Abstract
The goal of this systematic analysis is to provide a comprehensive review of the current cardiac magnetic resonance data on microvascular obstruction (MVO) and intramyocardial hemorrhage (IMH). Data related to the association of MVO and IMH in patients with acute myocardial infarction (MI) with left ventricular (LV) function, volumes, adverse LV remodeling, and major adverse cardiac events (MACE) were critically analyzed. MVO is associated with a lower ejection fraction, increased ventricular volumes and infarct size, and a greater risk of MACE. Late MVO is shown to be a stronger prognostic marker for MACE and cardiac death, recurrent MI, congestive heart failure/heart failure hospitalization, and follow-up LV end-systolic volumes than early MVO. IMH is associated with LV remodeling and MACE on pooled analysis, but because of limited data and heterogeneity in study methodology, the effects of IMH on remodeling require further investigation.
Keywords: cardiac magnetic resonance, ejection fraction, intramyocardial hemorrhage, left ventricular remodeling, microvascular obstruction
In the setting of an acute myocardial infarction (MI), persistence of coronary artery occlusion for >40 min can lead to irreversible myocardial damage that spreads as a “wave front phenomenon” progressing from endocardium to epicardium (1,2). Although timely reperfusion is presently the best mechanism to salvage ischemic myocardium and limit myocardial necrosis, revascularization also can have detrimental effects by triggering ischemic reperfusion injury that results in microvascular damage and further myocyte necrosis (3). Ischemic reperfusion injury can account for up to one-half of the size of the final MI (4). Depending on the severity of the ischemic injury, microvascular injury can lead to: 1) microvascular obstruction (MVO) only; and 2) MVO with intramyocardial hemorrhage (IMH) (4). The National Heart, Lung, and Blood Institute has emphasized microvascular damage and reperfusion injury after MI as important targets to improve outcomes (5). Although left ventricular ejection fraction (LVEF) traditionally has been used as a predictor of major adverse cardiac events (MACE), its use as the sole predictor has come under question (6).
Cardiac magnetic resonance (CMR) provides a comprehensive analysis of MI, including the assessment of myocardial scar, MVO, and IMH, and there is growing evidence that these parameters provide important information for predicting adverse left ventricular (LV) remodeling and MACE. This systematic state-of-the-art review will evaluate the literature examining the CMR parameters of MVO and IMH as biomarkers of adverse events after acute MI.
MICROVASCULAR OBSTRUCTION
MVO or “no reflow” refers to the small vessel changes that prevent adequate tissue perfusion despite revascularization and an open epicardial coronary artery (2). MVO is thought to be caused by an abrupt release of cytotoxic factors (7) that promote vasoconstriction, myocardial cellular edema (2,8), capillary endothelial cells swelling, and distal microembolization of atherosclerotic debris leading to plugging of vascular lumen with neutrophils, red blood cells, and platelets. MVO begins in the infarcted core and can increase in size for up to 48 h (9). MVO is reported to be present in up to 84% of the patients after ST-segment elevation myocardial infarction (STEMI) (10–12). The diagnosis of MVO can be made using angiography (13,14), echocardiography (15), nuclear scintigraphy (16), myocardial contrast echocardiography (17), or CMR. On angiography, microvascular blood flow is assessed using Thrombolysis In Myocardial Infarction flow grades, myocardial blush grade, and/ or corrected Thrombolysis In Myocardial Infarction frame count. The rate of myocardial uptake of microbubbles using contrast echocardiography has been used to assess MVO; however, this technique is limited by challenges of adequate acoustic windows, injection of microbubble contrast, and operator dependency (17). There are limited data using single photon emission computed tomography, and this has been used only in research applications (16). Of the available modalities, CMR provides the most comprehensive assessment of MVO.
MVO is detected on gadolinium-enhanced CMR as delayed or absent wash-in of contrast agent into the infarct zone. MVO as assessed by CMR is defined as “early” or “late” in reference to the timing of imaging relative to gadolinium administration (Figure 1). Early microvascular obstruction (EMVO) is identified by a prolonged perfusion defect on resting first-pass perfusion (FPP) imaging (18) or as a hypointense region in the core of the infarct on T1-weighted images obtained 2 to 5 min after contrast administration (19). Although FPP images have lower signal-to-noise ratio, spatial coverage, and ventricular coverage, a study comparing this technique with early T1-W imaging demonstrated concordance in 92% (20).
FIGURE 1. Cardiac Magnetic Resonance (CMR) Images From a 46-Year-Old Man With Diabetes and Chest Pain.
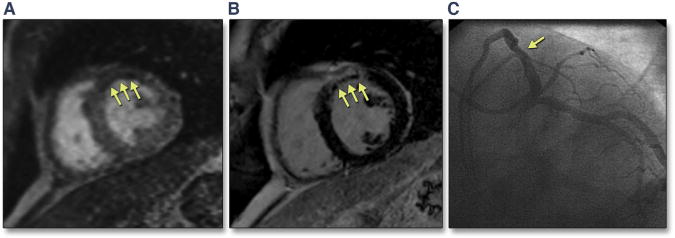
(A) First-pass perfusion (FPP) image shows a region of hypoperfusion in the anteroseptum (early microvascular obstruction [EMVO]). (B) Phase-sensitive inversion recovery (PSIR) sequence reveals presence of myocardial infarction (MI) with large area of late microvascular obstruction (LMVO). These findings were consistent with an acute MI in a diagonal branch that was originally missed on (C) cardiac catheterization (arrow shows proximal diagonal branch obstruction).
Depending on the severity of MVO, the absence of wash-in of gadolinium may persist for >10 min (21), resulting in a region of persistent hypoenhancement within the core of the infarct on conventional late gadolinium enhancement images, referred to as “late MVO” (LMVO). Late gadolinium enhancement imaging used for LMVO assessment has high spatial and contrast resolution (22) and enables full coverage of the LV myocardium. Because the wash-in of gadolinium into the infarct core is a dynamic process (23,24), it is presently unknown whether the rate of fill-in of the MVO area has prognostic importance and whether EMVO or LMVO is a better predictor of LV remodeling or MACE.
INTRAMYOCARDIAL HEMORRHAGE
IMH is considered a severe form of MVO and follows MVO development in the core of the infarct (25–27) with a tendency to expand for several hours after percutaneous coronary intervention (28,29). The cause includes vascular endothelial damage and accumulation of red blood cells in the myocardial extracellular space (30–34). It has been debated whether IMH is the cause or result of severe ischemic reperfusion injury (35). A high correlation between infarct size (IS) and IMH has been identified on histopathologic studies (r = 0.90); however, no correlation with the magnitude of early flow after revascularization (32,36,37) has been seen. Multiple factors contribute to the presence and severity of IMH, including the amount of collateral flow (25,38), ischemic preconditioning, extent of necrosis (25,33), distal coronary microembolization, and differences in individual risk factors, such as diabetes or smoking. IMH can be assessed with CMR using T2- or T2*-weighted imaging or parameter mapping sequences (Figure 2).
FIGURE 2. Example Images From a Study of Reperfused Acute MI in a Porcine Model.
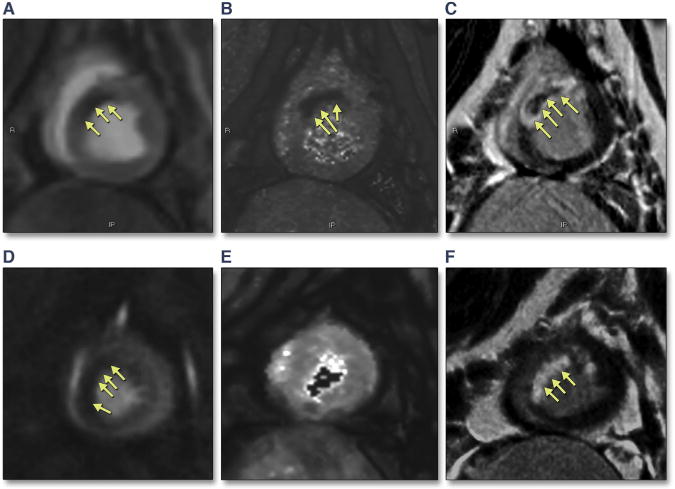
The images from the first animal (top) show (A) decreased perfusion in the mid-anteroseptal wall on FPP imaging, (B) an area of intramyocardial hemorrhage on T2* mapping, and (C) a corresponding hypointense area on phase-sensitive late gadolinium enhancement imaging, consistent with microvascular obstruction (MVO) with IMH. Images from the second animal (bottom) (D) demonstrate an area of reduced perfusion on FPP imaging, (E) T2* maps do not demonstrate any IMH, and (F) late gadolinium enhancement images demonstrate the absence of MVO or IMH.
Most studies have used T2-weighted short-tau inversion recovery (STIR) or T2*-weighted gradient echo pulse sequences to assess for IMH. IMH appears as a hypointense region within the infarct on T2-weighted sequences because the hemoglobin breakdown products shorten the myocardial T2-relaxation time. Because the paramagnetic effects of hemoglobin breakdown products more strongly affect T2* relaxation, T2*-weighted imaging is thought to be more sensitive for the detection of IMH (39,40). However, T2*-weighted images have lower signal-to-noise compared with T2-weighted images and are more sensitive to off-resonance artifacts. T2* values are lowest acutely in the IMH core, but gradually normalize to that of the rest of the infarct at 4 weeks because of extensive collagen deposition and absence of iron with resolution of MVO and IMH (41). IMH detected by both T2 (41,42) and T2* images has been correlated with the presence of hemorrhage on histopathologic analysis (kappa 0.96, p < 0.01) (43–45) (Figures 3 and 4). A recent study (46) in 14 patients with STEMI and 20 canines with acute reperfused MI suggests that T2* may be more suitable than T2 imaging techniques for assessing myocardial hemorrhage. The T2* decreased on average 54% in hemorrhagic infarctions and was 6% higher in nonhemorrhagic infarctions compared with remote myocardium. On the contrary, the T2 was increased by 17% in hemorrhagic infarcts and by 38% in nonhemorrhagic infarcts in the canine model, reflecting the competing effects of hemorrhage, which tends to shorten T2, with that of edema, which increases T2 (46). More studies performing direct comparison of T2 and T2* techniques will provide a promising approach to differentiate MVO with and without IMH. In some cases, it is difficult to adequately differentiate MVO from IMH because both may appear as a hypointense region within the infarct (44,47,48). Furthermore, MVO without hemorrhage can result in hypoenhanced regions seen on T2-weighted sequences because of low proton density in the infarct core (48). Thus, care must be used when distinguishing between MVO and IMH on T2-weighted images.
FIGURE 3. Transmural Anteroseptal and Lateral Hemorrhagic MI in a Patient Who Died of Cardiogenic Shock After Acute MI and Who Had Undergone Coronary Recanalization.
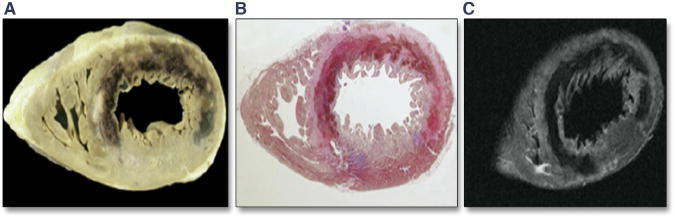
Gross anatomic image obtained at the time of autopsy (A), histology image after staining with Heidenhain trichrome stain (B), and ex vivo T2 CMR image from the short-axis slice (C) show the changes. Adapted with permission from Basso et al. (43).
FIGURE 4. Images From a Mongrel Dog in Which MI Was Experimentally Induced.
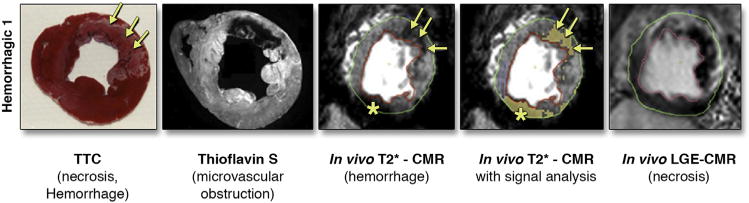
CMR was performed day 3 after reperfusion in which T2*-weighted gradient echo imaging was performed. Ex vivo, thioflavin S imaging, and triphenyl tetrazolium chloride staining were performed to assess for MVO, hemorrhage, and myocardial necrosis. Adapted with permission from Kumar et al. (45). CMR = cardiac magnetic resonance; LGE = late gadolinium enhancement; TTC = triphenyl tetrazolium chloride.
Pre-contrast T1-weighted inversion recovery images have demonstrated promise for detecting IMH in a porcine model of MI (49). As hemorrhage shortens T1, regions of hemorrhage appear bright on this sequence. In this study, the diagnostic sensitivity of the T1-weighted image was higher than that for T2*-weighted or T2-weighted imaging. This technique requires further clinical validation.
METHODS
We conducted a systematic review by searching PubMed for all published studies evaluating the relationship of MVO or IMH with LV remodeling and MACE through January 2014. The QUORUM diagram for our search methodology is presented in Figure 5. MACE are defined in studies as a composite of various secondary outcomes, including cardiac death, death, recurrent MI, congestive heart failure (CHF), CHF-related hospitalizations, unstable angina (50), embolic stroke (51), and atrial fibrillation, whichever occurred first. Variation in data presentation was identified.
FIGURE 5. QUORUM Diagram Showing Details of the PubMed Search Conducted Through January 2014 on 3 Topics.
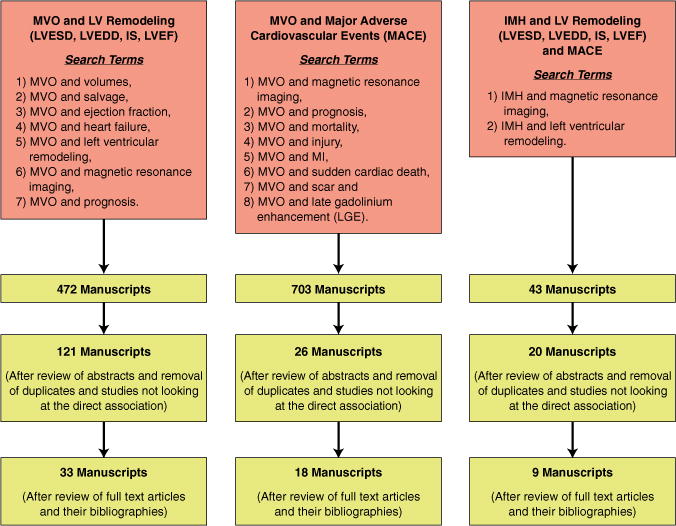
1) MVO and left ventricular (LV) remodeling; 2) MVO and major adverse cardiac events (MACE); and 3) IMH and LV remodeling. IMH = intramyocardial hemorrhage; IS = infarct size; LGE = late gadolinium enhancement; LV = left ventricular; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; MACE = major adverse cardiac events; MVO = microvascular obstruction.
We assessed the association of presence of EMVO or LMVO on clinical outcomes of MACE, cardiac death, CHF-related hospitalization, and recurrent MI. Studies were assessed by 2 reviewers (Y.H. and A.W.) to determine whether the studies met the specified inclusion criteria. A random effects meta-analysis using Mantel-Haenszel weighting was performed to calculate pooled odds ratios and 95% confidence intervals (CIs) for these outcomes. In studies that performed both EMVO and LMVO in the same subjects, a comparison of the difference in odds ratios for each endpoint between EMVO and LMVO was performed using an inverse-variance weighted meta-analysis with a variance estimate that took into account intra-study correlation between EMVO and LMVO (52).
We evaluated the association between EMVO and LMVO and LV function, volumes, and IS at baseline and on follow-up imaging. Because the majority of studies presented indexed LV volumes, indexed volumes were included in the analysis of LV volumes. Studies reporting only nonindexed volumes were excluded. Studies reporting data as median and interquartile range were excluded from the analysis, because the mean and variance could not be determined without using a normality assumption, which could not be verified without patient-level data. Inverse variance-weighted, random-effects meta-analysis of the mean differences in indexed LV volumes, ejection fraction (EF), and IS between patients with or without MVO on their initial imaging study after MI was performed for studies assessing EMVO or LMVO. To assess LV remodeling as a function of the presence or absence of MVO on the initial imaging study, a similar meta-analysis of the mean differences in volume EF and IS on follow-up studies obtained more than 4 months after the initial MI was performed.
Similar analysis was performed for IMH to assess the relationship between IMH and MACE and LV volumes and function. A random-effects meta-analysis with Mantel-Haenszel weights was performed assessing the hazard ratio for endpoints of cardiac death, recurrent MI, and CHF/CHF hospitalization.
Meta-analysis was performed using Review Manager (RevMan) 5 version 5.1.7 freeware package (The Nordic Cochrane Centre, The Cochrane Collaboration, 2008, Copenhagen, Denmark). Heterogeneity was assessed with the I2 statistic, and funnel plots were performed to assess for publication bias. No formal meta-regression was performed to explore heterogeneity because of the variation in reporting of important covariates and the scope of this review.
RESULTS
The pooled mean prevalence of EMVO, LMVO, and IMH in the studies reviewed was 65% (95% CI: 63% to 66%), 54% (95% CI: 52% to 56%), and 35% (95% CI: 31% to 38%), respectively (Online Table 1). Some of the variability in the prevalence of MVO between studies may be due to differences in study populations, contrast doses, pulse sequences, or timing of imaging post-contrast. LVEF, IS, and LV volumes were assessed on the baseline CMR in 12 of the 33 studies; however, only 7 studies analyzed LV functional information at 4 months to 1 year of follow-up. All the studies included patients presenting with acute MI who underwent thrombolysis or percutaneous coronary intervention between 12 and 72 h after symptom onset. One study exclusively included patients with non-STEMI (53).
EFFECT OF EMVO ON LV REMODELING AND MACE
Our review identified 10 studies (n = 698) examining the direct impact of EMVO on LV function, volumes, and remodeling, and 5 studies (791 patients) evaluating its impact on MACE. EMVO was assessed on 1.5-T CMR scanners in 9 of 10 studies. EMVO was assessed 24 h to 1 week after MI using the FPP technique in 6 studies and early post–gadolinium enhancement T1-weighted imaging in 5 studies (19,20,24,54–56). The study by Ørn et al. (55) is unique because it assessed the presence of EMVO at multiple time points after MI, but the results were similar to those of the companion studies.
The majority of the studies identified EMVO to be independently associated with LV remodeling and MACE (12,19,20,24,25,50,53,55–62). The pooled analysis is presented in Table 1, Figure 6, and Online Table 4. On review of studies that assessed LV remodeling both immediately after MI and at follow-up (20,24,50,55), there was a greater difference in EF and in particular indexed LV volumes at follow-up compared with baseline indicating differences in remodeling between cases with and without EMVO.
TABLE 1.
Mean Difference (IV and 95% CI) From Studies of Impact of EMVO and LMVO on Baseline and Follow-Up (4 Months to 1 Year) LVEF, LVEDV, LVESV, and IS
| Mean Difference (IV, Random, 95% CI) From Pooled Analysis (EMVO) |
Mean Difference (IV, Random, 95% CI) From Pooled Analysis (LMVO) |
|
|---|---|---|
|
Baseline
| ||
| EF (%) | −5.21 (−7.13 to −3.30)* | −5.82 (−8.21 to −3.43)* |
| IS (% LV) | 10.71 (8.49 to 12.92)* | 13.01 (9.95 to 16.07)* |
| LVEDVi (ml/m2) | 6.73 (3.32 to 10.14)† | 5.26 (−1.08 to 11.60)‡ |
| LVESVi (ml/m2) | 6.73 (6.10 to 7.37)* | 9.06 (1.76 to 16.3)§ |
|
| ||
|
Follow-Up (4 Months to 1 Yr)
| ||
| EF (%) | −7.44 (−9.07 to −5.80) | −7.76 (−9.63 to −5.90)* |
| IS (% LV) | 6.85 (3.65 to 10.06)† | 6.91 (0.35 to 13.47)‖ |
| LVEDVi (ml/m2) | 16.44 (13.10 to 19.77)* | 17.14 (7.20 to 27.08) |
| LVESVi (ml/m2) | 13.08 (10.26 to 15.90)* | 19.59 (6.76 to 32.42)# |
p < 0.00001.
p < 0.0001.
p = 0.10.
p = 0.02.
p = 0.04.
p = 0.0007.
p = 0.003.
CI = confidence interval; EDV = end-diastolic volume; EF = ejection fraction; EMVO = early microvascular obstruction; ESV = end-systolic volume; IS = infarct size; IV = inverse variance; LMVO = late microvascular obstruction; LV = left ventricular; LVEDVi = left ventricular end-diastolic volume index; LVESVi = left ventricular end-systolic volume index; MVO = microvascular obstruction.
FIGURE 6. Pooled Odds Ratios for the Association of EMVO and Adverse Cardiac Outcomes.
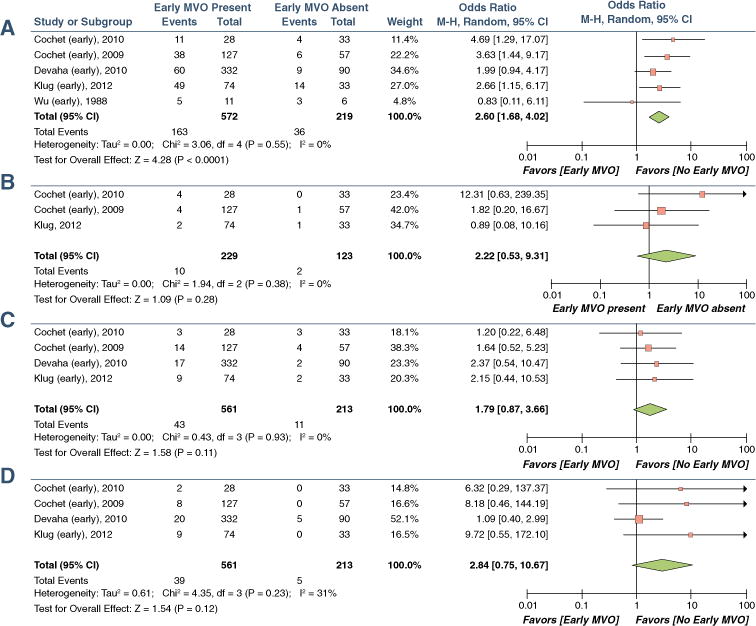
(A) MACE, (B) cardiac death, (C) recurrent MI, and (D) congestive heart failure (CHF) or CHF-related hospitalizations. CI = confidence interval; MVO = microvascular obstruction.
The study of 100 patients with acute MI by Weir et al. (12) noted that EMVO was associated with significantly more shrinkage of IS (delta IS) at follow-up. In our review of 3 studies inclusive of 106 patients (20,24,56) with both baseline and follow-up IS, a similar trend was identified. Infarct extent and infarct transmurality when studied along with IS were noted to be increased in the presence of EMVO (12,20,58).
Figure 6 shows the impact of EMVO on MACE, cardiac mortality, recurrent MI, and CHF/CHF hospitalization. Although there was a trend toward increased cardiac mortality, MI, and CHF hospitalization, the pooled odds ratios were not statistically significant.
Because studies used different methods for quantifying the size of EMVO, the effects due to the size of the region of MVO could not be assessed. In a study by de Waha et al. (63) in 438 patients, the extents of EMVO (hazard ratio: 1.03; 95% CI: 1.02 to 1.05) and EMVO/IS (hazard ratio: 2.22; 95% CI: 1.60 to 3.08) were identified as independent predictors of MACE (62). Bruder et al. (64) identified a cutoff EMVO value of >0.5 (% of LV mass) to predict MACE (odds ratio: 3.9; 95% CI: 1.1 to 13.9). A trend toward increased MACE in the presence of EMVO with increasing IS (% LV) from 18% to 30% to >30% was recognized by Wu et al. (50).
EFFECT OF LMVO ON LV REMODELING AND MACE
We identified 9 studies (n = 631) (12,19,24,53,65–68) looking at the direct impact of LMVO on LV remodeling and 7 studies (n = 2,132) (53,56,57,66,69–71) evaluating its impact on MACE (Online Tables 5A to 5D). The majority of these studies demonstrate an effect of LMVO on both LV remodeling and MACE in multivariate analysis (Table 1, Figure 7). The presence of LMVO predicted a greater reduction in LVEF at both baseline and follow-up. The point estimates for LV volume were greater at follow-up, which would be consistent with increased LV remodeling in the presence of LMVO (Table 1).
FIGURE 7. Pooled Odds Ratios for the Association of LMVO and Adverse Cardiac Outcomes.
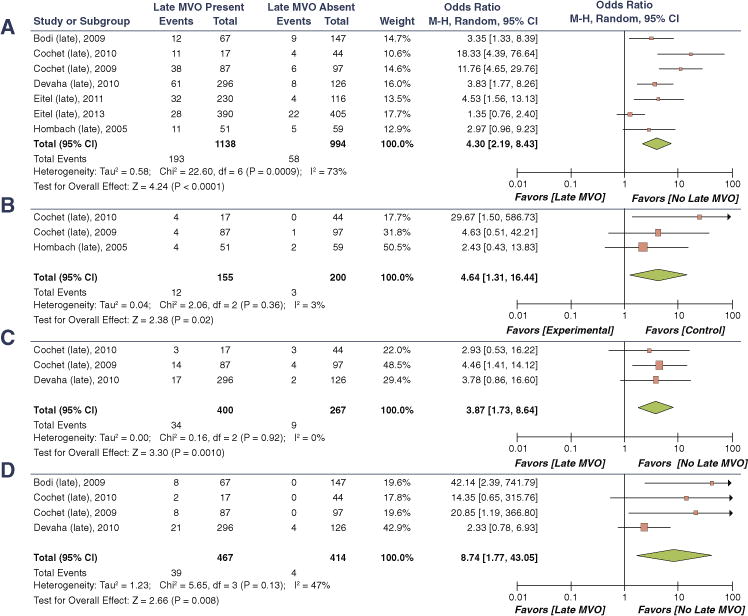
(A) MACE, (B) cardiac death, (C) recurrent MI, and (D) CHF or CHF-related hospitalizations. Abbreviations as in Figure 6.
LMVO has been independently associated with IS and reduction in IS at follow-up (60,65,66). In multiple studies there was a high correlation between LMVO and IS (r = 0.75 to 0.77) (65,66). However, in a study by Ørn et al. (55), when the presence of LMVO at 2 days versus 1 week after MI was compared, only its presence at 2 days predicted IS at 1-year follow-up. Ørn et al. (55) did not identify a correlation between size of LMVO and IS, but Cochet et al. (56) detected a significant correlation between them (r = 0.65, p <0.0001). Some studies recorded LMVO as present exclusively in the setting of transmural necrosis; thus, on multivariate analysis the predictive power of LMVO for LV remodeling was lower (72,73). Some studies have seen an independent predictive utility of presence and absence of MVO for LV end-diastolic volume (74,75) and LVEF beyond that of IS (76). Most studies (65,74,75,77–79) did not find an additional utility of assessing extent of LMVO to predict LV volumes or LVEF. In our meta-analysis, the presence of LMVO was significantly associated with cardiac death, recurrent MI, and CHF/CHF hospitalization (Figure 7). In studies that performed multivariate analysis, 4 (53,56,67) demonstrated an independent impact of LMVO on MACE, whereas 3 (69–71) did not.
A direct comparison of the studies in regard to LMVO size could not be performed because of variation in methods used to quantify the size of the LMVO (i.e., % LV mass to LV segments with LMVO [score] and MVO/IS ratio) as with EMVO. However, apart from 4 studies (69–71,80,81), most have demonstrated increasing LMVO size to correlate with MACE (51,57,66,78,82–84) on multivariate analysis. A study by Jensen et al. (82) suggested a cutoff of LMVO >3.9% of LV volume to predict MACE. Compared with the other measurements of size, the MVO/IS ratio was noted to have the strongest impact on MACE in a study by de Waha et al. (63). Of note, LMVO size emerged to be independently associated with MACE and IS in most of the studies. In the study by Hadamitzky et al. (84), in which multivariate analysis included GRACE score, IS by both CMR and single photon emission computed tomography and LMVO size, only GRACE score and LMVO size were independent predictors of MACE. Furthermore, LMVO allowed separation between a high-risk group (event rate 14%, 35% of patients) and a relatively low-risk group (event rate 5.5%, 65% of patients).
COMPARISON OF EMVO VERSUS LMVO
We identified 13 studies that assessed both EMVO and LMVO. Some compared the predictive value of EMVO versus LMVO for LV remodeling and MACE (53,55–57). A decrease in MVO from 70% to 62% to 59% from early, to intermediate, to late imaging has been observed in a study by Nijveldt et al. (24). A shrinkage in absolute MVO size, MVO:IS, and MVO transmurality between early and late imaging also is seen and is thought to be related to diffusion and collateral blood flow (69). Thus, EMVO is generally considered more sensitive (60,61), whereas LMVO is more specific for diagnosing microvascular damage (57,85). A high correlation is noted between EMVO and LMVO with correlation coefficients in the range of 0.52 to 0.78 (56,73).
In our meta-analysis of the data from 3 studies directly comparing EMVO and LMVO in the same patients, LMVO had a statistically higher odds ratios for predicting MACE (delta odds ratio: 2.56, p < 0.001), CHF death (delta odds ratio: 2.19, p = 0.035), and recurrent MI (delta odds ratio: 2.27, p = 0.009), with a trend toward a statistically higher estimate of cardiac death (delta odds ratio: 2.07, p = 0.10), assuming a correlation coefficient of 0.7 between EMVO and LMVO.
Although various studies have identified both EMVO and LMVO (56) or EMVO only (53) to be independently associated with MACE and LV remodeling, the preponderance of data favors LMVO to have the strongest relationship (57,76). In a study by Cochet et al. (56), LMVO had a greater predictive value for MACE (odds ratio for EMVO: 2.5; 95% CI: 1.0 to 6.2; p = 0.045 vs. odds ratio for LMVO: 8.7; 95% CI: 3.6 to 21.1; p < 0.001). The comparative sensitivity, specificity, and accuracy of EMVO versus LMVO for the prediction of MACE were 86%, 36%, and 48% versus 84%, 65%, and 70%, respectively, in this study.
EFFECT OF IMH ON LV REMODELING AND MACE
We identified 9 studies (1,106 patients) that examined the relationship between IMH and LV remodeling. The pooled mean differences in indexed volumes, IS, and EF between subjects with and without IMH are presented in Table 2 and demonstrate that IMH is associated with larger volumes, reduced EF, and increased IS.
TABLE 2.
Mean Difference (IV and 95% CI) From Studies Assessing the Impact of IMH on Baseline LVEF, LVEDV, LVESV, and IS
| Mean Difference (IV, Random, 95% CI) From Pooled Analysis (Baseline) |
Mean Difference (IV, Random, 95% CI) From Pooled Analysis (11 Days to 6 Months Follow-Up) |
|
|---|---|---|
| EF (%) | −8.81 (−11.13 to −6.49) | −10.86 (−13.08 to −8.64)* |
| IS (% LV) | 14.96 (11.54 to 18.37)* | 11.55 (8.25 to 14.85)* |
| LVEDVi (ml/m2) | 13.24 (9.32 to 17.16)* | 17.44 (10.91 to 23.97)* |
| LVESVi (ml/m2) | 14.62 (11.80 to 17.43)* | 17.33 (13.67 to 20.99)* |
p < 0.00001.
IMH = intramyocardial hemorrhage; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; other abbreviations as in Table 1.
There was variability in the imaging techniques used to assess IMH, and most studies have used T2-weighted STIR imaging, rather than T2* pulse sequences. Only 1 study (54) used both T2 and T2* to assess IMH, which was defined as present only when both T2 and T2* were positive. Two other studies (27,86) used T2* only. IMH was seen predominantly in anterior infarcts (27) and in infarcts involving >80% of LV thickness (86). O’Regan et al. (86) showed significantly reduced LVEF and increased LV volumes in patients with IMH on univariate analysis and a strong collinearity of IMH and MVO, whereas Ochiai et al. (27) detected no improvement in 1-month follow-up EF (LVEF 47 ± 9% vs. 51 ± 10%) and increased IS at baseline in the presence of IMH; however, multivariate adjusted analysis was not performed. Mather et al. (54) found an independent correlation of IMH with LV remodeling and a significant improvement of diagnostic area under the curve (from 0.699 to 0.826) by adding IMH to a multivariate model including LVEF and IS. Decreased EF acutely and dilated LV volumes without a significant recovery of EF and increased LV end-diastolic volume were seen at 3 months follow-up.
The remainder of the studies (75,81,87–89) used T2 STIR. IMH was more prevalent in patients undergoing rescue percutaneous coronary intervention (23% vs. 7%; p < 0.0001) compared with primary percutaneous coronary intervention (81). These studies identified higher IS in the presence of combined MVO and IMH versus MVO only, with a high correlation between IS and IMH (r = 0.53, p < 0.001) (87). One study identified IMH on multiple regression analysis (inclusive of IS, MVO, infarct location, and transmurality and time to percutaneous coronary intervention) to be strongly associated with adverse LV remodeling (87) at 4 months (R2 = 0.17, F-value: 20.19, p < 0.001). This study also showed that for all IS quartiles, IMH was associated with larger LV end-systolic volume. However, Husser et al. (81) showed that although IMH was univariate predictor of larger LV end-systolic volume on follow-up (odds ratio: 1.54; 95% CI: 1.15 to 2.07; p = 0.004) (81), it did not improve the area under the curve for predicting LV end-systolic volume when late gadolinium enhancement and LVEF data were incorporated into the model (OR: 0.914; 95% CI: 0.875 to 0.952 vs. OR: 0.913; 95% CI: 0.875 to 0.952; p = 0.9) (81). Likewise, IMH was not a predictor of baseline or follow-up LVEF in multivariate analysis in other studies (75,88). Thus, it is not entirely clear whether IMH will have significant incremental utility for predicting IS, ventricular volumes, and functions in models that include other parameters of the infarct or LV structural/functional parameters. Without a larger head-to-head study using both T2-W and T2* techniques, it is difficult to determine which technique has superior performance for detecting IMH.
Three studies, using T2 STIR (81,89,90), including a total of 991 patients with a follow-up of 6 months to 3 years, examined the impact of IMH on adverse cardiovascular outcomes. The pooled univariate hazard ratio for of IMH for predicting MACE was 3.88 (95% CI: 2.11 to 7.13). There are mixed results for the predictive utility of IMH in the presence of other infarct characteristics. One study demonstrated a trend toward more MACE in the presence of IMH with MVO compared with MVO without IMH (p = 0.09) (90). In the study by Eitel et al. (89), inclusion of IMH in the risk model (which included age, infarct location, time to treatment, LV function, and IS) increased the C statistic from 0.76 to 0.80 (p = 0.046). However, this study did not include MVO as a covariate. In the study by Husser et al. (81), although IMH independently predicted MACE, the addition of IMH to a model, which included late gadolinium enhancement and cine-functional parameters, did not improve the area under the curve for predicting MACE. Thus, although IMH may independently predict MACE, its incremental value over other CMR parameters requires further clarification.
ASSOCIATION BETWEEN IMH AND MVO
In most studies, IMH is identified only in the presence of MVO. The correlation of IMH has been reported to be 0.30 for EMVO and between 0.89 and 0.93 for LMVO (86,88). There are 2 theories about the association of MVO with IMH: 1) MVO leads to endothelial damage with subsequent leakage of red blood cells into the interstitial space, leading to IMH; and 2) IMH occurs as part of ischemia–reperfusion injury and hemorrhage, which in turn leads to greater myocardial swelling and compression of the microvasculature, thus worsening MVO (75,79,88). The study by Eitel et al. (89) that compared the graded impact of no MVO, MVO only, and MVO with IMH on LV remodeling or MACE detected a strong incremental association. Thus, there is likely a spectrum between MVO and MVO with IMH, with the presence of IMH suggesting greater cellular damage resulting in greater adverse remodeling and poorer outcomes.
STUDY LIMITATIONS
A principal limitation of the present analysis is that most studies of MVO and IMH are single center with small patient populations. In addition, variable imaging techniques and timing of imaging after MI have been used, leading to significant heterogeneity of results in the literature. MVO and IMH are dynamic processes after MI. Differential timing of assessment may lead to over- or underdiagnosis. The populations studied may suffer from selection bias because they may represent only the patients stable enough to undergo CMR assessment. Further bias may be introduced by the exclusion of patients with prior MI, severe heart failure, or cardiac arrhythmias. The estimates of effect sizes presented in this review examine only MVO and IMH as univariate factors without correction for other important covariates. However, the variation in techniques used and the heterogeneity in reporting of the data for these covariates limit the potential of meta-regression to determine the true prognostic utility of IMH and MVO in the presence of other factors, such as LV function and IS, which have been shown to affect MACE.
CONCLUSIONS
In overall pooled analysis, both EMVO and LMVO were associated with lower EF, larger ventricular volumes and infarct at baseline, and worse adverse LV remodeling at later time points after MI. LMVO was demonstrated to have a stronger relationship with MACE and the individual outcomes of cardiac mortality, recurrent MI, and CHF/CHF hospitalization compared with EMVO. IMH also predicted MACE; however, there is a smaller body of literature for IMH and limited direct comparisons of IMH and MVO. The current literature is limited by the preponderance of single-center studies using a variety of techniques with imaging at different time points after infarction. A multicenter study or registry with controlled inclusion criteria, standardized methodology, and timing relative to infarction is clearly needed to better assess the independent impact of both MVO and IMH on LV remodeling and MACE. Future advances in CMR pulse sequences will enable improved quantification of the extent and severity of MVO and IMH. CMR is well poised to study novel therapies to predict and reduce ischemic reperfusion injury, and provides a comprehensive multiparametric assessment of MI including MVO and IMH.
Supplementary Material
Acknowledgments
Dr. Kramer has received research support from Siemens Medical Systems and is a consultant to Synarc. Dr. Salerno has received research support from Siemens Medical Systems and the National Institutes of Health K23 HL112910–01. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CHF
congestive heart failure
- CI
confidence interval
- CMR
cardiac magnetic resonance
- EF
ejection fraction
- EMVO
early microvascular obstruction
- FPP
first-pass perfusion
- IMH
intramyocardial hemorrhage
- IS
infarct size
- LMVO
late microvascular obstruction
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiac events
- MI
myocardial infarction
- MVO
microvascular obstruction
- PSIR
phase-sensitive inversion recovery
- STEMI
ST-segment elevation myocardial infarction
- STIR
short-tau inversion recovery
APPENDIX
For supplemental tables, please see the online version of this article.
References
- 1.Reimer KA, Lowe JE, Rasmussen MM, et al. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 2.Kloner RA, Ganote CE, Jennings RB, et al. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilak GS, Hsu L-Y, Hoyt RF, et al. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol. 2008;43:7e15. doi: 10.1097/RLI.0b013e3181558822. [DOI] [PubMed] [Google Scholar]
- 4.Piper HM, Garcia-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291e300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–9. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Califf RM, Harrelson-Woodlief L, Topol EJ. Left ventricular ejection fraction may not be useful as an endpoint of thrombolytic therapy comparative trials. Circulation. 1990;82:1847–53. doi: 10.1161/01.cir.82.5.1847. [DOI] [PubMed] [Google Scholar]
- 7.Ye YX, Basse-Lüsebrink TC, Arias-Loza PA, et al. Monitoring of monocyte recruitment in reperfused myocardial infarction with intramyocardial hemorrhage and microvascular obstruction by combined fluorine 19 and proton cardiac magnetic resonance imaging. Circulation. 2013;128:1878–88. doi: 10.1161/CIRCULATIONAHA.113.000731. [DOI] [PubMed] [Google Scholar]
- 8.Reffelmann T, Kloner RA. The no-reflow phenomenon: a basic mechanism of myocardial ischemia and reperfusion. Basic Res Cardiol. 2006;101:359–72. doi: 10.1007/s00395-006-0615-2. [DOI] [PubMed] [Google Scholar]
- 9.Rochitte CE, Lima JA, Bluemke DA, et al. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998;98:1006–14. doi: 10.1161/01.cir.98.10.1006. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Iwakura K, Oh H, et al. Temporal changes in myocardial perfusion patterns in patients with reperfused anterior wall myocardial infarction: their relation to myocardial viability. Circulation. 1995;91:656–62. doi: 10.1161/01.cir.91.3.656. [DOI] [PubMed] [Google Scholar]
- 11.Lim YJ, Nanto S, Masuyama T, Kohama A, Hori M, Kamada T. Myocardial salvage: its assessment and prediction by the analysis of serial myocardial contrast echocardiograms in patients with acute myocardial infarction. Am Heart J. 1994;128:649–56. doi: 10.1016/0002-8703(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 12.Weir RA, Murphy CA, Petrie CJ, et al. Microvascular obstruction remains a portent of adverse remodeling in optimally treated patients with left ventricular systolic dysfunction after acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:360–7. doi: 10.1161/CIRCIMAGING.109.897439. [DOI] [PubMed] [Google Scholar]
- 13.van’t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–6. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 14.Gibson CM, Cannon CP, Murphy SA, Marble SJ, Barron HV, Braunwald E. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105:1909–13. doi: 10.1161/01.cir.0000014683.52177.b5. [DOI] [PubMed] [Google Scholar]
- 15.Schroder R, Dissmann R, Bruggemann T, et al. Extent of early ST segment elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol. 1994;24:384–91. doi: 10.1016/0735-1097(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 16.Kondo M, Nakano A, Saito D, Shimono Y. Assessment of “microvascular no-reflow phenomenon” using technetium-99 m macroaggregated albumin scintigraphy in patients with acute myocardial infarction. J Am Coll Cardiol. 1998;32:898–903. doi: 10.1016/s0735-1097(98)00435-5. [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Okamura A, Iwakura K, et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation. 1996;93:1993–9. doi: 10.1161/01.cir.93.11.1993. [DOI] [PubMed] [Google Scholar]
- 18.Trieb T, Mayr A, Klug G, et al. Patterns of myocardial perfusion in the acute and chronic stage after myocardial infarction: a cardiac magnetic resonance study. Eur J Radiol. 2012;81:767–72. doi: 10.1016/j.ejrad.2011.01.069. [DOI] [PubMed] [Google Scholar]
- 19.Bekkers SC, Backes WH, Kim RJ, et al. Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur Radiol. 2009;19:2904–12. doi: 10.1007/s00330-009-1489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogaert J, Kalantzi M, Rademakers FE, Dymarkowski S, Janssens S. Determinants and impact of microvascular obstruction in successfully reperfused ST-segment elevation myocardial infarction. Assessment by magnetic resonance imaging. Eur Radiol. 2007;17:2572–80. doi: 10.1007/s00330-007-0627-9. [DOI] [PubMed] [Google Scholar]
- 21.Mayr A, Klug G, Schocke M, et al. Late microvascular obstruction after acute myocardial infarction: relation with cardiac and inflammatory markers. Int J Cardiol. 2012;157:391–6. doi: 10.1016/j.ijcard.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 22.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 23.Rochitte CE. Microvascular obstruction: the final frontier for a complete myocardial reperfusion. J Am Coll Cardiol. 2008;51:2239e40. doi: 10.1016/j.jacc.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 24.Nijveldt R, Hofman MB, Hirsch A, et al. Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study. Radiology. 2009;250:363–70. doi: 10.1148/radiol.2502080739. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Dorado D, Théroux P, Solares J, et al. Determinants of hemorrhagic infarcts. Histologic observations from experiments involving coronary occlusion, coronary reperfusion, and reocclusion. Am J Pathol. 1990;137:301–11. [PMC free article] [PubMed] [Google Scholar]
- 26.Asanuma T, Tanabe K, Ochiai K, et al. Relationship between progressive microvascular damage and intramyocardial hemorrhage in patients with reperfused anterior myocardial infarction: myocardial contrast echocardiographic study. Circulation. 1990;96:448–53. doi: 10.1161/01.cir.96.2.448. [DOI] [PubMed] [Google Scholar]
- 27.Ochiai K, Shimada T, Murakami Y, et al. Hemorrhagic myocardial infarction after coronary reperfusion detected in vivo by magnetic resonance imaging in humans: prevalence and clinical implications. J Cardiovasc Magn Reson. 1999;1:247–56. doi: 10.3109/10976649909088337. [DOI] [PubMed] [Google Scholar]
- 28.Shishido T, Beppu S, Matsuda H, Miyatake K. Progression of intramural hemorrhage in the reperfused area: an experimental study assessed by myocardial contrast echocardiography (abstr) Circulation. 1993;88(Suppl I):I–402. [Google Scholar]
- 29.Ito H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2006;3:499–506. doi: 10.1038/ncpcardio0632. [DOI] [PubMed] [Google Scholar]
- 30.Kloner RA, Ganote CE, Jennings RB. The ‘no-reflow’ phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishbein MC, Y-Rit J, Lando U, Kanmatsuse K, Mercier JC, Gantz W. The relationship of vascular injury and myocardial hemorrhage to necrosis after reperfusion. Circulation. 1980;62:1274–9. doi: 10.1161/01.cir.62.6.1274. [DOI] [PubMed] [Google Scholar]
- 32.Kloner RA, Alker KJ. The effect of streptokinase on intramyocardial hemorrhage, infarct size, and the no-reflow phenomenon during coronary reperfusion. Circulation. 1984;70:513–21. doi: 10.1161/01.cir.70.3.513. [DOI] [PubMed] [Google Scholar]
- 33.Pislaru SV, Barrios L, Stassen T, Pislaru C, Van de Werf F. Infarct size, myocardial hemorrhage, and recovery of function after mechanical versus pharmacological reperfusion. Effects of lytic state and occlusion time. Circulation. 1997;96:659–66. doi: 10.1161/01.cir.96.2.659. [DOI] [PubMed] [Google Scholar]
- 34.Waller BF. Pathology of new investigation in the treatment of coronary heart disease. Curr Probl Cardiol. 1986;11:666–760. doi: 10.1016/0146-2806(86)90004-6. [DOI] [PubMed] [Google Scholar]
- 35.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 36.Massel DR. How sound is the evidence that thrombolysis increases the risk of cardiac rupture? Br Heart J. 1993;69:284–7. doi: 10.1136/hrt.69.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kloner RA, Alker K, Campbell C, Figures G, Eisenhauer A, Hale S. Does tissue plasminogen activator have direct beneficial effects on the myocardium independent of its ability to lyse intracoronary thrombi? Circulation. 1989;79:1125–36. doi: 10.1161/01.cir.79.5.1125. [DOI] [PubMed] [Google Scholar]
- 38.Higginson LA, White F, Heggtveit HA, et al. Determinants of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation. 1980;65:62–9. doi: 10.1161/01.cir.65.1.62. [DOI] [PubMed] [Google Scholar]
- 39.Linfante I, Llinas RH, Caplan LR, Warach S. MRI features of intracerebral hemorrhage within 2 hours from symptom onset. Stroke. 1999;30:2263–7. doi: 10.1161/01.str.30.11.2263. [DOI] [PubMed] [Google Scholar]
- 40.Wiesmann M, Mayer TE, Yousry I, Hamann GF, Bruckmann H. Detection of hyperacute parenchymal hemorrhage of the brain using echo-planar T2* weighted and diffusion weighted MRI. Eur Radiol. 2001;11:849–53. doi: 10.1007/s003300000649. [DOI] [PubMed] [Google Scholar]
- 41.Robbers LF, Eerenberg ES, Teunissen PF, et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34:2346–53. doi: 10.1093/eurheartj/eht100. [DOI] [PubMed] [Google Scholar]
- 42.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171e9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 43.Basso C, Corbetti F, Silva C, et al. Morphologic validation of reperfused hemorrhagic myocardial infarction by cardiovascular magnetic resonance. Am J Cardiol. 2007;100:1322–7. doi: 10.1016/j.amjcard.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 44.Lotan CS, Bouchard A, Cranney GB, et al. Assessment of postreperfusion myocardial hemorrhage using proton NMR imaging at 1.5 T. Circulation. 1992;86:1018–25. doi: 10.1161/01.cir.86.3.1018. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Green JD, Sykes JM, et al. Detection and quantification of myocardial reperfusion hemorrhage using T2* weighted CMR. J Am Coll Cardiol Img. 2011;4:1274–83. doi: 10.1016/j.jcmg.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Kali A, Tang RL, Kumar A, Min JK, Dharmakumar R. Detection of acute reperfusion myocardial hemorrhage with cardiac MR imaging: T2 versus T2. Radiology. 2013;269:387–95. doi: 10.1148/radiol.13122397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foltz WD, Yang Y, Graham JJ, Detsky JS, Wright GA, Dick AJ. MRI relaxation fluctuations in acute reperfused hemorrhagic infarction. Magn Reson Med. 2006;56:1311–9. doi: 10.1002/mrm.21079. [DOI] [PubMed] [Google Scholar]
- 48.Jackowski C, Christe A, Sonnenschein M, Aghayev E, Thali MJ. Postmortem unenhanced magnetic resonance imaging of myocardial infarction in correlation to histological infarction age characterization. Eur Heart J. 2006;27:2459–67. doi: 10.1093/eurheartj/ehl255. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen SF, Thrysøe SA, Robich MP, et al. Assessment of intramyocardial hemorrhage by T1-weighted cardiovascular magnetic resonance in reperfused acute myocardial infarction. J Cardiovasc Magn Reson. 2012;30:14–59. doi: 10.1186/1532-429X-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–72. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 51.de Waha S, Eitel I, Desch S, et al. Time-dependency, predictors and clinical impact of infarct transmurality assessed by magnetic resonance imaging in patients with ST-elevation myocardial infarction reperfused by primary coronary percutaneous intervention. Clin Res Cardiol. 2012;101:191–200. doi: 10.1007/s00392-011-0380-6. [DOI] [PubMed] [Google Scholar]
- 52.Al Khalaf MM, Thalib L, Doi SA. Combining heterogenous studies using the random-effects model is a mistake and leads to inconclusive meta-analyses. J Clin Epidemiol. 2011;64:119–23. doi: 10.1016/j.jclinepi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Cochet A, Lalande A, Lorgis L, et al. Prognostic value of microvascular damage determined by cardiac magnetic resonance in non ST-segment elevation myocardial infarction: comparison between first-pass and late gadolinium-enhanced images. Invest Radiol. 2010;45:725–32. doi: 10.1097/RLI.0b013e3181e6f45c. [DOI] [PubMed] [Google Scholar]
- 54.Mather AN, Fairbairn TA, Ball SG, Greenwood JP, Plein S. Reperfusion haemorrhage as determined by cardiovascular MRI is a predictor of adverse left ventricular remodelling and markers of late arrhythmic risk. Heart. 2011;97:453–9. doi: 10.1136/hrt.2010.202028. [DOI] [PubMed] [Google Scholar]
- 55.Ørn S, Manhenke C, Greve OJ, et al. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J. 2009;30:1978–85. doi: 10.1093/eurheartj/ehp219. [DOI] [PubMed] [Google Scholar]
- 56.Cochet AA, Lorgis L, Lalande A, et al. Prognostic impact of persistent microvascular obstruction as assessed by contrast-enhanced cardiac magnetic resonance in reperfused acute myocardial infarction. Eur Radiol. 2009;19:2117–26. doi: 10.1007/s00330-009-1395-5. [DOI] [PubMed] [Google Scholar]
- 57.de Waha S, Desch S, Eitel I, et al. Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J. 2010;31:2660–8. doi: 10.1093/eurheartj/ehq247. [DOI] [PubMed] [Google Scholar]
- 58.Klug G, Mayr A, Schenk S, et al. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:46. doi: 10.1186/1532-429X-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong DT, Leung MC, Richardson JD, et al. Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: a comparison of angiographic and cardiac magnetic resonance derived measurements. Int J Cardiovasc Imaging. 2012;28:1971–81. doi: 10.1007/s10554-012-0021-9. [DOI] [PubMed] [Google Scholar]
- 60.Yan AT, Gibson CM, Larose E, et al. Characterization of microvascular dysfunction after acute myocardial infarction by cardiovascular magnetic resonance first-pass perfusion and late gadolinium enhancement imaging. J Cardiovasc Magn Reson. 2006;8:831–7. doi: 10.1080/10976640600778049. [DOI] [PubMed] [Google Scholar]
- 61.Lund GK, Stork A, Saeed M, et al. Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology. 2004;232:49–57. doi: 10.1148/radiol.2321031127. [DOI] [PubMed] [Google Scholar]
- 62.Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083–9. doi: 10.1161/01.cir.0000027818.15792.1e. [DOI] [PubMed] [Google Scholar]
- 63.de Waha S, Desch S, Eitel I, et al. Relationship and prognostic value of microvascular obstruction and infarct size in ST-elevation myocardial infarction as visualized by magnetic resonance imaging. Clin Res Cardiol. 2012;101:487–95. doi: 10.1007/s00392-012-0419-3. [DOI] [PubMed] [Google Scholar]
- 64.Bruder O, Breuckmann F, Jensen C, et al. Prognostic impact of contrast-enhanced CMR early after acute ST segment elevation myocardial infarction (STEMI) in a regional STEMI network: results of the “Herzinfarktverbund Essen”. Herz. 2008;33:136–42. doi: 10.1007/s00059-008-3102-8. [DOI] [PubMed] [Google Scholar]
- 65.Vicente J, Mewton N, Croisille P, et al. Comparison of the angiographic myocardial blush grade with delayed-enhanced cardiac magnetic resonance for the assessment of microvascular obstruction in acute myocardial infarctions. Catheter Cardiovasc Interv. 2009;74:1000–7. doi: 10.1002/ccd.22157. [DOI] [PubMed] [Google Scholar]
- 66.Hombach V, Grebe O, Merkle N, et al. Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J. 2005;26:549–57. doi: 10.1093/eurheartj/ehi147. [DOI] [PubMed] [Google Scholar]
- 67.Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart. 2008;94:730–6. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
- 68.Mewton N, Bonnefoy E, Revel D, Ovize M, Kirkorian G, Croisille P. Presence and extent of cardiac magnetic resonance microvascular obstruction in reperfused non-ST-elevated myocardial infarction and correlation with infarct size and myocardial enzyme release. Cardiology. 2009;113:50–8. doi: 10.1159/000167042. [DOI] [PubMed] [Google Scholar]
- 69.Bodi V, Sanchis J, Nunez J, et al. Prognostic value of a comprehensive cardiac magnetic resonance assessment soon after a first ST-segment elevation myocardial infarction. J Am Coll Cardiol Img. 2009;2:835–42. doi: 10.1016/j.jcmg.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Eitel I, Blase P, Adams V, et al. Growth-differentiation factor 15 as predictor of mortality in acute reperfused ST-elevation myocardial infarction: insights from cardiovascular magnetic resonance. Heart. 2011;97:632–40. doi: 10.1136/hrt.2010.219543. [DOI] [PubMed] [Google Scholar]
- 71.Eitel I, Wöhrle J, Suenkel H, et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: cardiac magnetic resonance substudy of the AIDA STEMI trial. J Am Coll Cardiol. 2013;61:1447–54. doi: 10.1016/j.jacc.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 72.Tarantini G, Razzolini R, Cacciavillani L, et al. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98:1033–40. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 73.Shapiro MD, Nieman K, Nasir K, et al. Utility of cardiovascular magnetic resonance to predict left ventricular recovery after primary percutaneous coronary intervention for patients presenting with acute ST-segment elevation myocardial infarction. Am J Cardiol. 2007;100:211–6. doi: 10.1016/j.amjcard.2007.02.079. [DOI] [PubMed] [Google Scholar]
- 74.Nijveldt R, Beek AM, Hirsch A, et al. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52:181–9. doi: 10.1016/j.jacc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Beek AM, Nijveldt R, van Rossum AC. Intramyocardial hemorrhage and microvascular obstruction after primary percutaneous coronary intervention. Int J Cardiovasc Imaging. 2010;26:49–55. doi: 10.1007/s10554-009-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerber BL, Rochitte CE, Melin JA, et al. Microvascular obstruction and left ventricular remodeling early after acute myocardial infarction. Circulation. 2000;101:2734–41. doi: 10.1161/01.cir.101.23.2734. [DOI] [PubMed] [Google Scholar]
- 77.Małek ŁA, Œpiewak M, Kłopotowski M, Miśko J, Ruzyłło W, Witkowski A. The size does not matter – the presence of microvascular obstruction but not its extent corresponds to larger infarct size in reperfused STEMI. Eur J Radiol. 2012;81:2839–43. doi: 10.1016/j.ejrad.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 78.Amabile N, Jacquier A, Gaudart J, et al. Value of a new multiparametric score for prediction of microvascular obstruction lesions in ST-segment elevation myocardial infarction revascularized by percutaneous coronary intervention. Arch Cardiovasc Dis. 2010;103:512–21. doi: 10.1016/j.acvd.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105:656–62. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- 80.Eitel I, Nowak M, Stehl C, et al. Release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am Heart J. 2010;159:882–90. doi: 10.1016/j.ahj.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 81.Husser O, Monmeneu JV, Sanchis J, et al. Cardiovascular magnetic resonance-derived intramyocardial hemorrhage after STEMI: Influence on long-term prognosis, adverse left ventricular remodeling and relationship with microvascular obstruction. Int J Cardiol. 2013;167:2047–54. doi: 10.1016/j.ijcard.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 82.Jensen CJ, Eberle HC, Nassenstein K, et al. Impact of hyperglycemia at admission in patients with acute ST-segment elevation myocardial infarction as assessed by contrast-enhanced MRI. Clin Res Cardiol. 2011;100:649–59. doi: 10.1007/s00392-011-0290-7. [DOI] [PubMed] [Google Scholar]
- 83.Eitel I, Hintze S, de Waha S, et al. Prognostic impact of hyperglycemia in nondiabetic and diabetic patients with ST-elevation myocardial infarction: insights from contrast-enhanced magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:708–18. doi: 10.1161/CIRCIMAGING.112.974998. [DOI] [PubMed] [Google Scholar]
- 84.Hadamitzky M, Langhans B, Hausleiter J, et al. Prognostic value of late gadolinium enhancement in cardiovascular magnetic resonance imaging after acute ST-elevation myocardial infarction in comparison with single-photon emission tomography using Tc99m-Sestamibi. Eur Heart J Cardiovasc Imaging. 2014;15:216–25. doi: 10.1093/ehjci/jet176. [DOI] [PubMed] [Google Scholar]
- 85.Baks T, van Geuns RJ, Biagini E, et al. Recovery of left ventricular function after primary angioplasty for acute myocardial infarction. Eur Heart J. 2005;26:1070–7. doi: 10.1093/eurheartj/ehi131. [DOI] [PubMed] [Google Scholar]
- 86.O’Regan DP, Ariff B, Neuwirth C, Tan Y, Durighel G, Cook SA. Assessment of severe reperfusion injury with T2* cardiac MRI in patients with acute myocardial infarction. Heart. 2010;96:1885–91. doi: 10.1136/hrt.2010.200634. [DOI] [PubMed] [Google Scholar]
- 87.Ganame J, Messalli G, Dymarkowski S, et al. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J. 2009;30:1440–9. doi: 10.1093/eurheartj/ehp093. [DOI] [PubMed] [Google Scholar]
- 88.Bekkers SC, Smulders MW, Passos VL, et al. Clinical implications of microvascular obstruction and intramyocardial haemorrhage in acute myocardial infarction using cardiovascular magnetic resonance imaging. Eur Radiol. 2010;20:2572–86. doi: 10.1007/s00330-010-1849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eitel I, Kubusch K, Strohm O, et al. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ Cardiovasc Imaging. 2011;4:354–62. doi: 10.1161/CIRCIMAGING.110.960500. [DOI] [PubMed] [Google Scholar]
- 90.Amabile N, Jacquier A, Shuhab A, et al. Incidence, predictors, and prognostic value of intramyocardial hemorrhage lesions in ST elevation myocardial infarction. Catheter Cardiovasc Interv. 2012;79:1101–8. doi: 10.1002/ccd.23278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


