Abstract
We investigated the discrepancies in long-term sulfur measurements from 2000 to 2012 by two separate speciation methods, X-ray fluorescence (XRF) spectroscopy and ion chromatography (IC) across the United States (334 sites). Overall, there was a good correlation between sulfur measurements by XRF spectroscopy and IC (R ≥ 0.90 for most of the sites). However, the inorganic sulfate measured by ion chromatography was not sufficient to account for all the sulfur measured by XRF spectroscopy at many of the sites. Discrepancies were observed with the high ratios of sulfur measured by XRF spectroscopy to that by IC. Such high ratios also exhibited seasonal variation, and differed across land use types; significant differences occurred at locations classified as forest, agriculture, and mobile, but not in locations classified as commercial, desert, industrial, and residential. On average, the excess, or non-sulfate, sulfur (unmeasured organic sulfur or other inorganic species of sulfur) was variable and observed as high as ~13% of organic carbon and ~2% of PM2.5. The contribution of such assumed organosulfur was larger in the eastern region than other geographical locations in the United States. Besides the temporal and spatial trends, the additional sulfur was found to be related to other factors such as aerosol acidity and emission sources. The results suggest that these unmeasured sulfur species could have significant contribution to aerosol burden, and the understanding of these could help to control PM2.5 levels and to assess other effects of sulfur aerosols.
1. Introduction
Sulfur aerosols, mainly inorganic sulfate, play an important role in climate change because of their ability to scatter solar radiation. The majority of sulfur aerosols are found in the fine fraction (Whitby, 2007) and sulfate comprises a significant fraction of fine aerosol mass (Jimenez et al., 2009). Sulfur is emitted in the form of sulfur dioxide from combustion, biomass burning, and volcanoes, dimethylsulfide from oceans, and hydrogen sulfide from biological activities (Chin et al., 1996). Sulfates can affect aerosol acidity and also take part in acid deposition by the formation of sulfuric acid. Sulfur aerosols may also have adverse health effects (Pope and Dockery, 2006). Inorganic sulfates are formed in the aerosol from the oxidation of sulfur gases, and only a small fraction of sulfur (<3%) is emitted as sulfate from combustion sources in the United States (Chin et al., 1996).
Sulfur aerosol is usually measured in the form of sulfate ion, and it is often assumed to be the most important, if not the only, form of aerosol sulfur. However, an increasing body of evidence on organosulfate prevalance (Liggio et al., 2005; Liggio and Li, 2006; Iinuma et al., 2007; Surratt et al., 2008a) and recent studies on sulfur and sulfate measurements at several sites in the United States (Tolocka and Turpin, 2013; Shakya and Peltier, 2013) illustrate the significant contribution from non-inorganic sulfate in aerosols. The presence of organosulfates would lead to the underestimation of atmospheric sulfur mass, if an ion chromatography (IC) analysis alone is employed to quantify these components. Such discrepancies between these two measurements of sulfur and sulfate have also been reported from other locations (He et al., 2001; Wu et al., 2003). Aerosol organosulfates have been reported from several field studies, and are reported to contribute 2–14% of total sulfate mass (Lukács et al., 2009; Hawkins et al., 2010; Stone et al., 2012; Lin et al., 2012; Kundu et al., 2013); others have shown that organosulfates could contribute to as much as 30% of total organic mass fraction (Surratt et al., 2008b). Besides organosulfates, additional sulfur species such as methanesulfonates, hydroxymethanesulfonates, sulfites, sulphides, polycyclic aromatic sulfur heterocycles, and primary biological particles could also contribute to such discrepancies (Neubauer et al., 1996; Graham et al., 2003; Surratt et al., 2008b; Cozzi et al., 2009).
Detailed studies on the sulfur species other than sulfates are not common. These studies could be important for controlling PM2.5 (particulate matter with diameters smaller than 2.5 micrometers) emissions and understanding their direct and indirect effects on climate. Here, we investigate the discrepancies from long-term observations (2000 to 2012) and expand the fundamental understanding of a ubiquitous aerosol component. The main objectives of this study are to examine the discrepancies of sulfate and sulfur measurements by IC and XRF spectroscopy across the United States, and to investigate if any trends or relationships exist for the occurrences of such discrepancies. We hypothesize that the presence of organosulfates and inorganic sulfur compounds (other than inorganic sulfate) might lead to conditions where excess sulfur is detected that cannot be accounted for in a corresponding measure of sulfate.
2. Methods
Sulfur and sulfate measurement data (24 hours average) from years 2000 to 2012 were retrieved from the Air Quality System (AQS) database of United States Environmental Protection Agency (US EPA). Sulfur in PM2.5 was measured by X-ray fluorescence (XRF) spectroscopy, and sulfate in PM2.5 was measured by IC (USEPA, 1997, 1999). Both sulfur and sulfate were typically measured at EPA speciation sites across the United States at an interval of one filter every 3 or 6 days. Sulfur-content of sulfate (SS) was calculated by multiplying sulfate concentrations by 0.3337, which reflects sulfate stoichiometry, to adjust the measurement to reflect only the mass of elemental sulfur in sulfate ion. In addition to concentration data, measurement uncertainty was also collected for each of 160,309 measurement pairs analyzed. Other PM2.5 speciation data were also polled from the same AQS database.
We examined the discrepancies in sulfur (S) to SS concentrations at a total of 308 EPA sites by calculating the sulfur (S) to sulfate-sulfur (SS). 26 sites report duplicate, collocated measurements in a single geographic location, and are included in the analysis (334 sites in total). Uncertainties for the ratios were computed from the reported uncertainties for S and SS by applying an error propagation method (Danzer, 2007). As an example, if sulfur were present only as sulfate, the ratio calculated by detection from each method should compute as 1.0 but propagated measurement error often shifts this ratio within a small range of 1.0 – typically 0.9 and 1.1. Concentrations below detection limits were removed and were not included in the analyses. Of the ~160 thousand measurements we retrieved, 72% of measurement pairs were excluded because the propagated error bases crossed unity. Of the remaining ~45 thousand records, 0.8% of these reported ratios of greater than 2 or less than 0.5, which we indicated as a measure of gross measurement error, and were also excluded from the analysis. This leave ~44 thousand valid measurement pairs where the calculated ratio and uncertainty was significantly different than one, and these data are analyzed here. Ratios and corresponding uncertainty were calculated for every valid measurement pair in the dataset.
AQS was also polled for complimentary speciation data at every site possible for a broader surveillance effort in order to identify chemical associations with other measured elements. This included data reporting concentrations of components such as elemental carbon, organic carbon, other ions (e.g. ammonium, nitrate, chloride, etc.), and all available XRF metal speciation data from each site. A number of descriptive parameters were also collected, including site land use type, site types, geographic coordinates, and locations.
3. Results and discussion
A total of 334 sites (including 26 collocated sites) across the US were investigated for discrepancies in sulfur (S) and inorganic sulfate (SS) measured by XRF and IC, respectively. The S and SS measurements were strongly correlated with each other at almost all the sites. A total of 333 sites had r-value (Pearson correlation coefficient) greater than 0.90. One site had very few data points for both S and sulfate measurements (n = 4). In this regression analysis, we include all the data points where the S/SS ratio was observed to be greater than 0.5 but less than 2 and not considering the uncertainties. Slopes for the scatterplots were within 0.90 to 1.10 for most of the sites. There were 14 sites with regression slopes for these measurements greater than 1.10, and 15 sites with slopes less than 0.90.
3.1 Elemental sulfur (S) to sulfate-sulfur (SS) ratio
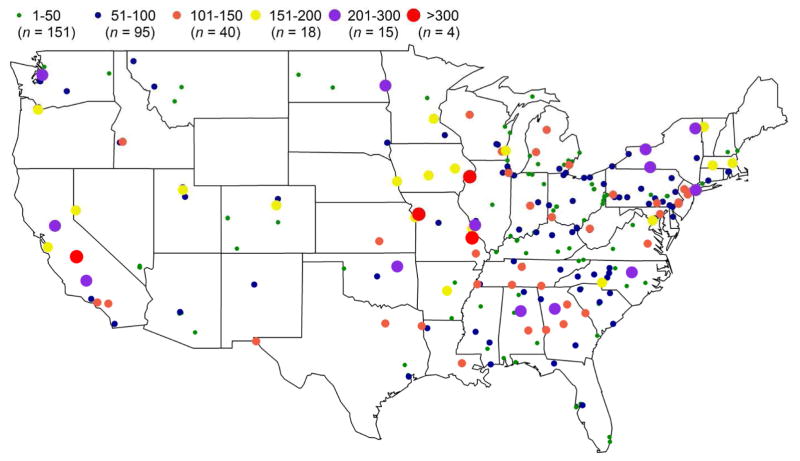
Out of 334 sites, 323 sites were observed with at least one significant measure of high S/SS ratio. No discernable geographic pattern was observed with analyzing high ratio frequency (Figure 1). More than 3/4 of the sites had frequency range of 1–100 (n = 246), and they were not limited to specific regions. Only four sites (Figure 2) had the highest frequency (>300 occurrences), and they were located in Fresno, California (suburban residential site), Davenport, Iowa (urban residential site), Kansas City, Missouri, and Bonne Terre, Missouri (rural agricultural sites). Out of 323 sites that had high S/SS ratio occurrences, high ratios were less than 20% of total measurements at most of the sites (~82% of all sites).
Figure 1.
Frequency of high S/SS ratio occurrences across the EPA sites in continental USA. Different colors illustrate the frequency of occurrences. Sites from non-continental US are not shown in this map.
Figure 2.
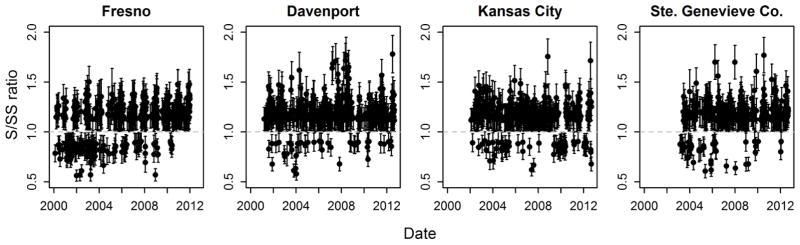
Sulfur to sulfur-sulfate ratios as the selected EPA sites: Fresno, California; Davenport, Iowa; Kansas city, Missouri; Ste. Genevieve Co., Missouri. Error bars are the uncertainties for the ratios computed from error propagation method. Any ratios with error bars crossing the unity (grey dashed line) are removed.
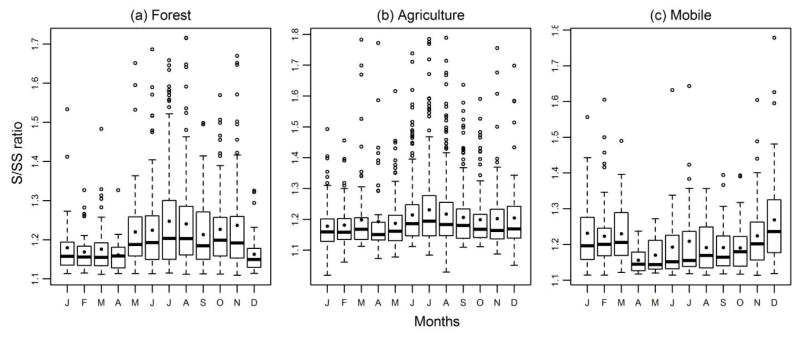
The four sites with the highest frequency of S/SS events were observed to exhibit seasonality for high ratios (Figure 3). The Fresno site had largest ratios during the winter while all three Midwestern sites had large ratios during summer and may indicate different aerosol conditions in Fresno than elsewhere. In total, there were 126 sites where mean S/SS ratios for summer were larger than the mean ratio during any other seasons, suggesting this relationship is seasonal. Because the highest ratios were typically observed during the summer, this may indicate the presence of organosulfate formation since organosulfate formation would be favored during summer with high photochemical activity, larger oxidant concentration, and increased concentration of biogenic secondary organic aerosol (SOA) precursor concentrations.
Figure 3.
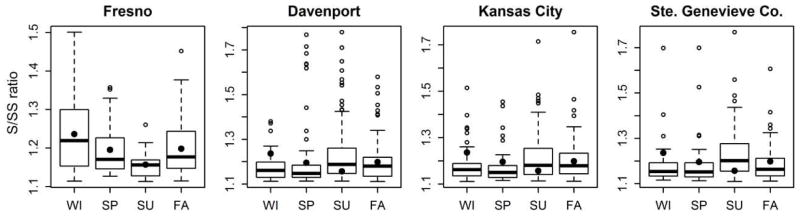
Seasonal variation of S/SS ratios for four sites with the most frequent high ratio occurrences. Boxes indicate quartiles and whisker bars indicate maxima and minima excluding the outliers, which are shown as open circles. Horizontal line and filled circle inside the box indicate median and mean, respectively.
In contrast, there were locations in which higher ratios occurred during winter, such as Fresno (Figures 2 and 3), a location that is often quite polluted. If organosulfates were the causes for such discrepancies during winter, organosulfates could also be formed from glyoxal and terpenes, both of which have distinct anthropogenic sources. However, photochemistry and oxidants might be the limiting factor during winter, and organic sulfur aerosol was observed at low levels in Fresno during winter (Ge et al., 2012). Major aerosol sources at Fresno during winter were traffic, cooking, and residential wood burning (Ge et al., 2012). At this location, high S/SS ratios were found to occur mainly when the water-soluble potassium ion (K+) to total potassium (K) ratio were around unity, and such a pattern was observed only during winter (Figure S1). Watson and colleagues (2001) report the K+/K ratio can vary with time and place, but approaches 1 when the predominant source of potassium is from wood smoke, Ratio less than one were explained by geologic sources of insoluble potassium that would result in lower measures of potassium ion compared to potassium by XRF. In Fresno, high S/SS ratios were observed when the K+/K ratio approached one which provides a line of evidence supporting that these nonsulfate sulfur species could be emitted during biomass burning (Graham et al., 2003). In our previous work, large wintertime S/SS ratios have been observed in regions such as Fairbanks, Alaska (Shakya and Peltier, 2013), a cold location also characterized by high concentrations of wood smoke. During winter, conversion of sulfur dioxide to sulfate is slower compared to summer because oxidants are usually at lower concentrations during winter (Chin et al., 1996). Lower oxidant concentrations and lower emissions of biogenic precursors during winter, would also result in lower contributions of any secondarily formed organic sulfates. However, there are also anthropogenic precursors for organosulfates (Stone et al., 2012; Kundu et al., 2013). We cannot, however, discard the possible contribution from unmeasured inorganic sulfur species.
To further investigate the differences among the sites in the US, monthly S/SS statistics were plotted for the seven specific land use types that are pre-defined within the AQS dataset. These classifications are qualitatively determined by local, state, and regional entities based on observed prevalent land use within ¼ mile of the site. A distinct seasonality for high ratio occurrences was observed for specific the land use types: forest, agriculture, and mobile (Figure 4); remaining sites with land use types such as commercial, desert, industrial, and residential, did not exhibit such distinct seasonality for S/SS ratios. This suggests there may be source-related differences among these types of locations. At forest and agriculture land-use types, the ratios were larger during summer months (i.e. July to August) suggesting the possible contribution of biogenically derived sulfur sources and the important role of photochemistry and oxidant concentrations. Many biogenic precursors such as isoprene, limonene, and pinenes could be such sources that are known as the potential precursors for organosulfates (Iinuma et al., 2007; Surratt et al., 2008a; Liggio et al., 2005).
Figure 4.
Monthly variation of S/SS ratios at the sites with land-use type as forest. Bottom axis are the initials of the months from January to December in a chronological order.
In contrast to these locations, mobile land-use type locations had largest ratios during the winter months of November to March (Figure 4). This was an unexpected finding, but it should be noted that there were only three sites with mobile land-use designations in the AQS database: Bakersfield, CA, Knoxville, TN, and Detroit, MI. In fact, these designations likely are not exclusive to a specific source type and reflect a mixed emissions profile. It is plausible that predominantly mobile source emissions at these sites are mixed with other sources, such as biomass burning, and higher ratios at these locations in winter could be consistent with wood smoke derived excess sulfur.
3.2 Additional sulfur
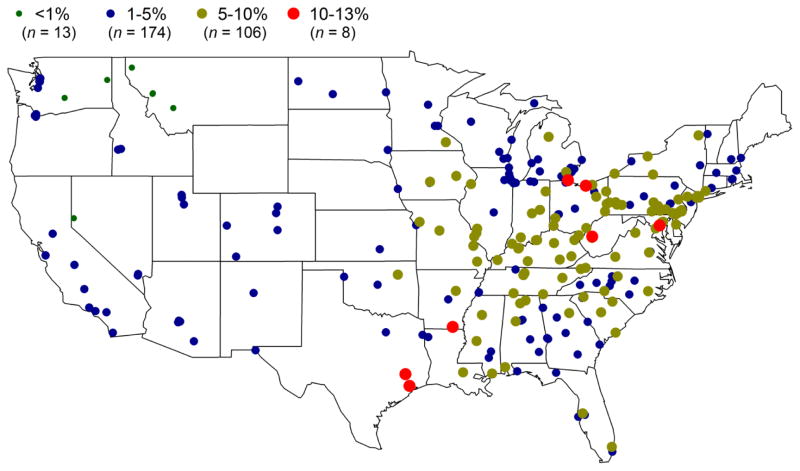
Additional sulfur (i.e. non-sulfate sulfur) calculated as the difference between S and SS shows that this sulfur could account for as much as 13% of organic carbon (OC) and 2.5% of PM2.5 mass, both observed in Crossett, AR. In this analysis, outliers for OC and PM2.5, defined as any value exceeding five times the mean for the individual site, were removed for this computation. Mean contributions of the additional sulfur to OC for the cases with only high ratios are shown in Figure 5. It exhibited the variability in its contributions based on the geographical locations. Out of 301 sites with the availability of both OC and S/SS ratio data, most sites (n = 280) had non-sulfate sulfur contribution of 1–10% of OC. Only a few sites (n = 8) had a contribution greater than 10% of OC. All of the sites with S/SS contribution of 5–10% of OC were located inside the eastern and central regions of the US. Based on the additional sulfur normalized by OC, western US sites had the least fraction of non-sulfate sulfur while the cities in eastern and central US locations had the largest non-sulfate sulfur contribution. This was also true for the contribution of the additional sulfur to PM2.5 mass showing higher contribution at the eastern region (1–2%) than other regions. Tolocka and Turpin (2012) recently reported the highest fraction of organosulfur to organic mass concentrations in the midwest and the eastern sites compared to the western sites, which is consistent with these results.
Figure 5.
Contribution of excess sulfur (inorganic sulfate subtracted from total sulfur) to OC at different EPA sites.
Besides organosulfates, the presence of other sulfur forms such as methanesulfonates, hydroxymethanesulfonates, sulfites (Neubauer et al., 1996), and primary organosulfur species (Graham et al., 2003) could also contribute to observation of such high ratios. Methanesulfonic acids (MSAs) are formed from dimethyl sulfide that has mainly oceanic sources (Barnes et al., 1994). Any contribution from MSA will likely be the largest during summer (Savoie and Prospero, 1989). However, sites along the coastal regions were not the sites with most frequent high ratios or the largest contributions of the additional sulfur, except for the one site located in Texas. Therefore, MSA might not be the major compound leading to the observations of high ratio events. Other sulfur containing organic compounds include alkyl and methane sulfonic acids, sulfonates, camphorsulfonic acid, and polar species such as benzothiazol, cyclic organic sulfites, and dimethyl sulfite (Blando et al., 1998).
We also investigated if the trend for high ratios occurs at the sites with larger sulfur concentration. A scatterplot (not shown) for the mean sulfur concentrations (by XRF) versus the mean S/SS ratios at the individual sites did not exhibit any such trends suggesting these events to be not associated with more sulfur-polluted conditions.
3.3 Case studies with high additional sulfur
We selected eight sites with highest additional sulfur contribution to OC (>10%) for a more thorough analysis (Table 1). On average, additional sulfur at these sites accounted for 1–3% of PM2.5. None of these sites have the land use types of forests, they were not the sites with the largest high ratio occurrences, and five of them had less than 13 occurrences for high ratios. Although there was seasonality for the ratios at these sites, only the sites in Houston, Arkansas, and West Virginia had the largest summer S/SS ratios. At the Houston site, the additional sulfur was strongly correlated with OC (r > 0.85, p<0.01; n = 20), a location where additional sulfur was also correlated with calcium (r = 0.47). The relationship between additional sulfur and Ca appears to have two association regimes (Figure S2), though there is no obvious explanation for this finding. At all sites - except for one site in Houston with just 9 measurements - the additional sulfur was strongly correlated with PM2.5 (r-values ranging from 0.69 to 0.91) suggesting these observations are likely to be real and not occurring due to a measurement bias from the instruments.
Table 1.
Details of the sites with highest additional sulfur contribution to OC
| Site ID | Location | Land-use | Setting | Frequency | S/OC % | S/PM2.5 % |
|---|---|---|---|---|---|---|
| C.482011039.5 | Deer Park, Houston-Sugarland, TX | Residential | Suburban | 9 | 10 | |
| C.482011039.6 | Ibid. | 94 | 10 | 1 | ||
| C.483390089.5 | Conroe, Houston- Sugarland | Commercial | Urban and Center City | 13 | 10 | 2 |
| C.050030005.5 | Crossett, AK | Commercial | Urban and Center City | 12 | 13 | 3 |
| C.240030019.6 | Fort Meade, Baltimore-Towson, MD | Commercial | Suburban | 11 | 11 | 2 |
| C.261150005.5 | Luna Pier, Monroe, MI | Agricultural | Rural | 73 | 11 | 2 |
| C.540390011.5 | Charleston, WV | Residential | Rural | 143 | 10 | 2 |
| C.390930016.5 | Lorain, Cleveland-Elyria-Mentor, OH | Commercial | Suburban | 8 | 13 | 2 |
S/OC- and S/PM2.5 are the mean ratios of additional sulfur to organic carbon and PM2.5 at the individual sites. Sites ‘C.482011039.5’ and ‘C.482011039.6’ were at the same location with the former having data from only the year 2000.
Though there was no direct correlation of excess sulfur with temperature (r = 0.38 and 0.39 at Houston and Charleston, respectively), most of these occurred during higher ambient temperature (>20 °C) periods. Except for the sites at Crossett, Arkansas (~51%) and Cleveland, Ohio (~62%), these high ratio events were occurring mainly in apparently acidic conditions i.e. above 83% of high ratios at these sites occurred when the molar ratios of ammonium to sulfate were less than two. One of the common factors for all these sites (except for the location in Arkansas) was their proximity to coal-fired power plant locations. All these sites (except Arkansas) were located within 80 miles of active coal-fired power plants (EPA, 2011). Previously, Eatough et al. (1981) have reported the presence of dimethyl sulfate and monomethyl sulfate in particulate matter emitted from coal- and oil-fired power plants. Particulate dimethyl sulfate was also found in the Los Angeles atmosphere (Eatough et al., 1986). Organosulfates were present in particle samples collected in a coal-fired power plant plume (Zaveri et al., 2010). None of these forms of sulfur would be detected by conventional chromatographic separation methods.
3.4 Aerosol acidity
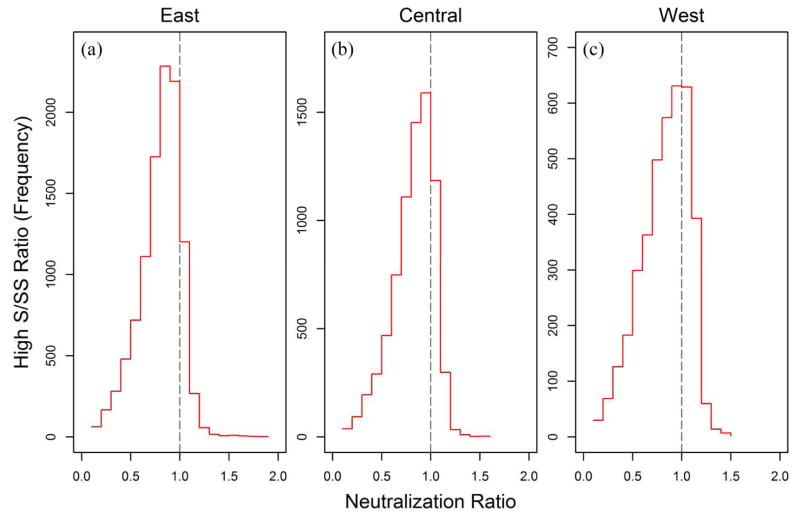
In aerosols, inorganic sulfate is usually associated with ammonium in the form of ammonium sulfate, (NH4)2SO4, or sometimes ammonium nitrate (NH4NO3). To investigate any links to aerosol acidity, the neutralization ratio (NR) was calculated for each data pair as the molar ratio of ammonium to the sum of sulfate and nitrate ([NH4+]/(2 ×[SO42−] + [NO3−]). The neutralization ratio is often used to estimate the aerosol acidity and it also incorporates nitrate concentrations and assumes that ammonium is the only cation counteracting the aerosol acidity (Shakya et al., 2010). The frequency distribution of high ratios exhibited at least 50% of occurrences during acidic conditions (i.e. NR<1) at many of the sites (222 of 312 sites analyzed). We observed similar patterns in our analyses from Fairbanks suggesting high ratios are likely to occur during acidic conditions (Shakya and Peltier, 2013). If organosulfate is the cause for such observation of high ratios, formation of organosulfate is expected to be enhanced during acidic conditions (Iinuma et al., 2007; Surratt et al., 2008b, Liggio and Li, 2006, Ervens et al., 2011). Smaller amount of organosulfates in humic-like substances in ambient aerosols has been attributed to the lower aerosol acidity (Lin et al., 2012).
The largest frequency of high S/SS ratio events occurred in the eastern- and central-region (Figure 6); this by itself is unsurprising as these locations have the highest number of monitoring stations. In the western region, most of the aerosols with high S/SS ratios appear to be neutralized. These data in Figure 6 appears to be skewed to occur more frequently under apparently acidic conditions in the Eastern and Central regions, and appear more normally distributed and centered around more neutralized conditions in the West. Mean additional sulfur contribution (Figure 5) also was the highest in the eastern US (>5%). Main sulfur dioxide (SO2) sources in the US are fuel combustion (including coal), industrial processes, fires and mobile (USEPA, 2013). The eastern US has the highest SO2 emission (USEPA, 2008) and consequently with high aerosol acidity levels. Most of the sites with the largest additional sulfur contribution were also found to be close to coal-fired power plant locations where the aerosols are likely to be more acidic. These findings suggest that aerosol acidity might play an important role in the formation of non-inorganic sulfate formation in the eastern and central region. There might be other important causes for this which includes biomass burning sources in the western region.
Figure 6.
Frequency of high ratio events at different bins of (separated by 0.1) neutralization ratio from (a) eastern (177 sites), (b) central (88 sites), and (c) western (63 sites) regions. The regions were separated based on time zone i.e. east region means the sites from eastern time zone, central from central time zone, and west from pacific and mountain time zones. Grey dashed line illustrates the completely neutralized aerosol.
3.5 Associations of high S/SS with other components
Calculated high S/SS ratios were compared against concentrations of other particulate components from the AQS database for all sites studied. Least square regression plots were created for high S/SS ratios against PM2.5, nitrate, potassium, and ammonium ions, OC by IMPROVE, OC by NIOSH, EC by IMPROVE, EC by NIOSH, and elemental Ni, Ca, K, and Fe. Where Pearson correlations coefficients were below 0.40, data were discarded. Of the remaining data, high S/SS appears to be greater than 0.40 at 72 different locations in 31 different states for at least one of the 11 comparison species in this analysis. This analysis assumes a Pearson coefficient greater than 0.40 reflects at least some statistically significant association between the different measures.
At three sites located in the Seattle, Washington metropolitan region (530330024, 530330048, and 530611007), high S/SS was associated with Fe, Ca (three sites), NH4, EC (two sites), K, and PM2.5. Ca and EC were most strongly associated (r = 0.59, 0.48, 0.54 for three sites measuring Ca; r = 0.5, 0.63 for two sites measuring EC) with high S/SS. Elemental K was also associated with high S/SS (r = 0.45). At a monitoring station in Helena, Montana (AQS site ID 300630024, 300630031, and 300930005, which are apparently co-located and operable at different times over this study period), high S/SS was associated K and K+, NH4, NO3, Ca, OC, and Fe. Both of these communities have a significant degree of wood smoke, and while we cannot exclude other sources of these species, it is likely that wood smoke plays an important role in this association.
Sites in Florida were also identified where there was an apparent association between S/SS and selected tracer compounds. At three sampling locations in Florida (Tampa, Miami, and Pensacola), high S/SS was associated with Ca, PM2.5, NH4, EC, OC, and K. Ca and K were only associated at the two northern Florida locations, Tampa and Pensacola, and not in Miami, where it is much less likely to encounter wood smoke.
It is interesting to note that the associations with Ca were observed in 22 different sites in 17 different states, none of which are typically thought to be highly influenced by high concentrations of crustal materials. In fact, some of the highest Pearson coefficients were observed for S/SS and Ca in locations such as Portland, Oregon (r = 0.82), Helena, Montana (r = 0.68), Manchester, New Hampshire (r = 0.63), and Dayton, Ohio (r = 0.62). All of these locations, however, are likely to have some degree of influence from winter time wood smoke, and it is possible that this observed calcium derives from this emission source.
3.6 Low S/SS ratios
It is interesting to note that there were many occurrences of significantly low S/SS ratios observed in this dataset. The frequency of low ratio events (~0.47%) was comparable to high S/SS ratio events (~0.53%). Ratios less than 1.0 are a peculiar finding in that XRF, which quantifies elemental sulfur, should measure all forms of sulfur present on a filter. Excluding data where propagated uncertainty crosses unity, there were still some ~21,000 measurement pairs significantly below 1.0.
There were no strong spatial or temporal patterns to low ratio events. On average, there was some indication that these events occurred in the spring and early summer season (Figure S4), though these findings are somewhat unconvincing. These events also occurred when aerosol was slightly acidic, but the results are weak at best.
It is noted, however, that the bulk of low S/SS ratio events appeared to occur when the K+/K ratio was greater than 1 (whereas the high S/SS events tended to occur when the K+/K ratio was one). This is an interesting finding and provides two lines of chemical evidence suggesting these ratios are likely attributed to either underreported uncertainty with one or both of the measurements, or at times XRF measurements of S and K are underreported. It is possible, but unlikely, that ion concentrations are biased where sulfate is contaminated while K+ is not or vice versa. If the measurement of a total element is reported to be less than one of its major constituents, the most likely explanation for this non-physical finding is in underreporting of XRF elemental concentrations, perhaps resulting from beam attenuation or spectral peak deconvolution, which are innate challenges in XRF speciation.
4. Conclusions
While inorganic sulfate is the major sulfur species in fine aerosols, there are a number of locations in the United States which report a significant concentration of nonsulfate sulfur species. Depending on location and season, this work demonstrates the likely presence of organic sulfur species in locations dominated by biogenic emissions, such as forested and agricultural regions, and this signal is most pronounced during the summer. Aerosol acidity is also linked to non-sulfate sulfur in the environment, and is likely to reflect a plausible mechanistic pathway for efficient organosulfur formation. In contrast, locations dominated by mobile source emissions appear to have the highest levels of non-sulfate sulfur in winter. A number of locations report high levels of non-sulfate sulfur in proximity to coal fired power plants, which are a significant source of gas phase sulfur precursors. Using elemental potassium and ionic potassium as tracers for biomass burning, we show that there is some evidence to suggest that excess sulfur is linked to biomass burning emissions, and high S/SS ratios were associated with K or K+ at 17 different sites in the United States. Calcium was also frequently associated with high S/SS and may indicate a link to crustal sources of S, or there is a significant contribution to Ca from wood burning activities. The additional sulfur contributed as much as 13% of OC and 2% of PM2.5 and thus is a significant missing component in the understanding of atmospheric sulfur chemistry.
Supplementary Material
Figure S1. K+/K ratio vs. S/SS ratio for four seasons at Fresno, California.
Figure S2. Correlation of additional sulfur with (a) organic carbon and (b) calcium at Houston. Grey point in figure (a) was excluded for correlation analyses.
Figure S3. K+/K ratio vs. S/SS ratio all the sites. Solid line at unity means the all K were K+.
Figure S4. Mean monthly ratio of S/SS. Labels in x-axis represent months January to December in chronological order. Individual lines represent the years from 2000 to 2012.
Highlights.
Discrepancies in sulfur measurement by IC and XRF were observed.
Non-sulfate (organic) sulfur could account for as much as 2% of PM2.5.
The additional sulfur was higher in central and eastern sites than other locations.
Acknowledgments
We greatly appreciate the resource support provided by the Fairbanks North Star Borough under a contract to the University of Massachusetts, and the National Institute of Health for resource support (ES017291), and to the Health Effects Institute for facilitation of AQS data access through the HEI Air Quality Database.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blando JD, Porcja RJ, Li TH, Bowman D, Lioy PJ, Turpin BJ. Secondary formation and the smoky mountain organic aerosol: An examination of aerosol polarity and functional group composition during SEAVS. Environ Sci Technol. 1998;32:604–613. [Google Scholar]
- Chin M, Jacob DJ, Gardner GM, Foreman-Fowler MS, Spiro PA. A global three-dimensional model of tropospheric sulfate. J Geophys Res. 1996;101:D13, 18667–18690. [Google Scholar]
- Cozzi F, Pellergrini I, Adami G, Reisenhofer E, Bovenzi M, Barbieri P. Sulphur speciation of PM10 samples by XANES spectroscopy. Central European Journal of Chemistry. 2009;7(3):395–401. [Google Scholar]
- Danzer K. Theoretical and Metrological Fundamentals. Springer; 2007. Analytical Chemistry. [Google Scholar]
- Eatough DJ, Lee ML, Later DW, Richter BE, Eatough NL, Hansen LD. Dimethyl sulfate in particulate matter from coal- and oil-fired power plants. Environ Sci Technol. 1981;15(12):1502–1506. [Google Scholar]
- Eatough DJ, White VF, Hansen LD, Eatough NL, Cheney JL. Identification of gas-phase dimethyl sulfate and monomethyl hydrogen sulfate in the Los Angeles atmosphere. Environ Sci Technol. 1986;20(9):867–872. doi: 10.1021/es00151a003. [DOI] [PubMed] [Google Scholar]
- Ervens B, Turpin B, Weber RJ. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos Chem Phys. 2011;11:11069–11102. [Google Scholar]
- Ge X, Setyan A, Sun Y, Zhang Q. Primary and secondary organic aerosols in Fresno, California during wintertime: Results from high resolution aerosol mass spectrometry. J Geophys Res. 2012;117:D19301. doi: 10.1029/2012JD018026. [DOI] [Google Scholar]
- Graham B, Guyon P, Maenhaut W, Taylor PE, Ebert M, Matthias-Maser S, Mayol-Bracero OL, Godoi RHM, Artaxo P, Meixner FX, Moura MAL, Rocha CHED, Grieken R, Glovsky MM, Flagan RC, Andreae MO. Composition and diurnal variability of the natural Amazonian aerosol. J Geophys Res. 2003;108(D24):4765. doi: 10.1029/2003JD004049. [DOI] [Google Scholar]
- Hawkins LN, Russell LM, Covert DS, Quinn PK, Bates TS. Carboxylic acids, sulfates, and organosulfates in processed continental organic aerosol over the southeast Pacific Ocean during VOCALS-Rex 2008. J Geophys Res. 2010;115:D13201. doi: 10.1029/2009jd013276. [DOI] [Google Scholar]
- He K, Yang F, Ma Y, Zhang Q, Yao X, Chan CK, Cadle S, Chan T, Mulawa P. The characteristics of PM2.5 in Beijing. China Atmos Environ. 2001;35:4959–4970. [Google Scholar]
- Iinuma Y, Müller C, Berndt T, Böge O, Claeys M, Herrmann H. Evidence for the existence of organosulfates from β-pinene ozonolysis in ambient secondary organic aerosol. Environ Sci Technol. 2007;41:6678–6683. doi: 10.1021/es070938t. [DOI] [PubMed] [Google Scholar]
- Jimenez, et al. Evolution of organic aerosols in the atmosphere. Science. 2009;326:1525–1529. doi: 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- Kundu S, Quraishi TA, Yu G, Suarez C, Keutsch FN, Stone EA. Evidence and quantification of aromatic organosulfates in ambient aerosols in Lahore, Pakistan. Atmos Chem Phys. 2013;13:4865–4875. [Google Scholar]
- Liggio J, Li SM, Mclaren R. Heterogenous reactions of glyoxal on particulate matter: Identification of acetals and sulfate esters. Environ Sci Technol. 2005;39:1532–1541. doi: 10.1021/es048375y. [DOI] [PubMed] [Google Scholar]
- Liggio J, Li SM. Organosulfate formation during the uptake of pinonaldehyde on acidic sulfate aerosols. Geophy Res Lett. 2006;33:L13808. doi: 10.1029/2006GL026079. [DOI] [Google Scholar]
- Lin P, Yu JZ, Engling G, Kalberer M. Organosulfates in humic-like substance fraction isolated from aerosols at seven locations in East Asia: A study by ultra-high resolution mass spectrometry. Environ Sci Technol. 2012;46:13118–13127. doi: 10.1021/es303570v. [DOI] [PubMed] [Google Scholar]
- Lukács H, Gelencsér A, Hoffer A, Kiss G, Horváth K, Hartyáni Z. Quantitative assessment of organosulfates in size-segregated rural fine aerosol. Atmos Chem Phys. 2009;9:231–238. [Google Scholar]
- Neubauer KR, Sum ST, Johnston MV, Wexler AS. Sulfur speciation in individual aerosol particles. J Geophys Res. 1996;101(D13):18701–18707. [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manage Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Savoie DL, Propsero JM. Comparison of oceanic and continental sources of non-sea-salt sulphate over the Pacific Ocean. Nature. 1989;339:685–687. [Google Scholar]
- Shakya KM, Ziemba LD, Griffin RJ. Characteristics and sources of carbonaceous, ionic, and isotopic species of wintertime atmospheric aerosols in Kathmandu Valley, Nepal. Aerosol and Air Quality Research. 2010;10(3):219–230. [Google Scholar]
- Shakya KM, Peltier RE. Investigating missing sources of sulfur at Fairbanks, Alaska. Environ Sci Technol. 2013;47:9332–9338. doi: 10.1021/es402020b. [DOI] [PubMed] [Google Scholar]
- Stone EA, Yang LM, Yu LYE, Rupakheti M. Characterization of organosulfates in atmospheric aerosols at four Asian locations. Atmos Environ. 2012;47:323–329. [Google Scholar]
- Surratt JD, Kroll JH, Kleindienst TE, Edney EO, Claeys M, Sorooshian A, Ng NL, Offenberg JH, Lewandowski M, Jaoui M, Flagan RC, Seinfeld JH. Evidence for organosulfates in secondary organic aerosol. Environ Sci Technol. 2008a;41:517–527. doi: 10.1021/es062081q. [DOI] [PubMed] [Google Scholar]
- Surratt JD, González YG, Chan AWH, Vermeylen R, Shahgholi M, Kleindienst TE, Edney EO, Offenberg JH, Lewandowski M, Jaoui M, Maenhaut W, Claeys M, Flagan RC, Seinfeld JH. Organosulfate formation in biogenic secondary organic aerosol. J Phys Chem A. 2008b;112:8345–8378. doi: 10.1021/jp802310p. [DOI] [PubMed] [Google Scholar]
- Tolocka MP, Turpin B. Contribution of organosulfur compounds to organic aerosol mass. Environ Sci Technol. 2012;46:7978–7983. doi: 10.1021/es300651v. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Compendium of methods for the determination of inorganic compounds in ambient air. U.S. Environmental Protection Agency; 1997. Sep, [Google Scholar]
- U.S. EPA. Final draft. U.S Environmental Protection Agency; 1999. Oct 7, Particulate matter (PM2.5) speciation guidelines. [Google Scholar]
- U.S. EPA. 2008 National Emissions Inventory: Review, analysis and highlights. U.S. Environmental Protection Agency; 2008. May, [Google Scholar]
- U.S. EPA. The toxics rule facilities. National electric energy data system (NEEDS 4.10 MATS) U.S. Environmental Protection Agency; 2011. Dec, [Google Scholar]
- U.S. EPA. 2011 National Emissions Inventory, version 1. Technical support document. U.S. Environmental Protection Agency; 2013. Nov, [Google Scholar]
- Watson JG, Chow JC, Houck JE. PM2.5 chemical source profiles for vehicle exhaust, vegetative burning, geological material, and coal burning in Northwestern Colorado during 1995. Chemosphere. 2001;43:1141–1151. doi: 10.1016/s0045-6535(00)00171-5. [DOI] [PubMed] [Google Scholar]
- Wu Y, Hao J, Fu L, Hu J, Wang Z, Tang U. Chemical characteristics of airborne particulate matter near major roads and at background locations in Macao, China. Sci Tot Environ. 2003;317:159–172. doi: 10.1016/S0048-9697(03)00331-0. [DOI] [PubMed] [Google Scholar]
- Whitby KT. The physical characteristics of sulfur aerosols. Atmos Environ. 2007;41:S25–S49. [Google Scholar]
- Zaveri RA, Berkowitz CM, Brechtel FJ, Gilles MK, Hubbe JM, Jayne JT, Kleinman LI, Laskin A, Madronich S, Onasch TB, Pekour MS, Springston SR, Thornton JA, Tivanski AV, Worsnop DR. Nighttime chemical evolution of aerosol and trace gases in a power plant plume: Implications for secondary organic nitrate and organosulfate aerosol formation, NO3 radical chemistry, and N2O5 heterogeneous hydrolysis. J Geophys Res. 2010;115:D12304. doi: 10.1029/2009JD013250. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. K+/K ratio vs. S/SS ratio for four seasons at Fresno, California.
Figure S2. Correlation of additional sulfur with (a) organic carbon and (b) calcium at Houston. Grey point in figure (a) was excluded for correlation analyses.
Figure S3. K+/K ratio vs. S/SS ratio all the sites. Solid line at unity means the all K were K+.
Figure S4. Mean monthly ratio of S/SS. Labels in x-axis represent months January to December in chronological order. Individual lines represent the years from 2000 to 2012.