Abstract
Molecular annotated patient-derived xenograft (PDX) models are useful for the preclinical investigation of anticancer drugs and individualized anticancer therapy. We established 23 PDXs from 88 surgical specimens of lung cancer patients and determined gene mutations in these PDXs and their paired primary tumors by ultradeep exome sequencing on 202 cancer-related genes. The numbers of primary tumors with deleterious mutations in TP53, KRAS, PI3KCA, ALK, STK11, and EGFR were 43.5%, 21.7%, 17.4%, 17.4%, 13.0%, and 8.7%, respectively. Other genes with deleterious mutations in ≥3 (13.0%) primary tumors were MLL3, SETD2, ATM, ARID1A, CRIPAK, HGF, BAI3, EP300, KDR, PDGRRA and RUNX1. Of 315 mutations detected in the primary tumors, 293 (93%) were also detected in their corresponding PDXs, indicating that PDXs have the capacity to recapitulate the mutations in primary tumors. Nevertheless, a substantial number of mutations had higher allele frequencies in the PDXs than in the primary tumors, or were not detectable in the primary tumor, suggesting the possibility of tumor cell enrichment in PDXs or heterogeneity in the primary tumors. The molecularly annotated PDXs generated from this study could be useful for future translational studies.
Keywords: Lung cancer, Gene mutations, Tumor models, Patient-derived xenografts, Biomarkers
Introduction
Lung cancer is the leading cause of cancer-related deaths both in the United States and worldwide, with an annual global incidence of about 1.6 million and mortality of 1.4–1.5 million [1–3]. Recent advances in genomic profiling have led to the identification of a number of frequently mutated genes in lung cancer [4–7]. Lung cancers with the same histological diagnosis and clinical stages can be classified into molecular subgroups based on gene mutations. Substantial efforts have been made to develop genotype-specific anticancer therapeutics. The finding that lung cancer cells with mutations in the epidermal growth factor receptor gene (EGFR) are highly susceptible to the EGFR inhibitors gefitinib [8–10], erlotinib [8,11] and afatinib has made these agents the first choice for treating EGFR mutant lung cancer. Both gefitinib and erlotinib have been reported to significantly prolong progression-free survival in patients with EGFR-mutant lung cancer [12,13]. Similarly, small molecular inhibitors for anaplastic lymphoma kinase (ALK) and ROS1 have been proven to be highly effective for treatment of lung cancers with ALK and ROS1 gene translocations [14–16]. However, despite the excitement accompanying the targeted therapeutics, only a subset of patients with the aberration respond and responses are often unfortunately brief. Furthermore, our knowledge of genetic alterations, their functional consequences and combinatorial effects in lung cancer is still not comprehensive. For most potential driver mutations identified in lung cancer, there are no effective therapeutic agents available. The success of the EGFR inhibitors underscores the urgency of developing effective genotype-specific anticancer therapeutics.
Anticancer drug development is often impeded by a lack of pre-clinical tumor models that are highly predictive of therapeutic effects in humans. Previous studies have shown that in vitro cell line models and in vivo xenograft tumors derived from established human cancer cell lines have limited predictive value for antitumor activity of a drug in clinical trials [17–19]. Anticancer agents that showed promising in vivo antitumor activity in xenograft tumor models have often been ineffective for the same type of cancer in clinical trials [20]. In fact, only about 5% of anticancer agents evaluated in human studies between 1991 and 2000 were successfully registered [20]. The majority of failures in late-phase clinical trials result from a lack of clinical efficacy caused primarily by the lack of efficacy proof of concept in humans, lack of predictive biomarkers to identify patient responders, and safety issues [20,21]. Thus, clinically relevant tumor models that accurately predict therapeutic efficacies would be highly valuable for anticancer drug development.
Evidence from recent studies has shown that patient-derived xenografts (PDXs) established directly from patients’ primary tumors preserve the histomorphologic features, heterogeneity, gene expression pattern (including cytokine expression by tumor stromal cells), DNA copy number alterations, and gene mutations of the original tumors [22–24]. These features were preserved after a series of passages of the tumorgrafts in mice [22,24]. When PDXs were treated with agents used in a parallel patient population, response rates similar to those reported in human studies were observed, suggesting that the PDX model is clinically relevant for evaluating the efficacy of anticancer drugs [22,25–28]. A remarkable correlation between drug activity in PDXs and clinical outcome was reported when patients with advanced cancer were treated with selected regimens based on the treatment responses of their PDX [29,30], suggesting that PDXs could provide robust models for identifying effective treatment for cancer patients and for predicting clinical efficacy of drug candidates. Consequently, PDXs derived from various types of cancers have been reported recently, including those established from lung cancer [23,26,28,31]. Those studies have demonstrated the feasibility of using PDXs for translational studies in drug development, for molecular characterization of cancer biology, and for strategic development of individualized therapy. Nevertheless, few molecularly-annotated lung cancer PDXs are reported in literature and are not readily available for preclinical studies.
Our purpose here was to develop molecularly annotated PDXs for evaluation of investigational anticancer agents and mechanistic characterization of lung cancers. We established PDXs from surgical specimens of lung cancer patients and characterized the gene mutations in those PDXs and the corresponding primary tumors. Our results show that some novel genes were frequently mutated in primary lung cancers and that the mutations in primary tumors can be recapitulated by their corresponding PDX.
Materials and methods
Human lung tissue specimens
Fresh lung cancer samples were collected in 2012 and 2013 from surgically resected specimens under approved research protocols with informed consent from the patients. This study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center.
Generation of patient-derived xenografts in immune-defective mice
All animal experiments were carried out in accordance with Guidelines for the Care and Use of Laboratory Animals (NIH publication number 85-23) and the institutional guidelines of MD Anderson Cancer Center. Six- to eight-week-old immune-defective non-obese or diabetic severe combined immunodeficiency (NOD-SCID) mice were obtained from Jackson Laboratory (Bar Harbor, Maine) or Charles River Laboratories, Inc. (Wilmington, MA).
Fresh surgical specimens of lung cancer were cut to about 2 mm3 in size, briefly soaked in matrigel, and implanted into the flank subcutaneous space of mice (2 or 3 mice/patient specimen), as described elsewhere within 1 hour of surgical resection [32]. The mice were monitored for up to 10 months for tumor growth. The tumors were harvested when they reached 1.5 cm in diameter. The tumors (labeled F1 for the first passage in animals) were divided into 2–3 mm3 specimens which were frozen in liquid nitrogen for future investigation, analyzed for molecular biological characterization, or reimplanted into mice to generate more tumorgrafts (F2, F3, etc., for subsequent passages).
Whole-exome sequencing for 202 cancer-related genes
Genomic DNA was isolated from primary tumor tissues and PDX tissues by proteinase K digestion and phenol extraction. The whole-exome sequencing for 202 cancer-related genes is shown in Supplement Table S1. Briefly, DNA samples were quantified by Qubit (Invitrogen, Grand Island, NY) and their quality was assessed using Genomic DNA Tape for the 2200 Tapestation (Agilent, Santa Clara, CA). DNA from each sample was sheared by sonication using an E220 instrument (Covaris, Woburn, MA). To ensure the proper fragment size, samples were checked on the TapeStation using the DNA High Sensitivity kit (Agilent). The sheared DNA proceeded to library preparation with the KAPA library preparation kit (KAPA Biosystems, Wilmington, MA) following the manufacturer's protocol. Samples were quantified using the KAPA qPCR quantification kit. Equimolar amounts of DNA were pooled for capture (8–12 samples per pool).
After library preparation, 202 genes predicted to be clinically relevant in cancer were selected for capture. Biotin-labeled probes were designed with Roche Nimblegen for capturing all exons in the 202 genes, following the manufacturer's protocol for the capture step. The cutoff for enrichment was 50-fold minimum. The captured libraries were sequenced on a HiSeq 2000 (Illumina Inc., San Diego, CA) on a version 3 TruSeq paired end flow cell according to the manufacturer's instructions at a cluster density between 700 and 1000 K clusters/mm2. Sequencing was performed on a HiSeq 2000 for 2 × 100 paired end reads with a 7-nt read for indexes using Cycle Sequencing v3 reagents (Illumina). The resulting BCL files containing the sequence data were converted to “.fastq.gz” files, and individual libraries within the samples were demultiplexed using CASAVA 1.8.2 software with no mismatches.
Data analysis
We aligned the T200 target-capture deep-sequencing data to human reference assembly hg19 by using Burrows–Wheeler Aligner software [33] and removed duplicated reads by using SAMtools [34] (both Sourceforge open source software, Slashdot Media, San Francisco, CA). We called single nucleotide variants and small insertions/deletions by using VarScan2 [35] and called copy number alterations by using a previously published algorithm [36] that reports gain or loss status of each exon. Genomic DNA from the SCID mouse was used to exclude nucleotide variants observed in the mouse genome. To ensure specificity, variants with an allele frequency less than 10% were not reported. To understand the potential functional consequence of detected variants, we compared them with the dbSNP, COSMIC [37], and TCGA databases and annotated them using SIFT [38], Polyphen [39], Condel [40], and Mutation Assessor [41].
Results
Establishing patient-derived xenografts from lung cancer specimens
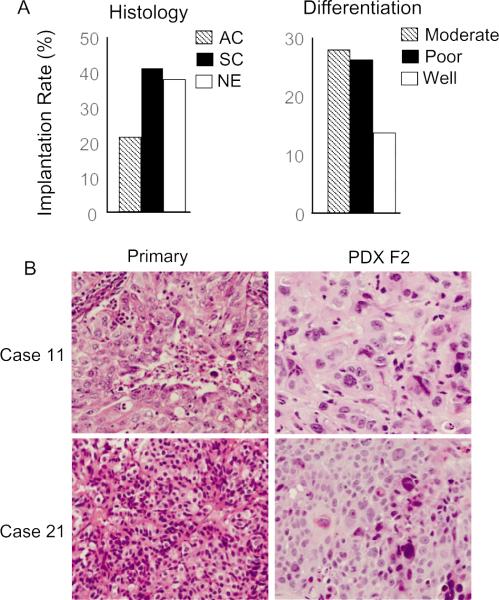
We collected surgically resected tumor samples from 88 NSCLC patients and implanted each specimen into 2–3 NOD-SCID mice to develop PDXs. We obtained 23 PDXs (Table 1). The overall implantation rate for development of a PDX was 26%. Squamous cancer and neuroendocrinal carcinoma had relatively higher implantation rates than adenocarcinoma. Moderately and poorly differentiated tumors had relative high implantation rates than well differentiated tumors (Fig. 1A). Nevertheless, the difference among those groups was not statistically significant (P = 0.09–0.35). The time from inoculation of the surgical specimen until harvest of the first generation of PDX (1.5 cm in diameter) ranged from 2 to 10 months, with an average of 4 months. The tumor engraftment rate is comparable to the rate reported for establishment of subcutaneous PDXs in SCID mice [31] but lower than that reported for engraftment from tumor specimens implanted under the renal capsule [26]. The tumors were harvested when they reach 1.5 cm in diameter. The tumors (labeled as F1 for the first passage in animals) were divided into several portions of about 2–3 mm3, which were frozen in liquid nitrogen for future investigation, or analyzed for biomarker and molecular biological characterization, or replanted into mice for generating additional generations of tumorgrafts (F2, F3, etc., for subsequent passages). Tumors capable of generating subsequent passages were used for molecular characterizations. All the F1 PDXs generated in this study could successfully generate F2–F4 tumors in nude mice. We performed histological analysis on two F2 PDXs and their corresponding primary tumors. The results showed that the histological morphology in these PDXs matched with that of primary tumors (Fig. 1B).
Table 1.
Patient demographic and clinical information for primary lung cancers (n = 23).
| Age/race/sex | Histology | Stage | Passage* | Differentiation | Smoking |
|---|---|---|---|---|---|
| 72/White/Female | SC | IIB (T2N0M0) | 2 | Moderate | Yes |
| 70/White/Male | SC | IB (T2AN0M0) | 2 | Moderate | Yes |
| 66/White/Female | SC | IB (T2N0M0) | 2 | Moderate | Yes |
| 63/White/Female | SC | IB (T2N0M0) | 2 | Poor | Yes |
| 38/White/Female | SC | IIB (T2BN1M0) | 2 | Moderate | No |
| 53/White/Female | SC | II (T2AN1M0) | 2 | Well | Yes |
| 49/White/Female | SC | IIIA (T3N1M0) | 2 | Poor | Yes |
| 57/African/Male | SC | IIB (T3N0M0) | 4 | Moderate | Yes |
| 63/Caucasia/Male | SC | IIA (T2N1M0) | 4 | Moderate | Yes |
| 54/Caucasia/Male | AC | IIIA(T2N2M0) | 3 | Poor | Yes |
| 58/White/Female | AC | II (T3N0M0) | 2 | Poor | Yes |
| 75/White/Male | AC | II (T2AN0M0) | 3 | Well | Yes |
| 55/Black/Female | AC | IB (T2N0M0) | 3 | Moderate | No |
| 73/White/Female | AC | IB (T2AN0M0) | 1 | Moderate | Yes |
| 55/Hispanic/Male | AC | IIB (T2N0M0) | 3 | Moderate | Yes |
| 62/Black/Female | AC | IB (T2N0M0) | 2 | Well | Yes |
| 68/Hispanic/Male | AC | IIIA(T2AN0M0) | 2 | Moderate | Yes |
| 51/White/Male | AC | IA(T1N0M0) | 2 | Poor | Yes |
| 64/Asian/Male | AC | IB (T2N0M0) | 2 | Moderate | Yes |
| 85/White/Male | AC | IA(T2AN0M0) | 2 | Poor | Yes |
| 54/White/Female | NE | IIIA | 2 | Moderate | Yes |
| 51/Hispanic/Male | Carcinoma/NE | IIIA(T3N1M0) | 3 | Poor | Yes |
| 43/White/Male | Large cell/NE | IIIA(T3N1M0) | 3 | Poor | Yes |
SC, squamous cell carcinoma; AC, adenocarcinoma; NE, neuroendocrine cancer.
Passage number indicates PDX passages (F) that were used in sequencing analysis.
Fig. 1.
Generation of lung cancer PDXs. (A) The implantation rates based on histo-pathological diagnosis and differentiations. AC: Adenocarcinoma; SC: squamous carcinoma; NE: neuroendocrine cancer. (B) Histological analysis of two PDXs and their corresponding tumors. The case numbers are the same as shown in Table 1 and Fig. 2. Case 11: poorly differentiated adenocarcinoma; Case 21: moderately differentiated neuroendocrine cancer.
Batch consistency of exome sequencing data
We isolated genomic DNA from 23 pairs of primary tumors and their corresponding PDXs (passages 1–4). Clinical information for the 23 patients is shown in Table 1. Eleven of the tumors were adenocarcinoma, 9 were squamous cell carcinoma, and 3 were neuroendocrine tumors or had neuroendocrine features. The DNA was subjected to exome sequencing analysis in 4 different batches of assays. The mean coverage for each data point was 500–700 reads. With a few exceptions, most data points had coverage of greater than 200 reads. One pair of samples was analyzed twice, and the two sets of results for this pair of samples are compared in Table 2. Except for the mutations with allele frequencies of about 10%, mutations were reliably detected in the same sample in the 2 different assays, with high consistency in their allele frequencies. This result demonstrated that, except for mutations detected at a borderline allele frequency, most mutations can be reliably detected by the assay approach.
Table 2.
Consistency of 2 different assay results for the same pair of samples.
| Genes | Mutation | Primary test1 allele frequent (%) | Primary test2 allele frequent (%) | PDX test1 allele frequent (%) | PDX test2 allele frequent (%) |
|---|---|---|---|---|---|
| NOTCH2 | A3V | 10.7 | ND | ND | ND |
| NOTCH2 | A3S | 10.8 | ND | ND | ND |
| RYR2 | A979S | 42.8 | 40.2 | 45.0 | 47.0 |
| TSC2 | A1141V | 40.6 | 40.1 | 47.4 | 55.8 |
| TP53 | T175H | 24.4 | 23.6 | ND | ND |
| RNF213 | V2806I | 67.8 | 64.3 | 49.2 | 52.4 |
| JAK3 | G284R | 59.1 | 61.1 | 41.3 | 43.9 |
| FLT4 | A628P | 53.2 | 55.2 | 50.0 | 50.0 |
| RELN | T444M | 61.0 | 58.5 | 44.4 | 50.0 |
| FAM135B | Q70Q | 48.2 | 46.3 | 48.5 | 48.6 |
| NOTCH1 | A2279V | 37.5 | 33.9 | 52.6 | 45.0 |
| KDM6A | 1421T | ND | ND | 10.2 | ND |
| AR | Q65L | ND | ND | 10.5 | ND |
ND, not detected.
Gene mutations in primary tumor tissues
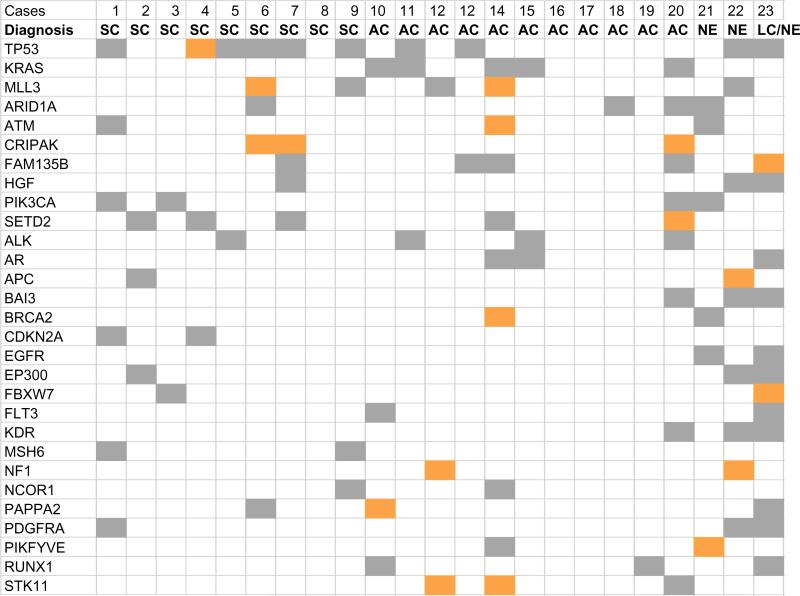
The number of mutations detected in primary tumor samples varied from 2 to 103. A total of 315 mutations were detected in 122 genes in the 23 primary tumors, an average of 13.7 mutations/ tumor. Possible deleterious or damaging mutations were detected in 91 genes, an average of about 8.8/tumor (range, 1–36). Fig. 2 shows the mutation status for the 23 primary tumors of 29 genes that had a potentially deleterious mutation in at least 2 primary tumors. Those genes were highly mutated in the tumors from patients 14, 20, 22, and 23, suggesting possible genomic instability in those samples. In contrast, the tumors of patients 8, 16–19 had very few mutations. Because only a few of these patients had disease recurrence or had died, we were not able to correlate gene mutations with recurrence or survival outcome.
Fig. 2.
Mutation status in 23 primary tumors of 29 genes with a deleterious mutation in at least 2 of the tumors. The 23 cases of lung cancer are arranged from left to right in the same order as in Table 1. SC: squamous cell carcinoma; AC: adenocarcinoma; NE: neuroendocrine cancer; LC: large cell cancer. Orange: stop or frame-shift mutations; gray: deleterious or damaging mis-sense mutations.
Table 3 lists the most frequently mutated genes in the primary tumors and Table 4 the mutated kinase genes detected in the primary tumors. The numbers of primary tumor with deleterious mutations previously implicated in therapeutic responses for lung cancer patients in TP53, KRAS, PI3KCA, ALK, STK11, and EGFR were 10, 5, 4, 4, 3, and 2, respectively. Other genes with deleterious mutations in ≥3 primary tumors were MLL3, SETD2, ATM, ARID1A, CRIPAK, FAM135B, HGF, AR, BAI3, EP300, KDR, PAPPA2, PDGRRA and RUNX1. Several of those genes, such as ATM [7], MLL3 [42], and SETD2 [6,43], were recently reported to be frequently mutated/changed in lung cancers, suggesting that their mutations may be used as biomarkers for these cancers. A number of large genes included in the analysis, such as CSDM3, CSDM1, HYDIN, LRP2, LRP1B, PCLO, and RYR2, most of them encoding proteins with >4000 amino acids, were also found to have high mutation frequencies. The frequent mutations detected in those large genes are consistent with a previous report on their mutations in various cancer samples, possibly due to mutational heterogeneity associated with gene expression levels and DNA replication times during cell cycles [44].
Table 3.
Common deleterious mutations detected in 23 primary tumors.
| Genes | Mutations number (%) | Proteins | Protein functions |
|---|---|---|---|
| TP53 | 10 (43.5) | Tumor protein p53 | Cell cycle, apoptosis, DNA repair |
| KRAS | 5 (21.7) | KRAS | RAS GTPase |
| MLL3 | 5 (21.7) | Histon-lysine N-methyltransferase | Mixed-lineage leukemia3, DNA repair |
| SETD2 | 5 (21.7) | Histon-lysine N-methyltransferase | DNA repair |
| ARID1A | 4 (17.4) | AT rich interactive domain 1A | Chromatin remodeling |
| PIK3CA | 4 (17.4) | PI3 kinase catalytic α unit | PI3K signaling |
| ALK | 4 (17.4) | Anaplastic lymphoma receptor kinase | Signal transduction |
| STK11 | 3 (13.0) | Liver kinase B1 (LKB1) | Signal transduction |
| ATM | 3 (13.0) | ATM Kinase | DNA repair |
Table 4.
Mutations in kinase and kinase regulators.
| Mutations number (%) | Genes of kinases and their regulators |
|---|---|
| ≥3 (13.0) | ALK, ATM, CRIPAK, IGF1R, KDR, PDGFRA, PIK3CA, STK11 |
| 2 (8.7) | CDK6, EGFR,FLT3, PIKFYVE,CDKN2A, PIK3CG, TGFBR2 |
| 1 (4.3) | ABL1, ARAF, ATR, AURKA, BRAF, CDK4, CHEK1, DDR2, FLT4, JAK1, JAK2, JAK3, KIT, MAP3K1, MAP3K4, PTK2, PTEN |
Gene mutations in patient-derived xenografts
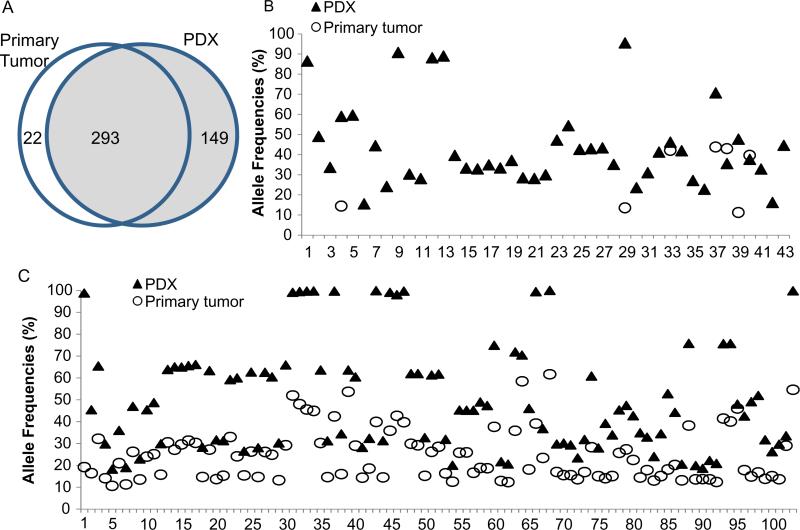
Of 315 mutations detected in primary tumors, 293 (93%) were also detected in their corresponding PDXs, suggesting that PDXs were able to recapitulate the majority of mutations in primary tumors. Nevertheless, there were 149 mutations detected in PDX that were not detected in primary tumors (Fig. 3A). A substantial number of the mutations detected in both primary tumors and PDXs had dramatic differences in their allele frequencies (>1.5 fold). Examples of those variations are shown in Fig. 3B and C. In the pair represented in Fig. 3B, 7 mutations were detected in the primary tumor but 43 mutations were detected in the PDX. In the pair shown in Fig. 3C, all 103 mutations were detected in both primary tumor and PDX, but a substantial number of the mutations had higher allele frequencies in the PDX than in the primary tumor. Those differences could be caused either by enrichment of tumor cells in the PDX or by heterogeneity in the primary tumor. It is important to note that the PDXs were derived from a region of the primary tumor different from that from which the DNA was isolated for sequencing.
Fig. 3.
Comparison of mutations in primary tumors and corresponding PDXs. (A) Mutations identified in 23 pairs of primary tumors and PDXs, showing numbers of unique and common mutations. (B and C) Allele frequencies of mutations in 2 pairs of primary tumors and their corresponding PDXs. The numbers on the X-axis represent individual mutations detected in primary tumor and/or PDX. As shown in the graphs, the allele frequencies in PDXs are often dramatically greater than in the primary tumor. In the pair shown in B, a substantial number of mutations detected in the PDX were not detected in the primary tumor.
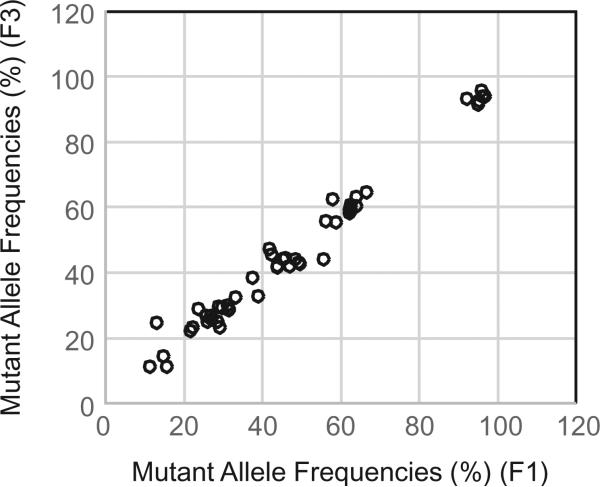
To determine whether mutant allele frequencies vary between different passages, we performed sequencing analysis on a pair of samples derived from the same PDXs but from different passages (F1 and F3, respectively). The results showed that all 48 mutations were detected in both the F1 and F3 tumors. Correlation analysis showed that the allele frequencies detected in these two samples were also highly consistent (r = 0.988, P = 0.000) (Fig. 4). This result indicates that mutational changes in PDXs were preserved at least in the early passages in mice, consistent with a previous report by others [22].
Fig. 4.
Mutations in different passages of the same PDXs. F1 and F3 passage samples from one PDX were analyzed for mutations in the 202 genes. The graph represents allele frequencies for 48 mutations detected in both F1 and F3 samples. Correlation analysis revealed that the allele frequencies detected in these two samples were well correlated (r = 0.988, P = 0.000).
Discussion
Our study resulted in 23 molecularly-annotated PDXs that will be useful for preclinical evaluation of investigational lung cancer-targeting agents and/or for molecular characterization of lung cancers. Although the number of cases where PDXs were established in this study is relatively small, our studies allowed us to detect a number of genes that were frequently mutated in lung cancer. Many of those genes were consistent with those reported previously by others. Some of those genes, such as TP53, KRAS, PI3KCA, STK11, EGFR, CDKN2A, and ALK are already known to be the tumor suppressor genes or oncogenes of relevance for lung cancer. Interestingly, two EGFR mutations (S811C and D855N) were detected in a neuroendocrine cancer, while another case with large cell/ neuroendocrine cancer had two other EGFR mutations (V674F and P959L). Whether those EGFR mutations contribute to tumorigenesis or sensitize cancers to anti-EGFR therapy remains to be determined. Several genes, such as ATM [7], MLL3 [42], and SETD2 [6,43], were recently reported to be frequently mutated/changed in lung cancers. In addition, several large genes that were included in the analysis, such as CSDM3, CSDM1, HYDIN, LRP2, LRP1B, PCLO, and RYR2, were also found to be frequently mutated in our lung cancer samples. Mutations in those large genes would be expected due to “chance”, or due to mutational heterogeneity associated with gene expression levels and/or DNA replication times during the cell cycles [44]. Moreover, presence of duplicated copies in human genome, as in the case for MLL3 [45], may also lead to increased rates of mutations detected by deep sequencing. Whether mutations in those genes may play roles in lung cancer initiation and progression remains undetermined, although ATM, MLL3, and SETD2 are included in the 125 cancer deriver genes affected by gene mutations [46]. Interestingly, both MLL3 and SETD2 encode histone methyltransferases that are required for DNA repair, genome stability, and p53-mediated checkpoint activation [47,48]. Functional insufficiency in either gene is recently identified to be the new drivers for development of leukemia [49,50].
Our study also revealed that lung cancer can vary greatly in numbers of gene mutations, which may reflect genome instability in some tumors. Moreover, our results demonstrate that PDXs usually effectively recapitulate gene mutations present in primary tumors. Nevertheless, some cancer cells may be enriched in the PDXs, leading to altered allele frequencies when compared with primary tumors. It is also possible that heterogeneity in the primary tumor may result in some mutations being detected in primary tumors and not detected in PDXs, or vice versa, or to have variations in allele frequencies between primary tumor and PDX. In fact, mutational intratumor heterogeneity can be readily detected in different regions of the same tumor [51].
Nevertheless, there are limitations in our current study. First, the surgical specimens used in this study were mostly from early stage patients. PDXs derived from late stage patients or from metastatic tumors will be desirable for preclinical drug efficacy studies, as those patients are most likely to receive systemic therapies. Second, levels of gene expressions or posttranscriptional modifications can drastically affect treatment responses and/or clinical outcomes [52–55]. Thus, PDXs with comprehensive molecular annotations, including whole-genome exome, whole-transcriptome, and global proteomic characterizations, will be highly valuable for future translational studies. Finally, all PDXs described in this study were established by inoculating tumors in subcutaneous areas. Evidence has shown that different anatomical sites affect the tumor microenvironment, tumor biology and responses to anticancer therapies [56–58]. PDXs established in orthotopic locations could be more appropriate for preclinical studies. Therefore, more resources and efforts will be needed in order to obtain lung cancer PDXs that cover majority of clinical characteristics and with comprehensive molecular annotations.
Supplementary Material
Acknowledgements
We thank Kathryn Hale of the Department of Scientific Publication at The University of Texas MD Anderson Cancer Center for editorial review of this manuscript.
Funding
This work was supported in part by the National Institutes of Health through Specialized Program of Research Excellence (SPORE) Grant CA070907 and The University of Texas MD Anderson Cancer Center Core Support Grant CA016672 (Lung Program DNA Analysis and Bioinformatics Core Facilities). Further support came from MD Anderson Cancer Center endowed funds, including the Moon Shot Program, the Staging Lung Cancer Research Fund, and the M.W. Elkins Endowed Fund for Thoracic Surgical Oncology.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2014.11.024.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Hammerman PS, Hayes DN, Wilkerson MD, Schultz N, Bose R, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd FA, Rodrigues PJ, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 12.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 13.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J. Clin. Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 14.Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davare MA, Saborowski A, Eide CA, Tognon C, Smith RL, Elferich J, et al. Foretinib is a potent inhibitor of oncogenic ROS1 fusion proteins. Proc. Natl. Acad. Sci. U.S.A. 2013;110:19519–19524. doi: 10.1073/pnas.1319583110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garber K. From human to mouse and back: ‘tumorgraft’ models surge in popularity. J. Natl. Cancer Inst. 2009;101:6–8. doi: 10.1093/jnci/djn481. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 20.Kola I. The state of innovation in drug development. Clin. Pharmacol. Ther. 2008;83:227–230. doi: 10.1038/sj.clpt.6100479. [DOI] [PubMed] [Google Scholar]
- 21.Arrowsmith J. Trial watch: phase II failures: 2008–2010. Nat. Rev. Drug Discov. 2011;10:328–329. doi: 10.1038/nrd3439. [DOI] [PubMed] [Google Scholar]
- 22.Sivanand S, Pena-Llopis S, Zhao H, Kucejova B, Spence P, Pavia-Jimenez A, et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci. Transl. Med. 2012;4:137ra75. doi: 10.1126/scitranslmed.3003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.John T, Kohler D, Pintilie M, Yanagawa N, Pham NA, Li M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin. Cancer Res. 2011;17:134–141. doi: 10.1158/1078-0432.CCR-10-2224. [DOI] [PubMed] [Google Scholar]
- 24.Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin. Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 25.Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goere D, Mariani P, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res. 2012;18:5314–5328. doi: 10.1158/1078-0432.CCR-12-0372. [DOI] [PubMed] [Google Scholar]
- 26.Dong X, Guan J, English JC, Flint J, Yee J, Evans K, et al. Patient-derived first generation xenografts of non-small cell lung cancers: promising tools for predicting drug responses for personalized chemotherapy. Clin. Cancer Res. 2010;16:1442–1451. doi: 10.1158/1078-0432.CCR-09-2878. [DOI] [PubMed] [Google Scholar]
- 27.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508–523. doi: 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Soler R, Kemp B, Wu QP, Mao L, Gomez J, Zeleniuch-Jacquotte A, et al. Response and determinants of sensitivity to paclitaxel in human non-small cell lung cancer tumors heterotransplanted in nude mice. Clin. Cancer Res. 2000;6:4932–4938. [PubMed] [Google Scholar]
- 29.Hidalgo M, Bruckheimer E, Rajeshkumar NV, Garrido-Laguna I, De OE, Rubio-Viqueira B, et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol. Cancer Ther. 2011;10:1311–1316. doi: 10.1158/1535-7163.MCT-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morelli MP, Calvo E, Ordonez E, Wick MJ, Viqueira BR, Lopez-Casas PP, et al. Prioritizing phase I treatment options through preclinical testing on personalized tumorgraft. J. Clin. Oncol. 2012;30:e45–e48. doi: 10.1200/JCO.2011.36.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fichtner I, Rolff J, Soong R, Hoffmann J, Hammer S, Sommer A, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. 2008;14:6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- 32.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat. Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonigro RJ, Grasso CS, Robinson DR, Jing X, Wu YM, Cao X, et al. Detection of somatic copy number alterations in cancer using targeted exome capture sequencing. Neoplasia. 2011;13:1019–1025. doi: 10.1593/neo.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer. 2004;91:355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 39.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez-Perez A, Lopez-Bigas N. Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score. Condel. Am. J. Hum. Genet. 2011;88:440–449. doi: 10.1016/j.ajhg.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu P, Morrison C, Wang L, Xiong D, Vedell P, Cui P, et al. Identification of somatic mutations in non-small cell lung carcinomas using whole-exome sequencing. Carcinogenesis. 2012;33:1270–1276. doi: 10.1093/carcin/bgs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn JW, Kim HS, Yoon JK, Jang H, Han SM, Eun S, et al. Identification of somatic mutations in EGFR/KRAS/ALK-negative lung adenocarcinoma in never-smokers. Genome Med. 2014;6:18. doi: 10.1186/gm535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruault M, Brun ME, Ventura M, Roizes G, De SA. MLL3, a new human member of the TRX/MLL gene family, maps to 7q36, a chromosome region frequently deleted in myeloid leukaemia. Gene. 2002;284:73–81. doi: 10.1016/s0378-1119(02)00392-x. [DOI] [PubMed] [Google Scholar]
- 46.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J, Kim DH, Lee S, Yang QH, Lee DK, Lee SK, et al. A tumor suppressive coactivator complex of p53 containing ASC-2 and histone H3-lysine-4 methyltransferase MLL3 or its paralogue MLL4. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8513–8518. doi: 10.1073/pnas.0902873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li F, Mao G, Tong D, Huang J, Gu L, Yang W, et al. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell. 2013;153:590–600. doi: 10.1016/j.cell.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C, Liu Y, Rappaport AR, Kitzing T, Schultz N, Zhao Z, et al. MLL3 is a Haploinsufficient 7q tumor suppressor in acute myeloid leukemia. Cancer Cell. 2014;25:652–665. doi: 10.1016/j.ccr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu X, He F, Zeng H, Ling S, Chen A, Wang Y, et al. Identification of functional cooperative mutations of SETD2 in human acute leukemia. Nat. Genet. 2014;46:287–293. doi: 10.1038/ng.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [Erratum appears in N Engl J Med. 2012 Sep 6;367(10):976]
- 52.Director's Challenge Consortium for the Molecular Classification of Lung Adenocarcinoma. Shedden K, Taylor JM, Enkemann SA, Tsao MS, Yeatman TJ, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat. Med. 2008;14:822–827. doi: 10.1038/nm.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thuerigen O, Schneeweiss A, Toedt G, Warnat P, Hahn M, Kramer H, et al. Gene expression signature predicting pathologic complete response with gemcitabine, epirubicin, and docetaxel in primary breast cancer. J. Clin. Oncol. 2006;24:1839–1845. doi: 10.1200/JCO.2005.04.7019. [DOI] [PubMed] [Google Scholar]
- 54.Cardnell RJ, Feng Y, Diao L, Fan YH, Masrorpour F, Wang J, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin. Cancer Res. 2013;19:6322–6328. doi: 10.1158/1078-0432.CCR-13-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Y, Zhou Z, Hofstetter WL, Zhou Y, Hu W, Guo C, et al. Aberrant expression of proteins involved in signal transduction and DNA repair pathways in lung cancer and their association with clinical parameters. PLoS ONE. 2012;7:e31087. doi: 10.1371/journal.pone.0031087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devaud C, Westwood JA, John LB, Flynn JK, Paquet-Fifield S, Duong CP, et al. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol. Ther. 2014;22:18–27. doi: 10.1038/mt.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vahle AK, Kerem A, Ozturk E, Bankfalvi A, Lang S, Brandau S. Optimization of an orthotopic murine model of head and neck squamous cell carcinoma in fully immunocompetent mice – role of toll-like-receptor 4 expressed on host cells. Cancer Lett. 2012;317:199–206. doi: 10.1016/j.canlet.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 58.Yoon S, Zhang R, Bucana C, Fidler I. Epigenetic regulation of multidrug-resistance and metastasis-related genes in human lung adenocarcinoma cells growing in ectopic or orthotopic organs of nude-mice. Int. J. Oncol. 1995;7:1261–1267. doi: 10.3892/ijo.7.6.1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.