Abstract
Aims
The SYMPLICITY HTN-3 randomized, blinded, sham-controlled trial confirmed the safety of renal denervation (RDN), but did not meet its primary efficacy endpoint. Prior RDN studies have demonstrated significant and durable reductions in blood pressure. This analysis investigated factors that may help explain these disparate results.
Methods and results
Patients with resistant hypertension were randomized 2 : 1 to RDN (n = 364) or sham (n = 171). The primary endpoint was the difference in office systolic blood pressure (SBP) change at 6 months. A multivariable analysis identified predictors of SBP change. Additional analyses examined the influence of medication changes, results in selected subgroups and procedural factors. Between randomization and the 6-month endpoint, 39% of patients underwent medication changes. Predictors of office SBP reduction at 6 months were baseline office SBP ≥180 mmHg, aldosterone antagonist use, and non-use of vasodilators; number of ablations was a predictor in the RDN group. Non-African-American patients receiving RDN had a significantly greater change in office SBP than those receiving sham; –15.2 ± 23.5 vs. –8.6 ± 24.8 mmHg, respectively (P = 0.012). Greater reductions in office and ambulatory SBP, and heart rate were observed with a higher number of ablations and energy delivery in a four-quadrant pattern.
Conclusions
Post hoc analyses, although derived from limited patient cohorts, reveal several potential confounding factors that may partially explain the unexpected blood pressure responses in both the sham control and RDN groups. These hypothesis-generating data further inform the design of subsequent research to evaluate the potential role of RDN in the treatment of resistant hypertension.
ClinicalTrials. gov identifier
Keywords: Renal denervation, Resistant hypertension, SYMPLICITY
See page 199 for the editorial comment on this article (doi:10.1093/eurheartj/ehu450)
Introduction
The recognition of the importance of the renal sympathetic and somatic nerves in modulating blood pressure and the development of a novel procedure intended to selectively interrupt the sympathetic contribution to hypertension has introduced an opportunity to provide meaningful benefit to patients with resistant hypertension. Early experience with surgical sympathectomy demonstrated positive reductions in blood pressure for some patients, but such procedures were abandoned because, in part, they had serious side effects.1,2 Additionally, the demonstration of high renal norepinephrine spill over into plasma of patients with untreated essential hypertension provides key evidence for the central role of renal sympathetic activation in the pathogenesis of hypertension.3,4 More recently, preclinical and early phase human evaluations of a catheter-based approach to renal denervation (RDN) have mechanistically correlated afferent sensory and sympathetic efferent denervation with decreased renal norepinephrine spill over, halving of renin activity and increased renal plasma flow.5 In parallel, RDN has also demonstrated clinically significant, sustained reductions in office systolic blood pressure (SBP) of ∼25 mmHg in unblinded studies.6,7 Furthermore, observed reductions in blood pressure in a real-world, less-selected patient population have been substantive across a broader range of baseline blood pressures and confirm the safety of radiofrequency RDN.8 These early promising results in patients with resistant hypertension led to the rapid adoption of this therapy and its inclusion as a potential treatment option in published guidelines.9,10
The recent results of the SYMPLICITY HTN-3 trial have challenged preclinical science, clinical anecdote, and the consistency observed across early phase trials. Although the trial demonstrated the safety of RDN, among 535 patients identified with treatment-resistant hypertension the difference in 6-month blood pressure decline between RDN and sham-treatment groups was not significantly different for office or ambulatory measures.11 Given such discordant findings between SYMPLICITY HTN-3 and prior experience, we investigated key factors that could have contributed to the greater than expected drop in blood pressure after a sham procedure and the less than expected blood pressure drop in the patients receiving RDN. Based on the results of multivariable analysis to identify predictors of SBP change, and analysis of pre-specified and post hoc subgroups to identify potential confounding factors that may have affected the trial results, three areas of investigation were pursued: (i) changes in antihypertensive medications, (ii) outcomes in selected subgroups, and (iii) detailed assessment of procedural data that may have impacted the delivery of effective RDN.
Methods
Patients
Details of the methods, patient eligibility, and primary results of SYMPLICITY HTN-3 have been published.11,12 Briefly, the trial enrolled patients with drug-resistant hypertension defined as an office SBP of at least 160 mmHg and an ambulatory SBP of at least 135 mmHg despite adherence to maximally tolerated doses of at least three antihypertensive medications including a diuretic. All patients provided written informed consent for participation in the trial. The primary safety endpoint was the rate of major adverse events compared with a performance goal.11 The primary efficacy endpoint compared the change in office SBP from baseline to 6 months between the denervation group and the control group using a 5 mmHg superiority margin.
Trial procedure
After manual documentation of medication use in patient diaries for at least 10 of 14 days prior to the second screening visit and confirmation of blood pressure criteria for randomization, patients underwent a renal angiogram to confirm acceptable renal artery anatomy. Eligible patients were randomized 2:1 to RDN (n = 364) or sham control (renal angiogram; n = 171). Randomization was stratified by study centre and by race (African American vs. non-African American). The protocol specified that four to six ablations should be delivered to each renal artery beginning at the distal end of the artery and rotating in a helical pattern as the catheter is pulled back. Trained non-physician proctors were present at all cases. The protocol specified that no changes to antihypertensive medications should be made during the 6-month follow-up period before the primary endpoint was assessed unless considered a clinical necessity by the treating physician.
For each patient randomized to RDN using the Symplicity™ renal denervation system (Medtronic, Inc., Santa Rosa, CA, USA), procedure data were collected including the number of ablations in each renal artery and the locations of the ablations. A ‘four-quadrant’ ablation was determined by at least one superior treatment, one inferior treatment, and a minimum of two anterior/posterior treatments in each artery.
Additional analysis
Multivariable predictors of change in SBP at 6 months from baseline were performed for office and 24-h ambulatory blood pressure measurements. Because of the importance of medication treatment in this study and the unique requirement for stable doses of antihypertensive drugs at maximally tolerated doses from baseline to 6-month follow-up, a detailed examination of medication adherence was undertaken. Following enrolment, blood pressure and medication use were documented between screening visit 1 and screening visit 2. Assessment of medication prescription between baseline (screening visit 2) and 6-month follow-up for all antihypertensive medications and for medications at maximally tolerated doses was completed as well. Blood pressure change from baseline was assessed for each group of patients on aldosterone antagonists, vasodilators, beta-blockers, and calcium-channel blockers.
The significant change in office SBP observed for the non-African-American subgroup but not observed in the African-American subgroup was further explored based on differences between subgroups in baseline antihypertensive medications prescribed, particularly vasodilator prescription at baseline.
Procedural considerations were analysed according to the number of radiofrequency ablation attempts documented, and the proportion of patients with a four-quadrant ablation in both renal arteries, one renal artery or not delivered to either renal artery based on data collected at the time of the procedure. Office, ambulatory and home blood pressure changes at 6 months were included for each of the procedural analyses. The mean number of ablation attempts and the number of treatments per length of both arteries were assessed according to the number of ablations attempted.
Statistical analysis
Factors that could potentially affect outcomes were tabulated, and outcomes related to those factors, including office and ambulatory blood pressures, and heart rates are presented. Effects related to baseline antihypertensive medications on blood pressure outcomes were compared between RDN and sham groups using the two-sample t-test.
Because RDN procedure data were available only in the patients randomized to the treatment group, a post hoc analysis was performed to match sham patients with RDN patients using the propensity score method (Figure 2). Specifically, propensity scores were calculated for each randomized patient using logistic regression, with treatment assignment as a dependent variable, and the baseline covariates as independent variables (age, gender, BMI, baseline eGFR, diabetes, race [African American, non-African American], obstructive sleep apnoea, heart failure, number of medication classes at baseline, baseline office SBP, baseline ambulatory SBP, and baseline medication classes). For each sham patient, one treatment patient with the closest baseline characteristics is selected as the matching patient.13 The clinical outcomes in each matched cohort based on the number of ablations delivered to the RDN treatment group are compared between the matched patients in the two randomization arms using the two-sample t-test. The change from baseline at 6 months for office and ambulatory SBP and heart rate was assessed for each of the matched groups.
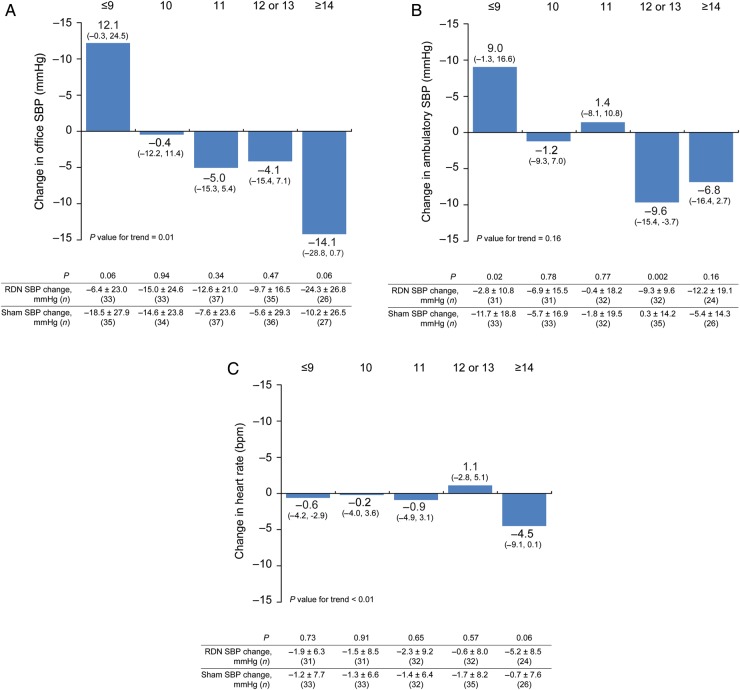
Figure 2.
The impact of number of ablation attempts on difference in 6-month change in office systolic blood pressure (A), 24-h ambulatory systolic blood pressure (B), and heart rate (C) between treated and matched sham patients. Baseline characteristics of the sham patients were propensity scored matched with the RDN patients. The SBP change measures for the RDN and matched sham patients, 95% confidence intervals, and P-values for the difference in change between the groups are shown.
Multivariable predictors of SBP change at 6 months were determined using multiple linear regressions. The following covariates were considered for each model: baseline office SBP ≥180 mmHg, African-American race, age <65 years, history of diabetes, eGFR ≥60 mL/min/1.73 m2, male gender, four-quadrant ablation pattern, ≥8 full 120-s ablations, ≥4 notches (defined as a visible depression in arterial wall at the site of ablation on fluoroscopic image), total number of ablation attempts, as well as baseline prescription of aldosterone antagonist, α-1-blocker, α-2 agonist, angiotensin-converting enzyme inhibitor, angiotensin-receptor blocker, beta-blocker, calcium-channel blocker, direct renin inhibitor, and vasodilator. The RDN procedural variables were included only in multivariable analyses of the RDN arm. A stepwise selection algorithm was used to select significant covariates with entry/stay significance levels of 0.2/0.1, respectively.
Results
Multivariable analysis of the overall group identified baseline office SBP ≥180 mmHg, and prescription of an aldosterone antagonist at baseline as positive predictors for increasing 6-month change from baseline in office SBP; prescription of a vasodilator at baseline was a negative predictor of office blood pressure reduction at 6 months (Table 1). The subgroup of patients <65 years in age was associated with SBP change in the RDN group in univariable analysis but not in the multivariable model. In the multivariable analysis, the total number of ablation attempts was an additional independent predictor for office SBP change in the RDN group. Aldosterone antagonist prescription at baseline was also a positive predictor for change in the 24-h ambulatory SBP change. Multivariable analysis of the control group also identified baseline office SBP ≥180 mmHg as a significant predictor of decreased office SBP. No variable predicted response in the control group for 24-h ambulatory SBP changes at 6 months.
Table 1.
Multivariable predictors of systolic blood pressure change at 6 months
| Covariate | Estimate | 95% Confidence interval | P-value |
|---|---|---|---|
| Pooled patients | |||
| Office SBP change (n = 518) | |||
| Randomized to RDN | −3.64 | −7.96, 0.69 | 0.100 |
| Baseline office SBP ≥180 mmHg | −14.94 | −19.06, −10.82 | <0.0001 |
| Aldosterone antagonist | −6.39 | −11.24, −1.54 | 0.010 |
| Vasodilator | 5.49 | 1.26, 9.72 | 0.011 |
| Ambulatory SBP change (n = 483) | |||
| Randomized to RDN | −2.11 | −5.10, 0.88 | 0.167 |
| Baseline eGFR ≥60 mL/min/1.73 m2 | −3.91 | −7.39, −0.44 | 0.028 |
| Aldosterone antagonist | −3.98 | −7.24, −0.72 | 0.017 |
| RDN group | |||
| Office SBP change (n = 318) | |||
| Baseline office SBP ≥180 mmHg | −14.31 | −19.23, −9.39 | <0.0001 |
| Total number of ablation attempts | −0.94 | −1.82, −0.05 | 0.040 |
| Aldosterone antagonist | −9.77 | −15.83, −3.72 | 0.002 |
| Vasodilator | 7.55 | 2.38, 12.72 | 0.005 |
| Ambulatory SBP change (n = 293) | |||
| Baseline eGFR ≥60 mL/min/1.73 m2 | −4.56 | −8.99, −0.13 | 0.044 |
| Aldosterone antagonist | −5.19 | −9.33, −1.06 | 0.014 |
| Sham groupa | |||
| Office SBP change (n = 169) | |||
| Baseline office SBP ≥180 mmHg | −8.00 | −16.42, 0.41 | 0.064 |
| African-American race | −11.97 | −19.81, −4.14 | 0.003 |
| Alpha-1 blocker use | −12.00 | −23.60, −0.40 | 0.044 |
P value needs to be <0.2 to enter the model, and needs to be <0.1 to stay.
aThere were no multivariable predictors of ambulatory SBP change in the sham group.
An average of 19.5 days transpired between the two screening visits. Between screening visit 1 and screening visit 2, 94% of patients were on stable doses of their antihypertensive medications and 78% of all patients were on maximum tolerated doses of at least three drugs for at least 6 weeks. After randomization, medication changes occurred more frequently with 39% of patients having a change in dose or drug class between baseline and the 6-month primary endpoint. The majority of the medication changes (69%) were for drugs prescribed at maximally tolerated dose, and this was often related to clinical symptoms or an adverse event, as permitted by the ‘medication escape’ criteria in the protocol (Table 2).
Table 2.
Antihypertensive medication use change analysis
| RDN group | Sham group | |
|---|---|---|
| Baseline number of medications | 5.1 ± 1.4 | 5.2 ± 1.4 |
| 6-month number of medications | 5.0 ± 1.4 | 5.2 ± 1.6 |
| Medication change SV1 to SV2 | 18 (4.9%) | 13 (7.6%) |
| Any medication change between baseline and 6 months | 139a (38.2%) | 72a (42.1%) |
| >1 change between baseline and 6 months | 119 (32.7%) | 60 (35.1%) |
| Decreased number of medication classes or doses | 52 (14.3%) | 23 (12.8%) |
| Increased number of medication classes or doses | 31 (8.5%) | 17 (9.9%) |
| Combination of increases and decreases in class and/or dose | 56 (15.3%) | 32 (18.7%) |
| Medication change related to an adverse event or symptom change | 98 (26.9%) | 53 (31.0%) |
| Medication change related to SBP <115 mmHg | 13 (3.6%) | 2 (1.2%) |
| Medication change related to SBP increase >15 mmHg | 14 (3.8%) | 7 (4.1%) |
| Other reasons | 72 (19.8%) | 41 (24.0%) |
Data is mean (SD) or n (%).
SV, screening visit.
aFour RDN group patients and two control group patients who had no net change for the 6-month period (i.e. the same medication changed and returned to previous dose).
Subgroup analysis
Medication classes
Office and 24-h ambulatory blood pressure change at 6 months for subgroups based on medication class prescription at baseline are shown in Table 3. There were no significant differences in blood pressure changes for patients on a vasodilator, beta-blocker, or calcium-channel blocker at baseline although there was a trend for greater office SBP change in the denervation group for the beta-blocker and calcium-channel blocker subgroups.
Table 3.
Blood pressure 6-month change from baseline according to baseline medication use
| Effectiveness measure | RDN group | Sham group | 95% CI | P-value |
|---|---|---|---|---|
| Aldosterone antagonists | ||||
| Office | (n = 76) | (n = 47) | ||
| SBP | −21.9 ± 25.0 | −13.8 ± 27.8 | −8.05 (−17.6, 1.5) | 0.10 |
| DBP | −10.3 ± 13.2 | −6.2 ± 17.5 | −4.06 (−10.0, 1.8) | 0.18 |
| 24-h ambulatory | (n = 73) | (n = 46) | ||
| SBP | −11.1 ± 15.4 | −5.4 ± 21.9 | −5.7 (−13.1, 1.7) | 0.13 |
| DBP | −7.0 ± 9.9 | −2.7 ± 12.0 | −4.3 (−8.3, −0.3) | 0.04 |
| Vasodilators | ||||
| Office | (n = 125) | (n = 76) | ||
| SBP | −11.0 ± 24.6 | −11.6 ± 26.7 | 0.6 (−6.7, 7.9) | 0.86 |
| DBP | −6.0 ± 12.6 | −4.7 ± 15.0 | −1.2 (−5.1, 2.7) | 0.53 |
| 24-h ambulatory | (n = 113) | (n = 73) | ||
| SBP | −7.2 ± 15.7 | −4.1 ± 17.0 | −3.1 (−7.9, 1.7) | 0.21 |
| DBP | −4.8 ± 9.9 | −2.9 ± 9.6 | −1.9 (−4.8, 1.0) | 0.20 |
| Beta-blockers | ||||
| Office | (n = 298) | (n = 145) | ||
| SBP | −15.6 ± 24.1 | −10.6 ± 27.0 | −5.0 (−10.0, 0.0) | 0.05 |
| DBP | −6.5 ± 12.0 | −4·7 ± 13.9 | −1.8 (−4.5, 0.8) | 0.18 |
| 24-h ambulatory | (n = 274) | (n = 135) | ||
| SBP | −6.8 ± 15.5 | −4.8 ± 16.7 | −2.0 (−5.3, 1.3) | 0.23 |
| DBP | −4.2 ± 9.5 | −3.1 ± 9.7 | −1.1 (−3.1, 0.9) | 0.28 |
| Calcium-channel blockers | ||||
| Office | (n = 242) | (n = 124) | ||
| SBP | −15.0 ± 22.9 | −9.6 ± 26.8 | −5.4 (−10.9, −0.2) | 0.06 |
| DBP | −6.6 ± 12.2 | −4.8 ± 13.7 | −1.8 (−4.5, 1.0) | 0.21 |
| 24-h ambulatory | (n = 225) | (n = 117) | ||
| SBP | −7.1 ± 15.8 | −4.7 ± 16.9 | −2.4 (−6.0, 1.2) | 0.20 |
| DBP | −4.3 ± 9.9 | −3.1 ± 9.8 | −1.2 (−3.4, 1.0) | 0.29 |
Values are mean ± SD.
Patient population
A significant difference in office SBP change was observed for the non-African-American subgroup, but this was not observed in the African-American population that comprised 26.2% of the overall population (Figure 1A). There was not a significant treatment interaction between the two subgroups (interaction P = 0.09). Although trends were similar, the differences between African-American and non-African-American subgroups were not significant for mean 24-h ambulatory or home SBP.
Figure 1.
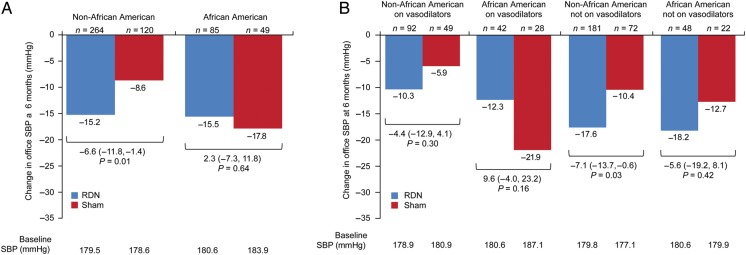
Change in office systolic blood pressure at 6 months for non-African-American and African-American subgroups (A) and for non-African-American and African-American subgroups according to baseline vasodilator use (B). P-values shown are for the difference between the 6-month change from baseline for the RDN group and the sham group. All 6-month change from baseline values are significant (P < 0.001).
Baseline medication prescription in the African-American and non-African-American subgroups was examined and found to be similar for all antihypertensive drug classes, except vasodilators: 56% of African-American sham patients and 46.7% of African-American RDN patients were receiving a vasodilator; 40.5% of non-African-American sham control patients and 33.7% of non-African-American patients were receiving a vasodilator at baseline. Overall 71% of the vasodilators prescribed were hydralazine and 27% were minoxidil; the remaining 2% were reserpine.
Further analysis of these subgroups based on baseline prescription of a vasodilator revealed a particularly large decline in SBP for the African-American subgroup prescribed vasodilators in the sham group (−21.9 ± 29.1 mmHg) which did not occur in either the African-American sham group not prescribed vasodilator therapy or in the non-African-American sham groups with or without vasodilator prescription at baseline (Figure 1B). In the non-African-American subgroup, patients not prescribed a vasodilator had a significantly greater change in office SBP (−17.6 mmHg in the RDN group and −10.4 mmHg in the sham group; 95% CI, −7.1 [−13.7, −0.6], P = 0.03).
Procedural analysis
Consistent and greater reductions in office and ambulatory blood pressure, and heart rate were identified with a higher number of renal artery ablations. When control patients and RDN patients were propensity score matched according to baseline characteristics, the difference in office and ambulatory SBP, and heart rate between the two groups, increased with increasing numbers of ablations delivered to the treatment group (Figure 2). This was statistically significant for the office SBP (P value for trend 0.01) and heart rate (P value for trend <0.01) and for the ambulatory SBP groups that received 12 or 13 ablations. The majority of patients received at least the minimum recommended number of ablations (4–6 per artery) but there were four patients who received less than eight ablations including two patients who only received one ablation. The higher numbers of ablations were related to repeated ablations when generator error codes appeared or in instances in which longer renal arteries permitted more ablations. The mean number of ablation attempts increased with increasing renal artery length. There was no increase in safety events corresponding to the increasing number of renal artery ablations (no MAEs occurred in patients receiving ≥13 ablations).
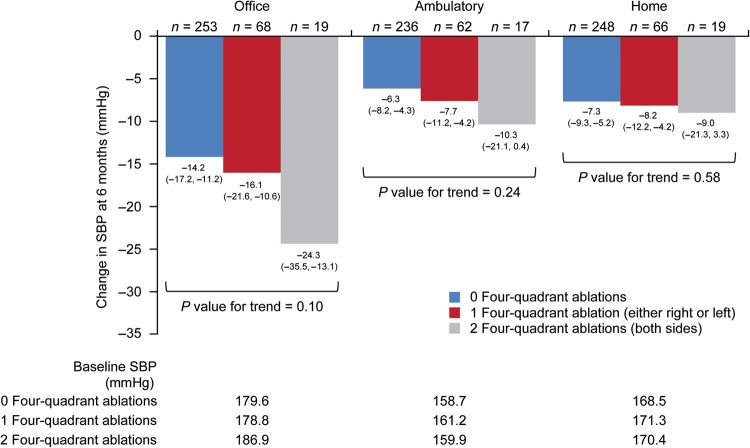
Delivery of ablations in a four-quadrant pattern to neither one or both renal arteries revealed a similar but not statistically significant pattern of increasing reduction in office (P value for trend 0.10), ambulatory (P value for trend 0.24), and home blood pressures (P value for trend 0.58) (Figure 3). These analyses revealed that only 19 treated patients received four-quadrant ablations in both renal arteries.
Figure 3.
Systolic blood pressure change at 6 months according to the ablation pattern. Change in office, ambulatory, and home systolic blood pressure at 6 months are shown based on delivery of ablations in four quadrants of the renal artery for both kidneys, one kidney, or neither kidney. A four-quadrant ablation is defined as one superior, one inferior, and two anterior/posterior ablations delivered.
Discussion
Amidst enthusiasm for a promising breakthrough therapy in treatment-resistant hypertension, the failure of SYMPLICITY HTN-3 to demonstrate a significant improvement in blood pressure compared with a sham procedure led to the examination of factors that might have contributed to the unexpected results. These post hoc analyses were conducted following completion of the primary analyses for the SYMPLICITY HTN-3 trial. The implementation of blinding and a sham control were expected to narrow but not eliminate the difference between experimental and control groups; what was observed was a less than expected RDN treatment effect and a more pronounced response in the sham group. Our initial multivariable analysis serves to guide further exploration of factors that may have affected the overall study efficacy result, since the result was similar between treatment groups, yet factors predicting changes in SBP differed between subgroups. Recognizing the limitations of additional exploratory testing in the context of an overall negative result, this preliminary analysis also provides the basis to identify and prioritize various factors for further study.
Variable adherence to and frequent revisions of antihypertensive therapy are well documented among hypertensive patients.14–18 However, in this trial, an analysis eliminating those with medication changes did not affect the primary outcome or pre-specified secondary outcome.14 Nevertheless, a substantial decrease in blood pressure among sham patients suggests a change in patient behaviour (despite self-reported documentation of medication adherence), or changes in prescribed antihypertensive medications during the course of trial participation. To the latter issue, although nearly all patients were prescribed maximal medical therapy at least 6 weeks prior to randomization, many patients (39%) underwent medication changes between the randomization and the 6-month endpoint assessment. These changes typically represented both alterations in dose and class of prescribed medications, a finding that challenges the premise that patients were actually receiving maximally tolerated doses at enrolment. Several randomized placebo-controlled pharmaceutical trials19–21 have shown much smaller reductions in ambulatory blood pressure than that observed in this trial, which also suggests that the observed sham response might be related to the maximum tolerated dose requirement and changing medication adherence patterns. The fact that there were eight clinical contact points with enrolled patients between the initial screening visit and the 6-month follow-up is clearly not representative of usual clinical practice and may also have impacted medication adherence. Moreover, the sham intervention and related hospitalization are not encountered in placebo-controlled pharmaceutical trials and may have had more impact than anticipated. This observation identifies the challenge for future RDN trials that, in spite of protocol mandate, patients with treatment-resistant hypertension can be maintained on a stable medication regimen to avoid confounding the assessment of device effectiveness. In fact, it is unclear whether a 2-week screening period for stabilization of antihypertensive medications is adequate for drugs that are not at maximal tolerated dose or whether the mandate for maintenance of a complex medical regimen under close supervision actually increased medication changes during the study.
Whether a differential blood pressure response following RDN exists relative to classes of antihypertensive therapy has been of particular interest, and in fact, outcomes among patients taking aldosterone antagonists represented a pre-specified analysis. The greater decline in blood pressure with RDN among patients already taking aldosterone antagonists seems initially counterintuitive and may be partially related to a higher baseline SBP for patients prescribed an aldosterone antagonist and differences in certain baseline characteristics (younger age and history of significantly more hypertensive crises). Alternatively, it may be that denervation contributes an additive effect to pre-existing neurohormonal blockade demonstrated with aldosterone antagonists22 and therefore results in the more exaggerated blood pressure response in this subgroup. However, it may be by chance alone that baseline aldosterone antagonist use appears as a predictor of blood pressure reduction.
Unlike previous SYMPLICITY trials, SYMPLICITY HTN-3 enrolled a substantial number of African-American patients who represent a significant proportion of hypertensive patients in the USA. The African-American sham patients demonstrated an unusually large decrease in SBP compared with non-African-American controls. Although a genetic basis has been postulated for differential response to hypertension and heart failure therapies among African Americans,23–25 the marked reduction in blood pressure in the sham group could be related to a change in medical adherence and/or type of therapy; notably, a higher proportion of African Americans were prescribed vasodilator therapy. The exact reasons for blood pressure differences observed between African-American and non-African-American control patients are unclear and highlight the importance of consistent and standardized BP care in subsequent denervation trials.
An especially challenging aspect of RDN therapy is that no practical and immediate measure of procedural success exists. Based on early experience, catheter-based RDN was expected to result in an ∼50% reduction in renal norepinephrine spillover.26,27 However, subsequent investigations regarding the effect of RDN on norepinephrine spillover as a surrogate marker of sympathetic activity have reported more modest and highly variable declines in sympathetic activity.26,28 Given this variability the possible contribution of a placebo effect in previous trials cannot be excluded. Assumption that the renal efferent and afferent nerves represent an isthmus for the sympathetic nervous system may be an oversimplification, and more detailed translational science was likely curtailed by clinical enthusiasm following early clinical trial results. These observations underscore the need for greater understanding of the pathophysiology of the sympathetic nervous system and histopathological insights to renal nerve ablation. Despite widespread clinical adoption, surprisingly little is known regarding the translation of RDN to sympathetic activity, and these results indicate that revisiting the basic science of RDN is essential to its advancement.26 Recently published research regarding the anatomical distribution of the peri-arterial sympathetic renal nerves in humans provides important new information that reinforces the need to refine the RDN procedure to target the renal arterial nerves more effectively and achieve more consistent denervation.29
An equally important procedural consideration was whether RDN was performed in the same manner as in early studies. For example, the recently presented experience from the Global SYMPLICITY Registry, with experienced operators in a similar population, demonstrated a mean SBP drop of −19.3 ± 22.4 at 6 months and delivered more ablations per patient (13.5 ± 4.1) than were delivered in the current trial.8 This issue was especially relevant given that more than half of operators performed at most two RDN procedures, and 31% performed only one RDN procedure during the trial. In our analysis, the lack of ablations in all four quadrants and the number of ablation attempts were highly correlated with blood pressure reductions. These two variables may be correlated and more ablation attempts may well increase the probability of ablating within all four quadrants. Additionally, the directional changes in blood pressure were consistent across all measures of blood pressure assessment as well as heart rate (an indicator of reduced hyper-sympathetic activity). Comparison of propensity score matched sham cohorts showed that this pattern of increasing response was less apparent in control patients and the RDN group receiving ≥14 ablations had significantly greater reductions in SBP. In addition, approximately three-quarters of patients did not receive ablations in all four quadrants.
Conclusions
The failure of RDN to significantly reduce blood pressure in a well-conducted study provides an opportunity to redefine methods of study and endpoints that may more carefully reveal its potential. The purpose of these analyses was to critically examine the results of the SYMPLICITY HTN-3 trial in the context of both existing RDN data and clinical trial design, systematically explore hypotheses related to confounding variables identified in either substudy or review of procedural technique, and directly address outstanding issues that have been postulated in the medical community as reasons for lack of efficacy. As this report represents both pre-specified and post hoc analyses from the SYMPLICITY HTN-3 trial, an important limitation is that these results are derived from more limited patient cohorts and from a trial that did not meet its primary efficacy endpoint. Thus, subgroup analyses should be considered exploratory and hypothesis-generating to inform the design of future RDN investigation. However, findings from these analyses will considerably influence both preclinical investigation and performance of clinical trials related to further RDN study.
Funding
This work was supported by Medtronic, Inc.
Conflict of interest: D.E.K. receives research and grant support and consulting honoraria from Medtronic CardioVascular and Boston Scientific. D.L.B. is on Advisory Boards for Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; is on the Board of Directors for Boston VA Research Institute, Society of Cardiovascular Patient Care; is Chair American Heart Association Get With The Guidelines Steering Committee; is on Data Monitoring Committees for Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and Population Health Research Institute (including for EnligHTNment) and receives Honoraria from American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology); Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Associate Editor); Journal of the American College of Cardiology (Section Editor, Pharmacology); Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic (co-PI of SYMPLICITY HTN-3), Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda. S.B. and M.F. are employees of Medtronic, Inc. C.M.D. serves on an Advisory Board for Medtronic. M.E. receives grant support and consulting fees from Medtronic, Inc. J.M.F. reports grants, personal fees and non-financial support from NIH, Novartis, and Medtronic, and personal fees from Back Beat Hypertension, and NIVasc. R.R.T., W.W.O., B.T.K. receive consultation fees from Medtronic. D.P.L. receives honoraria as a member of an advisory board for Medtronic. M.B.L. has nothing to disclose. S.O. receives grant support and fees from Medtronic and Bayer, Honoraria and consultant fees from Daiichi Sankyo, Inc., grant support from Merck and is a consultant to Forest and Takeda. P.A.S. receives consultant fees from Abbott Ventures, Inc., Boston Scientific, Inc., Medtronic Cardiovascular, Inc., and Rainbow Medical, Inc. P.A.S. is Chief Medical Officer for Cibiem, Inc. and Rox Medical Inc. and receives royalties and salary from Ardian. K.R.-S. receives personal fees from Medtronic, Boston Scientific Corporation, Cordis Corporation, and CardioSonic. G.L.B. is a consultant to AbbVie, Takeda, Medtronic, Relypsa, Daichi-Sankyo, Janssen, Novartis and Bayer (fees paid to institution) and receives grant support from Takeda.
Acknowledgements
We thank Xiaohua Chen, MS, and Lanyu Lei, MS, from Harvard Clinical Research Institute for statistical analyses funded by Medtronic, and Colleen Gilbert, PharmD, from Medtronic for editorial support. We would also like to thank Minglei Lui, PhD, Lilian Lee, PhD, and Juan Wu, MS, for data analysis support and Manuela Negoita, MD, Sidney A Cohen, MD, PhD, Vanessa DeBruin, MS, Denise Jones, RN, BSN, Dan Jolivette, MD and the entire SYMPLICITY HTN-3 study team for dedicated research support.
References
- 1.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 2.Longland CJ, Gibb WE. Sympathectomy in the treatment of benign and malignant hypertension; a review of 76 patients. Br J Surg. 1954;41:382–392. doi: 10.1002/bjs.18004116814. [DOI] [PubMed] [Google Scholar]
- 3.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Esler M, Lambert G, Jennings G, Turner A, Kaye D. Central and peripheral norepinephrine kinetics in heart failure, coronary artery disease, and hypertension. Adv Pharmacol. 1998;42:650–653. doi: 10.1016/s1054-3589(08)60835-2. [DOI] [PubMed] [Google Scholar]
- 5.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 6.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. for the Symplicity HTN-2 Investigators. [DOI] [PubMed] [Google Scholar]
- 7.Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 8.Mahfoud F, Mancia G, Schlaich MP, Narkiewics K, Ruilope L, Schmieder R, Böhm M. Reduction in office blood pressure after renal denervation in a large real world patient population with uncontrolled hypertension: interim 12-month results from the Global SYMPLICITY registry. Presented at the European Society of Cardiology; Barcelona: 2014. p. p62. [Google Scholar]
- 9.Mahfoud F, Luscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefevre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Böhm M European Society of Cardiology. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34:2149–2157. doi: 10.1093/eurheartj/eht154. [DOI] [PubMed] [Google Scholar]
- 10.Schmieder RE, Redon J, Grassi G, Kjeldsen SE, Mancia G, Narkiewicz K, Parati G, Ruilope L, van de Borne P, Tsioufis C. ESH position paper: renal denervation—an interventional therapy of resistant hypertension. J Hypertens. 2012;30:837–841. doi: 10.1097/HJH.0b013e328352ce78. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 12.Kandzari DE, Bhatt DL, Sobotka PA, O'Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin D. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Statist. 1985;39:33–38. [Google Scholar]
- 14.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–774. doi: 10.1097/HJH.0b013e32835e2286. [DOI] [PubMed] [Google Scholar]
- 15.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 17.Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control—continued disparities in adults: United States, 2005–2006. NCHS Data Brief. 2008:1–8. [PubMed] [Google Scholar]
- 18.Conlin PR, Gerth WC, Fox J, Roehm JB, Boccuzzi SJ. Four-year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Ther. 2001;23:1999–2010. doi: 10.1016/s0149-2918(01)80152-1. [DOI] [PubMed] [Google Scholar]
- 19.Bakris GL, Lindholm LH, Black HR, Krum H, Linas S, Linseman JV, Arterburn S, Sager P, Weber M. Divergent results using clinic and ambulatory blood pressures: report of a darusentan-resistant hypertension trial. Hypertension. 2010;56:824–830. doi: 10.1161/HYPERTENSIONAHA.110.156976. [DOI] [PubMed] [Google Scholar]
- 20.Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1423–1431. doi: 10.1016/S0140-6736(09)61500-2. [DOI] [PubMed] [Google Scholar]
- 21.Oxlund CS, Henriksen JE, Tarnow L, Schousboe K, Gram J, Jacobsen IA. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens. 2013;31:2094–2102. doi: 10.1097/HJH.0b013e3283638b1a. [DOI] [PubMed] [Google Scholar]
- 22.Wray DW, Supiano MA. Impact of aldosterone receptor blockade compared with thiazide therapy on sympathetic nervous system function in geriatric hypertension. Hypertension. 2010;55:1217–1223. doi: 10.1161/HYPERTENSIONAHA.109.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cubeddu LX, Aranda J, Singh B, Klein M, Brachfeld J, Freis E, Roman J, Eades T. A comparison of verapamil and propranolol for the initial treatment of hypertension. Racial differences in response. JAMA. 1986;256:2214–2221. [PubMed] [Google Scholar]
- 24.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 25.Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med. 2001;344:1351–1357. doi: 10.1056/NEJM200105033441802. [DOI] [PubMed] [Google Scholar]
- 26.Esler M. Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8:593–598. doi: 10.1016/j.jash.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 27.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 28.Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension. 2014;64:118–124. doi: 10.1161/HYPERTENSIONAHA.113.03098. [DOI] [PubMed] [Google Scholar]
- 29.Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, Virmani R, Joner M. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. doi: 10.1016/j.jacc.2014.03.059. [DOI] [PubMed] [Google Scholar]