Abstract
Background
Early-afterdepolarizations (EADs) are triggers of cardiac arrhythmia driven by L-type Ca2+ current (ICaL) reactivation or sarcoplasmic reticulum (SR) Ca2+ release and Na+/Ca2+ exchange. In large mammals the positive action potential (AP) plateau promotes ICaL reactivation, and the current paradigm holds that cardiac EAD dynamics are dominated by interaction between ICaL and the repolarizing K+ currents. However, EADs are also frequent in the rapidly repolarizing mouse AP, which should not readily permit ICaL reactivation. This suggests that murine EADs exhibit unique dynamics, which are key for interpreting arrhythmia mechanisms in this ubiquitous model organism. We investigated these dynamics in myocytes from arrhythmia-susceptible CaMKIIδC-overexpressing mice (Tg), and via computational simulations.
Methods and Results
In Tg myocytes, β-adrenergic challenge slowed late repolarization, potentiated SR Ca2+ release, and initiated EADs below the ICaL activation range (−47±0.7 mV). These EADs were abolished by caffeine and tetrodotoxin (but not Ranolazine), suggesting that SR Ca2+ release and Na+ current (INa), but not late INa, are required for EAD initiation. Simulations suggest that potentiated SR Ca2+ release and Na+/Ca2+ exchange triangulate late AP repolarization, which permits non-equilibrium reactivation of INa, and thereby drives the EAD upstroke. AP clamp experiments suggest that lidocaine eliminates virtually all inward current elicited by EADs, and that this effect occurs at concentrations (40-60 μM) for which lidocaine remains specific for inactivated Na+ channels. This strongly suggests that previously inactive channels are recruited during the EAD upstroke, and that non-equilibrium INa dynamics underlie murine EADs.
Conclusions
Non-equilibrium reactivation of INa drives murine EADs.
Keywords: arrhythmia, cardiac dynamics, calcium, INa, EADs
Introduction
It is generally accepted that lethal ventricular arrhythmias occur when a triggering event is able to propagate into electrophysiologically susceptible tissue1. Premature ventricular contractions are prototypical triggers in the myocardium, and are thought to emerge from arrhythmogenic cellular events known as early and delayed afterdepolarizations (EADs or DADs)2. DADs occur in the diastolic interval and result from Na+/Ca2+exchange secondary to spontaneous sarcoplasmic reticulum Ca2+ release (SCR)3. In contrast, EADs initiate during action potential (AP) repolarization and involve complex dynamical interaction among the currents that remain available (or regain availability) late in the AP4. Humans and other large mammals exhibit ventricular APs with a prolonged plateau phase (~100-200 ms) at slightly positive membrane potentials (Em). This imposes two key constraints on the inward currents capable of driving EADs in these species: (1) the fast sodium current (INa) is largely excluded as it remains entirely inactivated at plateau Em5; (2) while Na+/Ca2+exchange can act as the proximal cause of EADs in large mammals6, 7, peak inward Na+/Ca2+exchange current (INaCa) is thermodynamically limited because plateau Em is relatively close to exchange equilibrium3. Thus, the L-type Ca2+ current (ICaL) eventually carries the majority of inward charge during the EAD upstroke. As a consequence of these constraints, a series of recent studies has established that EADs in large mammals rely largely upon the dynamics governing reactivation of ICaL1,5,8.
In contrast, the murine ventricular AP repolarizes rapidly (tens of ms) and exhibits a brief plateau at negative Em (~ −50 mV). These characteristics sharply limit the potential for ICaL reactivation, yet numerous studies have observed EADs in the mouse9-13, and most have implicated ICaL11-14. This presents a paradox regarding the electrophysiologic conditions required to elicit EADs in the mouse ventricle, which has important implications for interpreting arrhythmia mechanisms in a large number of disease models. To clarify the dynamics capable of driving murine EADs, we have combined experimental and computational approaches to assess EAD mechanisms in myocytes from arrhythmia-susceptible CaMKIIδC-overexpressing mice (Tg)15.
EADs appearing during β-adrenergic stimulation were initiated by non-equilibrium reactivation of INa secondary to exaggerated sarcoplasmic reticulum (SR) Ca2+ release and INaCa. In some instances this evoked subsequent reactivation of ICaL and dynamical behavior similar to that described for larger species. However, the majority of events remained reliant upon non-equilibrium INa dynamics. Thus, we suggest that the mouse AP imposes unique constraints on EAD dynamics that rely upon an INa-dependent mechanism of initiation.
Methods
A detailed description of all methods and analyses are provided in the Supplemental Information (SI).
CaMKIIδC transgenic mice
Male and female cardiac-specific CaMKIIδC transgenic mice (n=135) and WT littermates (n=130) were studied at 60.4±0.6 days of age (both groups). These mice have previously been described in detail15, 16, however, we note that they now exhibit a slower time course of HF development than originally published (Figure S4). All procedures received prior approval from the UCSD Animal Subjects Committee.
Myocyte Isolation, Electrophysiology, and Ca2+ imaging
CaMKIIδC myocytes were isolated via enzymatic dissociation, and stored for at least 2 hours at 37°C prior to undergoing one of two protocols: (1) Current clamp AP recordings in whole cell patch configuration, or (2) pause-induced spontaneous Ca2+ release under field-pacing (SCR). For patch clamp experiments, simultaneous Ca2+ imaging was performed via Fura-2 with internal solution containing (mmol/L) 120 K-Aspartate, 10 KCl, 10 NaCl, 5 Mg-ATP, 1 MgCl2, 0.3 Li-GTP, and 125 μM Fura-2 potassium salt (pH 7.2, KOH). The external solution for both patch-clamp and field-pacing experiments (Normal Tyrode’s, NT) contained (mM) 140 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 10 Hepes (pH: 7.4, NaOH). Isoproterenol (100 nM) was added from frozen stock (1 mM in 0.01N HCl), and caffeine (10 mM) was prepared fresh from powder. For both experiments cells were preloaded with Ca2+-sensitive dyes in 37°C media: Fura-2 AM for patch-clamp (15 minutes, 5 μM, Life Technologies: F1225) and Fluo-3 AM for SCR (40 minutes, 5 μM, Life Technologies: F1241). Cells were then twice rinsed and stored in NT for 20 minutes to allow de-esterification. During current clamp experiments, cells were paced for 50 cycles (1 Hz) in NT before Iso was applied focally by micro-bore tubing. Another 50 cycles were acquired at least 1 minute after beginning Iso superfusion, as such all patch-clamp recordings are paired analyses. In SCR experiments, cells were paced for 30 seconds (again 1 Hz), and followed by a 30-second pause for SCR before rapid caffeine contracture (10 mM in 0 Na+/0 Ca2+ Tyrode’s). For SCR, Iso containing NT was applied to a separate group of cells from the same isolation. The bathing solution was maintained at 37°C for all experiments, and the experimenter was blinded to the genotype of the cells.
Computational Models
Our mouse ventricular cell model was a modification of the Bondarenko myocyte17. Alterations were made primarily to permit implementation of acute and chronic effects of CaMKII. These included phosphoregulation of the Ryanodine receptor (RyR), ICaL, INa, and late INa (INaL)18-20, as well as transcriptional regulation of the transient outward current (Ito), inward rectifier current (IK1), INaCa, and sarco-endoplasmic reticulum Ca2+-ATPase (SERCA)16, 20, 21. β-adrenergic stimulation was simulated via established effects at ICaL, the ultra-rapid delayed rectifier current (IKur), and on function of the Na+/K+-ATPase and SERCA. Importantly we did not implement any direct effects of βAR stimulation at INa. While previous work has suggested that βAR agonists induce both a leftward shift in voltage-dependent activation and inactivation, and increased peak conductance 22, these effects may be partially explained by CaMKII. In particular, CaMKII has recently been shown to be responsible for the Iso-dependent leftward shifted inactivation and increased INaL accompanying Iso challenge 22, and these actions are already incorporated in our INa model. Additionally, the effects of β-adrenergic stimulation on peak conductance and activation have previously been shown to occur relatively slowly (appearing after ~ 5 minutes), and at least partly depend upon translocation of NaV1.5 to the cell surface 23, 24. Because our experiments were generally complete 2-3 minutes after application of Iso, it is unlikely that these increases in channel density contributed to the EADs we’ve measured.
Statistics
Fisher’s exact test was used for EAD susceptibility in terms of proportion of cells. The Kruskal-Wallis test was used for percent of cycles with EADs or DADs. This test was applied across all 4 groups (WT, Tg, WT + Iso, Tg + Iso), and followed by either Mann-Whitney (unpaired data) or Wilcoxon (paired data) post hoc tests, with Bonferroni correction. Due to larger sample sizes for SCR experiments, differences in incidence of SCR were determined by Chi-squared tests, and mean event frequencies (events/cell) were assessed by the Mann-Whitney test. Repeated measures ANOVA was used for action potential and Ca2+-handling comparisons after separating the transgenic cells into EAD+ and EAD− subsamples (see Results). Student’s t-tests were applied with Bonferroni correction for all post hoc comparisons.
Results
Cellular arrhythmogenesis in CaMKIIδC transgenic myocytes
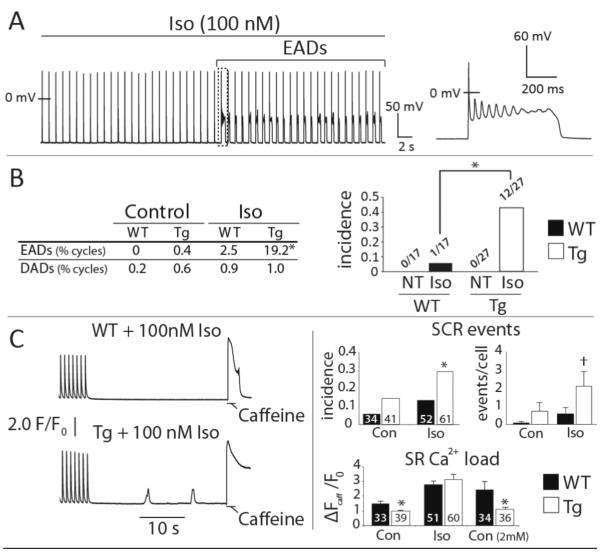
The cardiac-specific CaMKIIδC transgenic mouse develops heart failure (HF), is arrhythmia-susceptible, and presents increased cellular susceptibility to both EADs and DADs10, 16, 20, 21 (Figure S4). Here we elicited EADs through β-adrenergic challenge by isoproterenol (100 nM, Iso; Figure 1A & B), and while DADs and SCR were almost completely absent in patch-clamp experiments (19 events in 1881 cycles, Tg vs. WT: p = 0.34), Tg myocytes were more susceptible to pause-induced SCR during Iso treatment (Figure 1C). Importantly, the ability of Iso to induce both arrhythmogenic behaviors was associated with normalization of a baseline deficit in SR Ca2+ content in Tg cells (Figure 1C, bottom right). This suggests that an Iso-induced increase in SR Ca2+ load may be central to both forms of electrophysiologic instability.
Figure 1.
CaMKIIδC myocytes are susceptible to EADs and SCR. A. Representative Tg cell transitioning to EADs during Iso challenge. B. EAD frequency (% of all cycles, left) and incidence (right) was increased in Tg compared to WT cells during Iso. Neither group exhibited EADs in Normal Tyrode’s (NT). C. Left: representative Ca2+ recordings during pause-induced SR Ca2+ release experiments. Right: summary data for SCR incidence (fraction of cells with 1 or more SCR events), SCR frequency (events/cell), and SR Ca2+ content at baseline, during Iso, and in elevated extracellular Ca2+ (2 mM, SR Ca2+ content only). Cell numbers are inset to each bar. **p < 0.01, *p < 0.05, † p = 0.06. Data are mean±SEM.
Enhanced Ca2+ handling and prolonged repolarization precede EAD initation
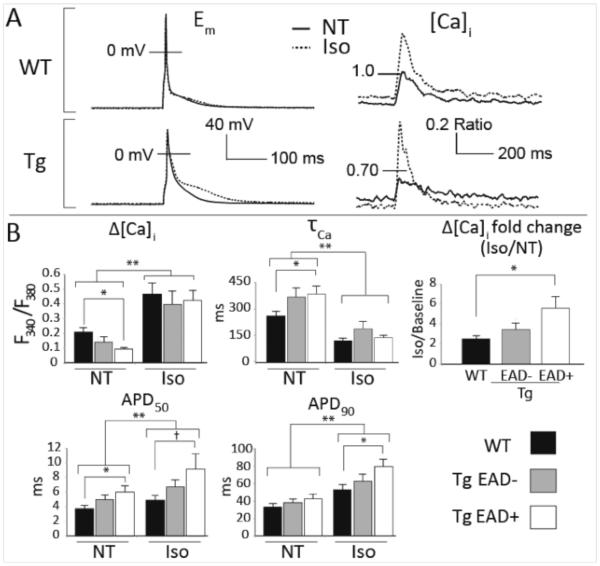
To describe the changes in steady-state Ca2+ handling and AP morphology associated with EADs, we separated the Tg cells into those that did exhibit EADs (EAD+, n=12), and did not exhibit EADs (EAD−, n=15) in at least 10% of cycles. These two Tg cell populations were compared with all WT cells (n=17). In EAD+ recordings, steady-state data were taken from the 5-10 cycles immediately prior to the first EAD, for all other cells we used the final 5-10 cycles of the recording. These analyses suggest that murine EAD susceptibility is associated with exaggerated Ca2+ cycling and slowed late repolarization immediately before EAD initiation (Figure 2). At baseline (normal Tyrode’s), Ca2+ transients were smaller and slower to decay in EAD+ cells compared with WT. The baseline AP in EAD+ cells also exhibited slower early repolarization, although late repolarization was not different from WT (p = 0.13). As expected, Iso increased Ca2+ transient amplitude and accelerated Ca2+ decay in WT cells. These effects were exaggerated in EAD+ cells such that, immediately before initiation of EADs, Iso had normalized the baseline deficits in both Ca2+ transient amplitude and decay rate. This resulted in an Iso-induced increase in transient amplitude that was more than doubled in EAD+ cells relative to WT (Figure 2A, bottom right, and Figure 2B, top right). AP prolongation accompanied these Iso-dependent Ca2+ handling changes in both WT and Tg cells, and again this effect was amplified in EAD+ cells, particularly for late repolarization where APD90 was 53% longer than in Iso-treated WT myocytes (p < 0.05).
Figure 2.
EAD susceptibility is associated with exaggerated Ca2+ cycling and slowed late repolarization. A. Representative steady-state APs and Ca2+transients. The smaller and more slowly decaying baseline Ca2+ transients in EAD+ cells are normalized by Iso, and associated with suspended late repolarization. B. Summary data for steady-state APs and Ca2+handling. † pairwise contrast p = 0.05, * pairwise contrast p < 0.05, ** main effect of Iso p < 0.05. Data are mean±SEM.
SR Ca2+ release is required for murine EADs
These steady-state effects suggest that Iso elicits EADs in Tg cells by enhancing Ca2+ cycling in a manner that destabilizes repolarization. However, all observed Ca2+ handling and AP changes could be due to gain-of-function regulation at either ICaL or SR Ca2+ release, both of which are recognized consequences of CaMKII activation16, 25, 26, and are the primary drivers of EADs in large mammals6, 7, 27.
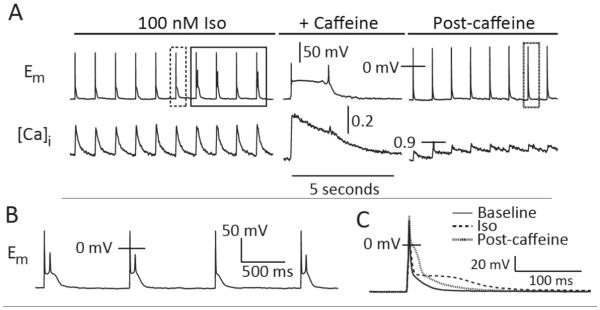
The low late AP plateau in Iso (Figure 2B, top) is probably due to greater SR Ca2+ release and prolonged inward INaCa, as suggested by earlier investigations28, 29. To test whether this plays a role in driving the observed EADs, we rapidly applied caffeine to a cell exhibiting Iso-induced EADs. This maneuver simultaneously eliminates inward currents driven by SR Ca2+ release, and potentiates ICaL by reducing Ca2+-dependent inactivation - thus exaggerating any ICaL-dependent EAD mechanism. Figure 3 shows that caffeine caused the expected large Ca2+ release, and thereby triggered an AP. The sustained elevation of [Ca2+]i during the contracture greatly prolonged repolarization (Figure 3A). However, while caffeine lingered after the return to pacing (preventing SR Ca2+ reloading) EADs were abolished. Thus, SR Ca2+ release is essential for EAD initiation, but potentiated ICaL is not sufficient to generate EADs in the absence of SR Ca2+ release.
Figure 3.
SR Ca2+ release is required for EAD initiation. A. Caffeine (10 mM) eliminates EADs by depleting the SR Ca2+ store. B. EADs from the solid boxed region in panel A. C. AP morphologies from the NT (solid line), Iso (dashed line), and post-caffeine (dotted line) records. Iso and post-caffeine APs are boxed in (A).
The changes in AP morphology resulting from caffeine also support the contention that SR Ca2+ release promotes EADs by altering the trajectory of late repolarization. Figure 3C shows that caffeine elicits inverse effects upon early and late repolarization. APD50 is dramatically increased, presumably due to less Ca2+-dependent inactivation of ICaL, whereas the INaCa-driven late plateau is removed (with repolarization parallel to the baseline AP trajectory). Thus, Iso causes larger SR Ca2+ release and inward INaCa, which suspends terminal repolarization.
Unique dynamics of murine EADs
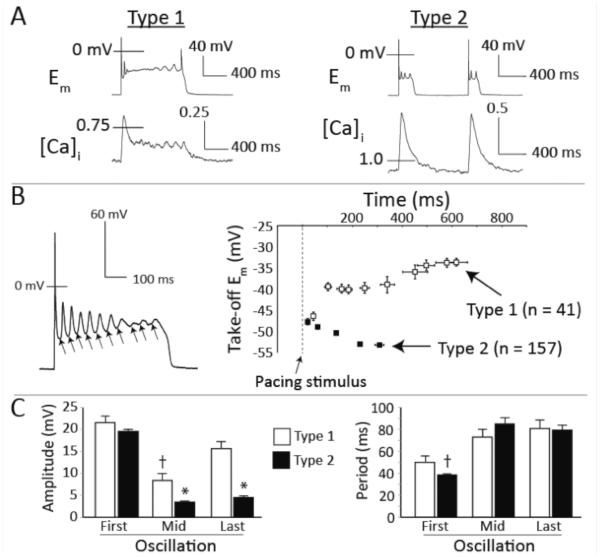
The observed EADs could be discriminated by whether or not they exhibited sustained Ca2+ transients, and we reasoned that this may indicate two populations of EADs caused by distinct mechanisms. Of the 198 EAD-containing cycles, 20.7% exhibited a sustained Ca2+ transient - type 1 EAD morphology (Figure 4A, left). The remaining EADs (79.3%) exhibited normal monotonic Ca2+ transient decay, and shorter Em plateaus with fewer oscillations (Figure 4A, right) - type 2 EADs. Importantly, the take-off (TO) potentials of the first oscillations in both types were below the ICaL activation range, at −46.4 and −47.7 mV for types 1 and 2, respectively (Figure 4B, right). This further indicates that ICaL reactivation is unlikely to contribute to EAD initiation in either type of event. For type 1 EADs, subsequent oscillations took off from more positive potentials, whereas they became progressively more negative in type 2 EADs. Together, these observations indicate two characteristics of murine EADs that distinguish them from EADs in large mammals: (1) the initiating event relies upon dynamics that cannot be explained by ICaL reactivation, and (2) while ICaL reactivation may contribute to EAD maintenance in cycles with sustained Ca2+ transients (type 1), a separate dynamical mechanism can support multiple oscillations in type 2 EADs.
Figure 4.
Multiple mechanisms contribute to murine EADs. A. Representative examples of type 1 (left) and type 2 (right) EADs. B. Left: Representative trajectory for TO potentials within a single AP. Right: Mean trajectories for TO potentials in type 1 and type 2 EAD cycles. C. Average oscillation amplitudes (left) and periods (right) for the initial (First), middle (Mid), and final (Last) oscillations in all type 1 ( n=33) and type 2 EAD (n=48) cycles with more than 3 oscillations. * p < 0.05 vs. Type 1 (same oscillation) and Type 2 (First oscillation); † p < 0.05 vs. Type 1 (First oscillation). Data are mean±SEM.
Temporal changes in oscillation amplitude and period provide further support for the existence of multiple dynamical mechanisms in the mouse. A signature of ICaL-dependent EAD dynamics in large mammals is that, within a given AP, EAD oscillations increase in both amplitude and period before spontaneously terminating. These properties have been described in terms of non-linear dynamics, which suggest that the ICaL and K+ current interactions evolve through a destabilizing Hopf bifurcation, from which oscillation amplitude and period increase before terminating at a homoclinic bifurcation5. Figure 4C (left) shows that, while both types of EAD exhibit similarly large initiating oscillations, only type 1 EADs also present the characteristic increase in amplitude from the mid-point oscillation to the final oscillation of the cycle. Oscillation period increases from the first oscillation to the last in both types of EAD, although the first oscillation is more rapid in the type 2 events compared to type 1 (Figure 4C, right). In sum, these analyses suggest that the two types of EAD are initiated by a common dynamical mechanism that does not require ICaL, but that type 1 EADs then recruit ICaL and exhibit late dynamics that are very similar to those observed in larger mammals.
SR Ca2+ release slows late repolarization and recruits INa to initiate EADs
Because the mechanism of EAD initiation requires SR Ca2+ release, and occurs at negative potentials which favor forward-mode Na+/Ca2+exchange, INaCa is an obvious candidate for driving EAD initiation. However, it is unlikely that the conventionally small, slow, and monotonically decaying forward mode INaCa could elicit the large and fast depolarizations observed here. Thus, it is more likely that Ca2+ release and INaCa contribute by "conditioning" late repolarization to permit reactivation of another major inward current6. To interrogate these dynamics quantitatively, we developed a computational model of the mouse ventricular myocyte (see Methods and SI for details of this implementation).
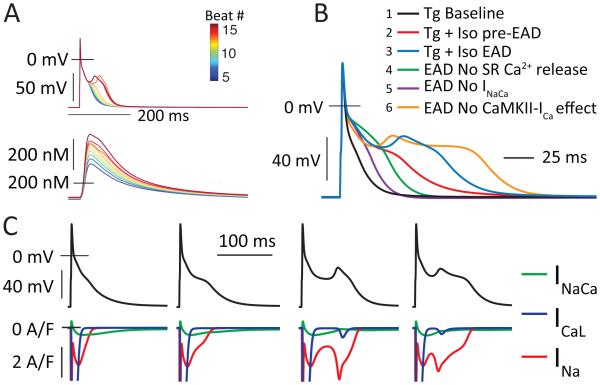
The EADs produced by this model recapitulated the more frequent type 2 EAD morphology. Figure 5A shows the transition to EAD behavior (at beat 13 of 50), as Ca2+ transient amplitude increases during simulated Iso. These EADs exhibit variable morphology but initiate at potentials between −40 and −45 mV, and occur 30 to 50 ms after the current injection used to trigger the AP (Figure 5B, 2 vs. 3). Further, they appear quasi-stochastically for the first several beats after transition, which is a signature of the chaotic determinism that is proposed to be a general characteristic of EAD dynamics8. Acutely removing the CaMKII-specific effects on ICaL was not sufficient to abolish EADs in the model (6 vs. 3), whereas computational ablation of either SR Ca2+ release (4 vs. 3), or INaCa (5 vs. 3) did eliminate EADs. Notably, INaCa elicits both AP prolongation and EAD initiation via recruitment of INa, which exhibits a progressive induction and then clear bifurcation (Figure 5C) as Ca2+ transient amplitude, SR Ca2+ release, and INaCa increase. While INaCa is well known to mediate Ca2+-dependent modulation of late AP duration in the mouse9, 30-32, this interaction between INaCa and INa reactivation is not widely appreciated as contributor to AP prolongation in the mouse. However, these simulations suggest that, even prior to EAD initiation, inward current contributed by reactivating INa is responsible for much more of the observed AP prolongation than INaCa (Figure 5C, first and second columns). ICaL was also reactivated to a small extent during EADs, but this is secondary to INa. We were unable to find an EAD regime that replicated the less frequent type 1 EAD morphology, and we suggest that this behavior may require a more complex representation of EC coupling than is present in this model, which does not permit prolonged or reactivating SR Ca2+ release (see SI 2.1.2).
Figure 5.
INa drives EAD initiation in murine ventricular myocytes. A. A sequence of simulated beats in Iso showing transition through AP prolongation to EAD initiation as whole-cell Ca2+ load and SR Ca2+ release increases. B. Simulations of the first EAD+ beat in the model where specific components were deleted (only in this beat) to test their acute effects in EAD initiation. The deleted components were: (4) SR Ca2+ release (Grel = 0); (5) INaCa (VNCX = 0); (6) CaMKII effects at ICaL (GCaL = 0.3458, Kpc,max=0.1162). C. Four APs and corresponding inward currents, showing the role of INa in both AP prolongation and EAD initiation.
EAD initiation is carried by non-equilibrium reactivation of INa
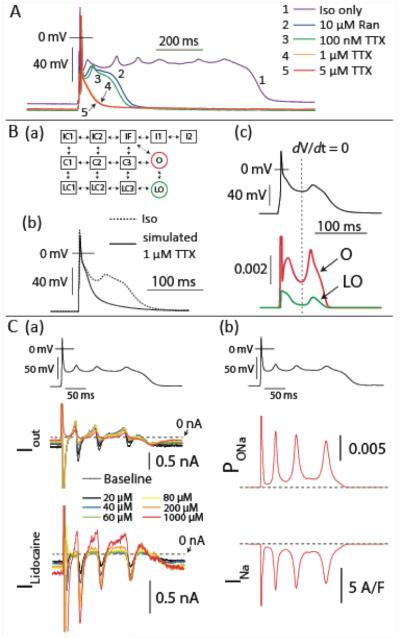
To experimentally test the model prediction that INa is responsible for driving EAD initiation, we attempted to interrupt Iso-induced EADs by applying INa inhibitors of differing selectivity for the late and fast components of INa. In the same cell we first rapidly applied 10 μM Ranolazine, and after brief washout, introduced tetrodotoxin (TTX) at 100 nM, 1 μM, and 5 μM (Figure 6). Ranolazine exhibits greater selectivity for INaL33, and at 1 Hz, 10 μM Ranolazine achieves approximately 70% attenuation of INaL but near negligible inhibition of peak INa34. Figure 6A shows that, while this treatment clearly inhibited prolonged EADs, it did not prevent EAD initiation (trace 2 vs. 1). Similarly, TTX at a dose capable of inhibiting neuronal INa (100 nM35) did not prevent the EAD upstroke (Figure 6A, trace 3). However, TTX eliminated
Figure 6.
Non-equilibrium reactivation of INa carries the EAD upstroke. A. EADs were interrupted first by rapid application of 10 μM Ranolazine. After washout, TTX was progressively introduced to the bath and eliminated EADs at 1 μM. B. (a) The states of the INa model: C1-C3 are closed states, LC1-LC3 are burst-mode closed states, IF is the fast inactivation state, I1 and I2 represent intermediate and deeply inactivated channels, and finally, O and LO are the normal and burst-mode open states, respectively. (b) EADs were eliminated by simulated 1 μM TTX (50% reduction in GNa). (c) The time course of LO and O occupancies during a simulated EAD, where the canonical open state (O) repopulates before the late open state LO and supports a role for non-equilibrium reactivation of INa in EAD initiation. C. (a) lidocaine blockade during AP clamp of a WT mouse myocyte indicates that a substantial lidocaine-sensitive current is recruited by the EAD upstroke. At top is the clamped AP waveform (collected in a Tg cell), in the middle is the corresponding raw current (Iout), and at bottom is the lidocaine-sensitive difference current (Ilidocaine). Lidocaine concentrations are shown inset. (c) The same experimental AP waveform is applied to the WT INa model, and yields similar current reactivation.
EADs at a dose (1 μM) approximately sufficient for half inhibition of peak myocardial INa and 30% inhibition of INaL36 (Figure 6A, traces 4 and 5 - full recordings are provided in Figure S5). The ability of TTX to eliminate EADs was reproducible - 3/3 cells were returned to normal AP morphology upon rapid application of TTX (Figure S6). Thus, while INaL may contribute to the dynamics of plateau EADs by contributing inward current late in the AP, this component of the Na+ current is not responsible for initiating murine EADs. Instead it appears that EAD initiation is carried by reactivation of canonical fast INa.
While these reactivations initiate above the Em range that typically permits steady-state INa availability, non-equilibrium reactivation of myocardial INa is known to occur in both normal and pathologically mutated NaV1.5 channels37. Returning to our model, we confirmed that an acute 50% reduction in INa conductance eliminated EADs as in the experiments (Figure 6B(b)), and observed that the state occupancies of the INa model support the contention that non-equilibrium INa reactivation is responsible for EAD initiation. Figure 6B(c) shows that the fast open state (O) repopulates prior to the burst-mode open state (LO), and achieves ~5 times greater peak state occupancy during the EAD upstroke. These channel reopenings are fueled by slight recovery through the canonical closed states (C1-C3, Figure S7).
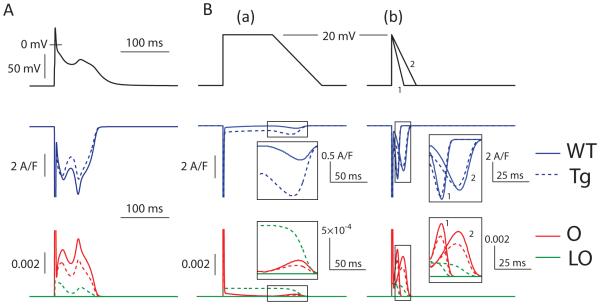
To test this model result experimentally we performed AP clamp experiments with one of the recorded EAD waveforms in the presence of various concentrations of lidocaine. This INa antagonist is largely selective for inactivated channels38, 39 and as shown in Figure 6C(a) it is clear that the EAD upstroke in these AP clamps induces a significant lidocaine-sensitive inward current, even below the concentration range that inhibits non-inactivated channels39. Further, applying the same experimental AP waveform to clamp the INa model achieves similar reactivation characteristics (Figure 6C(b)). Importantly, these reactivation dynamics were not attributable to the modeled effects of CaMKII at the Na+ channel. Rather the established leftward-shifts in steady-state activation and inactivation21, and slower recovery from inactivation, both of which are incorporated into the Tg INa model, serve to limit non-equilibrium reactivation. Figure 7A shows that reactivation is exaggerated in the isolated WT INa model compared to the Tg model during AP clamp of the first Tg EAD waveform. As described previously, the propensity for this reactivation is highly dependent on the trajectory of repolarization37.
Figure 7.
Simulated CaMKII hyperphosphorylation inhibits rather than promotes non-equilibrium reactivation of INa. A. Simulated AP clamp of the WT and Tg INa models20, using the first AP with an EAD from the Tg+Iso cell model and showing exaggerated reactivation in the WT model. B. Voltage clamp ramp simulations of the WT and Tg INa models. (a) The slow repolarizing ramp (similar to that used by Clancy et al.) to simulate human repolarization, permits little non-equilibrium reactivation from either INa model. (b) More rapid repolarizations (1 = 4V/s, 2 = 2V/s) without a preceding plateau permits much greater reactivation, although this is blunted in the Tg INa model.
To investigate whether differences between the mouse and large mammal APs should be expected to alter the impact of CaMKII on these reactivation dynamics, we applied repolarizing voltage ramps of differing trajectories to the INa models37. As shown in Figure 7B, a slowly repolarizing ramp (− 1 V/s) after prolonged depolarization (panel a) elicited much less non-equilibrium reactivation than more rapid (~ −2 or −4 V/s) repolarizations with no preceding plateau (panel b). In both protocols the Tg model exhibited reduced reactivation of the canonical open state (O), but because these reactivations were much smaller for both Tg and WT in the slower ramp, the enhanced late current (LO) in the Tg model caused the overall INa to be much larger for Tg in the slower, pseudo-large mammal waveform. Finally, when the WT INa model is substituted into the current-clamped Tg cell model, the exaggerated reactivation described in Figure 7 further disrupts normal repolarization (Figure S8).
Discussion
Here we have used an established arrhythmogenic mouse model and computational simulations to describe the unique constraints that murine electrophysiology imposes on mechanisms of EAD generation. We found that, unlike EADs in large mammals, the rapidly repolarizing murine AP dramatically reduces the potential for ICaL reactivation to drive EADs. Instead EADs are initiated by non-equilibrium reactivation of INa in the mouse ventricle, and this depends critically on the ability of SR Ca2+ release to modulate the trajectory of late repolarization. These findings provide a key framework for interpreting mechanisms of arrhythmogenesis in existing and future mouse models of disease.
The role of SR Ca2+ release in EAD dynamics has been appreciated for more than 20 years40, 41. In large mammals, triggered Ca2+ release is thought to promote EADs in one of two ways: (1) potentiated, but still synchronous, Ca2+ release and inward INaCa can be sufficient to disrupt the balance of currents during the AP plateau, and thereby "condition" repolarization to enter the dynamical regime involving ICaL (see Volders et al., for review6), or (2) Ca2+ release becomes spatiotemporally disco-ordinated, such that Ca2+ waves occur during AP repolarization, which again drives INaCa and may recruit accessory inward currents7. While we have not assessed the potential for Ca2+ waves to contribute to the EADs observed here, the time-scale upon which these events develop suggest that they are unlikely to have contributed to EAD initiation. The first EAD oscillation typically occurred between 30 and 50 ms after the triggering current pulse in our experiments, whereas spontaneous Ca2+ waves exhibit slower kinetics in which peak [Ca2+]i develops over hundreds of milliseconds.
Thus, any spontaneous event not detected before the AP was stimulated would quickly be overwhelmed by the Ca2+ release triggered by excitation. It is possible that wavelike dynamics contribute to plateau oscillations in type 1 EADs, but these events still require initiation by the faster dynamical mechanism. Thus, we contend that the suspended repolarization due to sustained INaCa is more likely to describe how SR Ca2+ release contributes to EAD dynamics in the mouse. This mechanism also explains how gain-of-function changes to ICaL gating and/or expression can promote murine EADs without eliciting ICaL reactivation. That is, by potentiating SR Ca2+ release through increased SR Ca2+ load and fractional release. It is for this reason, and the well known promiscuity of available Ca2+ channel antagonists42, 43 that we have avoided experiments involving ICaL antagonists in this study. As such, we strongly recommend against interpreting the ability of ICaL inhibitors to reduce EAD incidence in the mouse as inferring a role for ICaL reactivation in driving murine EADs.
While cardiac EADs have most commonly been associated with the late component of INa33, previous studies have also recognized that reactivation of fast INa may be involved in certain contexts. Most clearly, the LQT3 mutation I1768V exhibits exaggerated non-equilibrium reactivation during slow ramp repolarization, but appears innocuous by standard square-pulse characterization. Simulation of these properties within the Luo-Rudy guinea pig ionic model recapitulated AP prolongation at normal sinus rates and yielded EADs in simulated bradycardia37. More generally, early studies suggested that reactivation of INa may drive EADs initiating at more negative potentials in large mammals6, 40. However, these mechanisms were quickly thought to be less physiologically important because they required more severe conditions to be induced40 - as might be expected of any mechanism that depends on INa recovery after the prolonged AP plateau. Together, these prior studies suggest that, in large mammals, the conditions required to achieve non-equilibrium reactivation of INa are probably supra-physiologic in all but a few disease contexts. In contrast, the triangular mouse AP both accelerates recovery of INa and reduces the potential for ICaL reactivation, hence shifting EAD dynamics to favor reactivation of INa. As a result, we conclude that, for any conditioning stimulus (exaggerated SR Ca2+ release and INaCa in the CaMKIIδC transgenic model used here), non-equilibrium INa dynamics are the most likely basis for EAD initiation in the mouse. Furthermore, our simulations suggest that these INa dynamics are responsible for the majority of late AP prolongation in the mouse under conditions of exaggerated Ca2+ cycling, particularly β-adrenergic challenge. This finding may be important for many murine simulation studies, where INa models that are not capable of representing non-equilibrium reactivation are unlikely to capture Ca2+-induced AP prolongation within reasonable constraint of INaCa and other currents that are active late in the AP.
A key question for the general importance of these findings is whether non-equilibrium reactivation is facilitated by CaMKII, and thus, whether this behavior may be specific to this Tg mouse model. To date, no data are available to describe a role for CaMKII in non-equilibrium reactivation, and we have not assessed it experimentally in this study. However, the loss-of-function effects that CaMKII exerts on steady state INa would be expected to impair rather than facilitate non-equilibrium reactivation21, and our simulations in the WT and Tg INa models support this assertion (Figure 7). Thus, while investigation of the potential for CaMKII to alter non-equilibrium gating of INa is warranted, there is currently little reason to expect such an outcome. Rather we believe these INa dynamics are a general property of murine electrophysiology, which depends on the trajectory of AP repolarization rather than CaMKII-dependent alteration of INa gating properties.
Supplementary Material
4.1 Acknowledgements
We thank Kevin P. Vincent for his careful auditing of our computational model.
4.2 Sources of Funding
This work was supported by a National Institutes of Health (5P01HL46345-12, A.M; P01-HL080101, J.H.B and D.B; R01-HL105242, A.M and D.B), the Fondation Leducq Translatlantic CaMKII Alliance (D.B), and postdoctoral fellowships from the American Heart Association and Heart Rhythm Society (A.E).
Footnotes
4.3 Disclosures
D.B received a research grant from Gilead Sciences in May 2013. Gilead Sciences was in no way involved in the design, funding, execution, or interpretation of this study.
References
- 1.Sato D, Xie LH, Nguyen TP, Weiss JN, Qu Z. Irregularly appearing early afterdepolarizations in cardiac myocytes: Random fluctuations or dynamical chaos? Biophysical journal. 2010;99:765–773. doi: 10.1016/j.bpj.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: Heart failure, myocardial infarction, and atrial fibrillation. Physiological reviews. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annual review of physiology. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7:1891–1899. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran DX, Sato D, Yochelis A, Weiss JN, Garfinkel A, Qu Z. Bifurcation and chaos in a model of cardiac early afterdepolarizations. Physical review letters. 2009;102:258103. doi: 10.1103/PhysRevLett.102.258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: Time to revise current concepts. Cardiovascular research. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, Wen H, Fefelova N, Allen C, Baba A, Matsuda T, Xie LH. Revisiting the ionic mechanisms of early afterdepolarizations in cardiomyocytes: Predominant by ca waves or ca currents? American journal of physiology. Heart and circulatory physiology. 2012;302:H1636–1644. doi: 10.1152/ajpheart.00742.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, Karagueuzian H, Garfinkel A, Weiss JN, Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2983–2988. doi: 10.1073/pnas.0809148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pott C, Muszynski A, Ruhe M, Bogeholz N, Schulte JS, Milberg P, Monnig G, Fabritz L, Goldhaber JI, Breithardt G, Schmitz W, Philipson KD, Eckardt L, Kirchhof P, Muller FU. Proarrhythmia in a non-failing murine model of cardiac-specific na+/ca 2+ exchanger overexpression: Whole heart and cellular mechanisms. Basic research in cardiology. 2012;107:247. doi: 10.1007/s00395-012-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase ii contributes to cardiac arrhythmogenesis in heart failure. Circulation. Heart failure. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Said M, Becerra R, Valverde CA, Kaetzel MA, Dedman JR, Mundina-Weilenmann C, Wehrens XH, Vittone L, Mattiazzi A. Calcium-calmodulin dependent protein kinase ii (camkii): A main signal responsible for early reperfusion arrhythmias. Journal of molecular and cellular cardiology. 2011;51:936–944. doi: 10.1016/j.yjmcc.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas G, Gurung IS, Killeen MJ, Hakim P, Goddard CA, Mahaut-Smith MP, Colledge WH, Grace AA, Huang CL. Effects of l-type ca2+ channel antagonism on ventricular arrhythmogenesis in murine hearts containing a modification in the scn5a gene modelling human long qt syndrome 3. The Journal of physiology. 2007;578:85–97. doi: 10.1113/jphysiol.2006.121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase ii and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 14.Killeen MJ, Gurung IS, Thomas G, Stokoe KS, Grace AA, Huang CL. Separation of early afterdepolarizations from arrhythmogenic substrate in the isolated perfused hypokalaemic murine heart through modifiers of calcium homeostasis. Acta physiologica. 2007;191:43–58. doi: 10.1111/j.1748-1716.2007.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr., Bers DM, Brown JH. The deltac isoform of camkii is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circulation research. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 16.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic camkiideltac overexpression uniquely alters cardiac myocyte ca2+ handling: Reduced sr ca2+ load and activated sr ca2+ release. Circulation research. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 17.Bondarenko VE, Rasmusson RL. Transmural heterogeneity of repolarization and ca2+ handling in a model of mouse ventricular tissue. American journal of physiology. Heart and circulatory physiology. 2010;299:H454–469. doi: 10.1152/ajpheart.00907.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of ca-calmodulin-dependent protein kinase ii on rabbit ventricular myocyte ion currents and action potentials. Biophysical journal. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, Leymaster ND, Dun W, Wright PJ, Cardona N, Qian L, Mitchell CC, Boyden PA, Binkley PF, Li C, Anderson ME, Mohler PJ, Hund TJ. Ca2+/calmodulin-dependent protein kinase ii-based regulation of voltage-gated na+ channel in cardiac disease. Circulation. 2012;126:2084–2094. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner S, Hacker E, Grandi E, Weber SL, Dybkova N, Sossalla S, Sowa T, Fabritz L, Kirchhof P, Bers DM, Maier LS. Ca/calmodulin kinase ii differentially modulates potassium currents. Circulation. Arrhythmia and electrophysiology. 2009;2:285–294. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase ii regulates cardiac na+ channels. The Journal of clinical investigation. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dybkova N, Wagner S, Backs J, Hund TJ, Mohler PJ, Sowa T, Nikolaev VO, Maier LS. Tubulin polymerization disrupts cardiac beta-adrenergic regulation of late ina. Cardiovascular research. 2014 doi: 10.1093/cvr/cvu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba S, Dun W, Boyden PA. Can pka activators rescue na+ channel function in epicardial border zone cells that survive in the infarcted canine heart? Cardiovascular research. 2004;64:260–267. doi: 10.1016/j.cardiores.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Yi J, Hu N, George AL, Jr., Murray KT. Activation of protein kinase a modulates trafficking of the human cardiac sodium channel in xenopus oocytes. Circulation research. 2000;87:33–38. doi: 10.1161/01.res.87.1.33. [DOI] [PubMed] [Google Scholar]
- 25.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of l-type calcium channels. Nature cell biology. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 26.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase ii promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase ii signaling. Circulation research. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noble D. Influence of na/ca exchange stoichiometry on model cardiac action potentials. Annals of the New York Academy of Sciences. 2002;976:133–136. doi: 10.1111/j.1749-6632.2002.tb04731.x. [DOI] [PubMed] [Google Scholar]
- 29.Schouten VJ, ter Keurs HE. The slow repolarization phase of the action potential in rat heart. The Journal of physiology. 1985;360:13–25. doi: 10.1113/jphysiol.1985.sp015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreiro M, Petrosky AD, Escobar AL. Intracellular ca2+ release underlies the development of phase 2 in mouse ventricular action potentials. American journal of physiology. Heart and circulatory physiology. 2012;302:H1160–1172. doi: 10.1152/ajpheart.00524.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pott C, Ren X, Tran DX, Yang MJ, Henderson S, Jordan MC, Roos KP, Garfinkel A, Philipson KD, Goldhaber JI. Mechanism of shortened action potential duration in na+-ca2+ exchanger knockout mice. American journal of physiology. Cell physiology. 2007;292:C968–973. doi: 10.1152/ajpcell.00177.2006. [DOI] [PubMed] [Google Scholar]
- 32.Yao A, Su Z, Nonaka A, Zubair I, Lu L, Philipson KD, Bridge JH, Barry WH. Effects of overexpression of the na+-ca2+ exchanger on [ca2+]i transients in murine ventricular myocytes. Circulation research. 1998;82:657–665. doi: 10.1161/01.res.82.6.657. [DOI] [PubMed] [Google Scholar]
- 33.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac "late sodium current. Pharmacology & therapeutics. 2008;119:326–339. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas G. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brette F, Orchard CH. No apparent requirement for neuronal sodium channels in excitation-contraction coupling in rat ventricular myocytes. Circulation research. 2006;98:667–674. doi: 10.1161/01.RES.0000209963.02720.70. [DOI] [PubMed] [Google Scholar]
- 36.Wu L, Ma J, Li H, Wang C, Grandi E, Zhang P, Luo A, Bers DM, Shryock JC, Belardinelli L. Late sodium current contributes to the reverse rate-dependent effect of ikr inhibition on ventricular repolarization. Circulation. 2011;123:1713–1720. doi: 10.1161/CIRCULATIONAHA.110.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clancy CE, Tateyama M, Liu H, Wehrens XH, Kass RS. Non-equilibrium gating in cardiac na+ channels: An original mechanism of arrhythmia. Circulation. 2003;107:2233–2237. doi: 10.1161/01.CIR.0000069273.51375.BD. [DOI] [PubMed] [Google Scholar]
- 38.Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. The Journal of general physiology. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno JD, Zhu ZI, Yang PC, Bankston JR, Jeng MT, Kang C, Wang L, Bayer JD, Christini DJ, Trayanova NA, Ripplinger CM, Kass RS, Clancy CE. A computational model to predict the effects of class i anti-arrhythmic drugs on ventricular rhythms. Science translational medicine. 2011;3:98ra83. doi: 10.1126/scitranslmed.3002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.January CT, Chau V, Makielski JC. Triggered activity in the heart: Cellular mechanisms of early after-depolarizations. European heart journal. 1991;12(Suppl F):4–9. doi: 10.1093/eurheartj/12.suppl_f.4. [DOI] [PubMed] [Google Scholar]
- 41.Priori SG, Corr PB. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. The American journal of physiology. 1990;258:H1796–1805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- 42.Hata T, Makino N, Nakanishi H, Yanaga T. Modulation of na+-ca2+ exchange in cardiac sarcolemmal vesicles by ca2+ antagonists. Molecular and cellular biochemistry. 1988;84:65–76. doi: 10.1007/BF00235194. [DOI] [PubMed] [Google Scholar]
- 43.Rusck M, Juhsz O, Orlick J, Zachar J. Inhibition of na+-ca2+ exhcange by calcium antagonists in rat brain microsomal membranes. General Physiology Biophysics. 1986;5:529–535. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.