L, Cotter A, Black SE. A SPECT study of apathy in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2007;24(1):65–72. doi: 10.1159/000103633. [DOI] [PubMed] [Google Scholar]
- Lanctot KL, Herrmann N, Black SE, Ryan M, Rothenburg LS, Liu BA, Busto UE. Apathy associated with alzheimer disease: Use of dextroamphetamine challenge. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry. 2008;16(7):551–557. doi: 10.1097/JGP.0b013e318170a6d1. [DOI] [PubMed] [Google Scholar]
- Lee D, Seo H, Jung MW. Neural basis of reinforcement learning and decision making. Annual Review of Neuroscience. 2012;35:287–308. doi: 10.1146/annurev-neuro-062111-150512. doi:10.1146/annurev-neuro-062111-150512; 10.1146/annurev-neuro-062111-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, Ruth TJ, et al. Stoessl AJ. Effects of expectation on placebo-induced dopamine release in parkinson disease. Archives of General Psychiatry. 2010;67(8):857–865. doi: 10.1001/archgenpsychiatry.2010.88. [DOI] [PubMed] [Google Scholar]
- Loewenstein G, Rick S, Cohen JD. Neuroeconomics. Annual Review of Psychology. 2008;59(1):647–672. doi: 10.1146/annurev.psych.59.103006.093710. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60(7):1094–1097. doi: 10.1212/01.wnl.0000055861.95202.8d. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Dale CL, Hough MG. Preparatory allocation of attention and adjustments in conflict processing. NeuroImage. 2007;35(2):949–958. doi: 10.1016/j.neuroimage.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45(8):1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125(3):624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR. Reward-based decision-making and aging. Brain Research Bulletin. 2005;67(5):382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mattox ST, Valle-Inclan F, Hackley SA. Psychophysiological evidence for impaired reward anticipation in parkinson's disease. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2006;117(10):2144–2153. doi: 10.1016/j.clinph.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Meier SL, Charleston AJ, Tippett LJ. Cognitive and behavioural deficits associated with the orbitomedial prefrontal cortex in amyotrophic lateral sclerosis. Brain : A Journal of Neurology. 2010;133(11):3444–3457. doi: 10.1093/brain/awq254. [DOI] [PubMed] [Google Scholar]
- Mell T, Heekeren HR, Marschner A, Wartenburger I, Villringer A, Reischies FM. Effect of aging on stimulus-reward association learning. Neuropsychologia. 2005;43(4):554–563. doi: 10.1016/j.neuropsychologia.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia (Basel, Switzerland) 1995;6(4):195–199. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- Mimura M, Oeda R, Kawamura M. Impaired decision-making in parkinson's disease. Parkinsonism & Related Disorders. 2006;12(3):169–175. doi: 10.1016/j.parkreldis.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, et al. Shoda Y. ‘Willpower’ over the life span: Decomposing self-regulation. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. Re-valuing the amygdala. Current Opinion in Neurobiology. 2010;20(2):221–230. doi: 10.1016/j.conb.2010.02.007. doi:http://dx.doi.org/10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41(2):147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonabarraga J, García-Sánchez C, Llebaria G, Pascual-Sedano B, Gironell A, Kulisevsky J. Controlled study of decision-making and cognitive impairment in parkinson's disease. Movement Disorders. 2007;22(10):1430–1435. doi: 10.1002/mds.21457. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Perretta JG, Pari G, Beninger RJ. Effects of parkinson disease on two putative nondeclarative learning tasks: Probabilistic classification and gambling. Cognitive and Behavioral Neurology : Official Journal of the Society for Behavioral and Cognitive Neurology. 2005;18(4):185–192. doi: 10.1097/01.wnn.0000187939.81541.1d. [DOI] [PubMed] [Google Scholar]
- Persico AM, Reich S, Henningfield JE, Kuhar MJ, Uhl GR. Parkinsonian patients report blunted subjective effects of methylphenidate. Experimental and Clinical Psychopharmacology. 1998;6(1):54–63. doi: 10.1037//1064-1297.6.1.54. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms KE, Omar R, Hailstone JC, Warren JD. Flavour processing in semantic dementia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46(6):761–768. doi: 10.1016/j.cortex.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluck GC, Brown RG. Apathy in parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73(6):636–642. doi: 10.1136/jnnp.73.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Frosini D, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Decision making in de novo parkinson's disease. Movement Disorders : Official Journal of the Movement Disorder Society. 2010;25(10):1432–1436. doi: 10.1002/mds.23098. [DOI] [PubMed] [Google Scholar]
- Rahman S, Robbins TW, Hodges JR, Mehta MA, Nestor PJ, Clark L, Sahakian BJ. Methylphenidate (‘ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2006;31(3):651–658. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain : A Journal of Neurology. 1999;122(Pt 8):1469–1493. doi: 10.1093/brain/122.8.1469. Pt 8. [DOI] [PubMed] [Google Scholar]
- Rami L, Loy CT, Hailstone J, Warren JD. Odour identification in frontotemporal lobar degeneration. Journal of Neurology. 2007;254(4):431–435. doi: 10.1007/s00415-006-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Laine M, Kaasinen V, Norvasuo-Heila MK, Nagren K, Helenius H. Striatal dopamine transporter and extrapyramidal symptoms in frontotemporal dementia. Neurology. 2002;58(10):1489–1493. doi: 10.1212/wnl.58.10.1489. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19(20):9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57(12):1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cerebral Cortex (New York, N Y: 1991) 2003;13(3):308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Progress in Neurobiology. 2008;86(3):216–244. doi: 10.1016/j.pneurobio.2008.09.001. doi:http://dx.doi.org/10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, De Araujo IET. Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience. 2003;18(3):695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain : A Journal of Neurology. 2005;128(Pt 11):2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, et al. Owen AM. Parkinson's disease and dopaminergic therapy--differential effects on movement, reward and cognition. Brain : A Journal of Neurology. 2008;131(Pt 8):2094–2105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RB, Lazzaro SC, Lau B, Myers CE, Gluck MA, Glimcher PW. Dopaminergic drugs modulate learning rates and perseveration in parkinson's patients in a dynamic foraging task. The Journal of Neuroscience. 2009;29(48):15104–15114. doi: 10.1523/JNEUROSCI.3524-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10(6):787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychological Science : A Journal of the American Psychological Society / APS. 2008;19(4):320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310(5752):1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, Duzel E. Ageing and early-stage parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain : A Journal of Neurology. 2007;130(Pt 9):2412–2424. doi: 10.1093/brain/awm147. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: Basic and recent data. Behavioral and Brain Functions : BBF. 2010;6:24. doi: 10.1186/1744-9081-6-24. 9081-6-24. doi:10.1186/1744-9081-6-24; 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science (New York, N Y) 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology. 2008;65(2):249. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher J. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2013;37(4):681–696. doi: 10.1016/j.neubiorev.2013.02.002. doi:http://dx.doi.org/10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2007;27(18):4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Singer T, Dolan R. The neurobiology of punishment. Nature Reviews Neuroscience. 2007;8(4):300–311. doi: 10.1038/nrn2119. [DOI] [PubMed] [Google Scholar]
- Sienkiewicz-Jarosz H, Scinska A, Kuran W, Ryglewicz D, Rogowski A, Wrobel E, et al. Bienkowski P. Taste responses in patients with Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(1):40–46. doi: 10.1136/jnnp.2003.033373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Howard JH, Howard DV. Adult age differences in learning from positive and negative probabilistic feedback. Neuropsychology. 2010;24(4):534–541. doi: 10.1037/a0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinz H, Zamarian L, Benke T, Wenning GK, Delazer M. Impact of ambiguity and risk on decision making in mild alzheimer's disease. Neuropsychologia. 2008;46(7):2043–2055. doi: 10.1016/j.neuropsychologia.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Sjogren M, Wikkelso C, Ostling S, Wallin A, Blennow K. Biological correlates of clinical subgroups of alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2002;14(4):191–197. doi: 10.1159/000066025. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rodawalt WC, Siemers ER. Risky decision making in huntington's disease. Journal of the International Neuropsychological Society : JINS. 2001;7(1):92–101. doi: 10.1017/s1355617701711095. [DOI] [PubMed] [Google Scholar]
- Thiel A, Hilker R, Kessler J, Habedank B, Herholz K, Heiss WD. Activation of basal ganglia loops in idiopathic parkinson's disease: A PET study. Journal of Neural Transmission (Vienna, Austria : 1996) 2003;110(11):1289–1301. doi: 10.1007/s00702-003-0041-7. [DOI] [PubMed] [Google Scholar]
- Torralva T, Dorrego F, Sabe L, Chemerinski E, Starkstein SE. Impairments of social cognition and decision making in alzheimer's disease. International Psychogeriatrics / IPA. 2000;12(3):359–368. doi: 10.1017/s1041610200006463. [DOI] [PubMed] [Google Scholar]
- Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, et al. Manes F. The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia. 2007;45(2):342–349. doi: 10.1016/j.neuropsychologia.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain : A Journal of Neurology. 2009;132(Pt 5):1299–1309. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: A trigger for pathological gambling in parkinson's disease? Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2009;34(13):2758–2766. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, et al. Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D2Receptors. Journal of Pharmacology and Experimental Therapeutics. 1999;291(1):409. [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of huntington's disease. Journal of Neuropathology & Experimental Neurology. 1985;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Watkins LH, Rogers RD, Lawrence AD, Sahakian BJ, Rosser AE, Robbins TW. Impaired planning but intact decision making in early huntington's disease: Implications for specific fronto-striatal pathology. Neuropsychologia. 2000;38(8):1112–1125. doi: 10.1016/s0028-3932(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Sampson EL, Loy CT, Warren JE, Rossor MN, Fox NC, Warren JD. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. NeuroImage. 2007;35(1):207–213. doi: 10.1016/j.neuroimage.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini ML, Seeley WW, Rankin K, Lee SS, Matthews BR, Miller BL. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69(14):1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of parkinson's disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology : Official Journal of the Society for Behavioral and Cognitive Neurology. 2003;16(4):193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133(5):1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]