Abstract
Objectives
A fast and accurate diagnosis is necessary to control and eliminate tuberculosis (TB). In Korea, TB continues to be a serious public health problem. In this study, diagnostic tests on clinical samples from patients suspected to have TB were performed and the sensitivity and specificity of the various techniques were compared. The main objective of the study was to compare various diagnostic tests and evaluate their sensitivity and specificity for detecting tuberculosis.
Methods
From January 2013 to December 2013, 170,240 clinical samples from patients suspected to have TB were tested with smear microscopy, acid-fast bacilli culture, and real-time polymerase chain reaction (PCR). The test results were compared and data were analyzed.
Results
A total of 8216 cultures tested positive for TB (positive detection rate, 4.8%). The contamination rate in the culture was 0.6% and the isolation rate of nontuberculous mycobacteria was 1.0%. The sensitivity and specificity of smear microscopy were 56.8% and 99.6%, respectively. The concordance rate between the solid and liquid cultures was 92.8%. Mycobacterium isolates were not detected in 0.4% of the cases in the liquid culture, whereas no Mycobacterium isolates were detected in 6.8% of the cases in the solid culture. The sensitivity and specificity of real-time PCR for the solid culture were 97.2% and 72.4%, respectively, whereas the corresponding data for the liquid culture were 93.5% and 97.2%.
Conclusion
The study results can be used to improve existing TB diagnosis procedure as well as for comparing the effectiveness of the assay tests used for detecting Mycobacterium tuberculosis isolates.
Keywords: acid-fast bacilli culture, real-time polymerase chain reaction, smear microscopy, tuberculosis
1. Introduction
A fast and accurate diagnosis is necessary to control and eliminate tuberculosis (TB). To diagnose TB, clinical tests are performed by obtaining a specimen such as sputum from the person with symptoms suggestive of TB. The tests used for diagnosing TB are microscopic examination of acid-fast bacilli (AFB) smear, culture tests, nucleic acid amplification tests, and drug-susceptibility tests. Microscopic examination of sputum samples (AFB smear staining) and their culture with subsequent drug-susceptibility testing is the standard method for diagnosing TB; however, this method has insufficient sensitivity and specificity. Nucleic acid amplification is a useful supplement to improve the rapidity and accuracy of TB diagnosis [1,2]. In Korea, TB continues to be a serious public health problem. In countries with a high prevalence of TB, patients whose sputum sample tests are positive for AFB on direct microscopic examination or those displaying chest radiographic findings suggestive of TB are generally presumed to have pulmonary TB and are treated empirically with anti-TB drugs.
In Korea, laboratory data of all suspected TB patients who visit public health centers are registered in the Korean TB Information Management System (TBnet). Based on these data, diagnostic tests on clinical samples from patients suspected to have TB were performed and the sensitivity and specificity of the various techniques were compared. The study results can be used for further improving existing TB diagnosis procedures as well as for comparing the effectiveness of the assay tests used for detecting Mycobacterium tuberculosis isolates.
2. Materials and methods
2.1. Study patients
From January 2013 to December 2013, 170,240 clinical samples recovered from patients in whom TB was suspected were prospectively collected from the 255 regional public health centers. The samples were tested for TB in 13 regional diagnostic centers of the Korean Institute of Tuberculosis (Table 1).
Table 1.
Locationwise data of total samples tested and positivity rates for Mycobacterium tuberculosis.
| Location | No. of samples tested | No. of positive cultures | Rate of positive culture (%) |
|---|---|---|---|
| Seoul | 24,000 | 1,855 | 7.7 |
| Busan | 16,595 | 790 | 4.8 |
| Incheon | 9,626 | 483 | 5.0 |
| Gyeonggi-do | 26,788 | 1,554 | 5.8 |
| Gangwon-do | 13,815 | 295 | 2.1 |
| Chungcheongbuk-do | 6,230 | 211 | 3.4 |
| Chungcheongnam-do | 14,090 | 567 | 4.0 |
| Jeollabuk-do | 6,982 | 291 | 4.2 |
| Jeollanam-do | 18,319 | 460 | 2.5 |
| Gyeongsangbuk-do | 12,899 | 679 | 5.3 |
| Gyeongsangnam-do | 16,000 | 723 | 4.5 |
| Jeju-do | 4,104 | 117 | 2.9 |
| Osong (central center) | 792 | 191 | 24.1 |
| Total | 170,240 | 8,216 | 4.8 |
2.2. Smear microscopy
All samples were processed and concentrated by centrifugation (3000 g, 20 minutes). The samples were tested with smear microscopy, culture (used as reference technique), and polymerase chain reaction (PCR). For smear examination, fixed preparations were stained with auramine fluorochrome and examined under a fluorescence microscope (magnification, 200×; number of fields observed, 30). Smears tested were confirmed to be positive by the Ziehl–Neelsen (ZN) staining method.
2.3. AFB culture and identification
The respiratory samples were digested and decontaminated using 4% sodium hydroxide and 2% N-acetyl-l-cysteine sodium hydroxide. The samples were then separately cultured in solid (3% Ogawa solid egg-based medium kit; KIT, Cheongju, Korea) and liquid medium (Mycobacteria Growth Indicator Tube liquid medium; Becton Dickinson, Sparks, MD, USA) systems. Liquid cultures were monitored using the automated MGIT 960 system (Becton Dickinson) for up to 6 weeks, whereas the solid medium was monitored for up to 8 weeks. If an AFB culture tests positive for M. tuberculosis in either the liquid or the solid medium, an immunochromatographic assay was performed to confirm the culture result (TB Ag MPT64 RAPID; Standard Diagnostics, Yongin, Korea).
2.4. Real-time PCR
DNA extract from each sample was amplified using the AdvanSure TB/NTM real-time PCR kit (LG Life Science, Seoul, Korea) according to the manufacturer's protocol. Based on the instructions supplied by the manufacturer, Ct < 35 was considered positive for M. tuberculosis.
2.5. Data analysis
Data management and statistical analyses were performed using IBM SPSS Statistics 19 (SPSS Inc., Chicago, IL, USA). Sensitivity and specificity of the diagnostic tests were calculated using culture results as gold standard. A patient is confirmed to have TB if mycobacterial growth was observed on the culture and the AFB smear was confirmed to be positive by the ZN staining method. Multivariate logistic regression was used to estimate associations between the covariates and the outcomes, adjusting for potential confounders that showed a significant effect in crude odds ratios at the 5% significance level.
3. Results
Smear microscopy and solid culture tests were performed on all the samples collected (n = 170,240). Table 1 presents data on the number of samples tested in various regions in Korea (n = 13) and the rate of positivity. The positive rates of culture varied from 2.1% to 24.1% (based on location). The number of samples tested was high during the period between March and May, whereas it was low in December (Figure 1). The culture positivity rates reported each month during the study period were between 4.1% and 5.7%. Liquid culture was performed for 10,607 samples and real-time PCR was performed for 5,275 samples. The smears were positive in 5,962 cases (positive detection rate, 3.5%), with 8,216 cultures testing positive for TB (positive detection rate, 4.8%). The contamination rate in the cultures was 0.6% (996/170,240) and the isolation rate of nontuberculous mycobacteria (NTM) was 1.0% (1,706/170,240). The contamination rate and NTM isolation rate were 0.5% and 0.8% for the solid culture, respectively, and the corresponding rates for the liquid culture were 2.4% and 3.4%. The contamination rate and the NTM isolation rate of the liquid culture were higher than that of the solid culture. The sensitivity and specificity were calculated for each assay and the values were compared, except for contaminated cultures and NTM isolations. The sensitivity and specificity of smear microscopy were 56.8% and 99.6%, respectively. Based on solid culture results, the sensitivity and specificity of smear microscopy were 54.3% and 99.6%, respectively. However, when liquid culture was included in the calculation, the sensitivity of the culture results improved to 62.6% and 98.6%, respectively. The concordance rate between the solid and liquid culture was 92.8%. No Mycobacterium isolates were detected in 0.4% and 6.8% of the cases in the liquid culture and solid culture, respectively.
Figure 1.
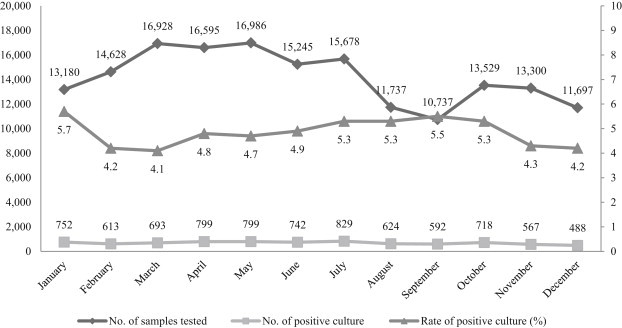
Monthly distribution of sample numbers tested and detections of Mycobacterium tuberculosis (culture results).
Real-time PCR identified 3360 positive cases (positive detection rate, 63.7%). Compared with culture results, real-time PCR had sensitivity and specificity of 83.7% and 96.2%, respectively. The sensitivity and specificity of real-time PCR were 97.2% and 72.4% for the solid culture, and the corresponding values for the liquid culture were 93.5%, and 97.2% (Table 2). The TB detection rates of real-time PCR were 26.2% (11/42) in samples detected to be positive in solid culture but negative in liquid culture.
Table 2.
Comparisons of smear microscopy, real-time PCR, and culture results.
| Solid culture |
Liquid culture |
|||
|---|---|---|---|---|
| Smear microscopy | Real-time PCR | Smear microscopy | Real-time PCR | |
| No. of samples | 167,538 | 3,692 | 10,144 | 1,583 |
| True positive | 4,633 | 2,185 | 1,524 | 826 |
| False positive | 684 | 298 | 105 | 51 |
| True negative | 158,697 | 1,146 | 7,604 | 649 |
| False negative | 3,524 | 63 | 911 | 57 |
| Sensitivity (95% CI) | 56.8% (55.7–57.9) | 97.2% (96.4–97.84) | 62.6% (60.6–64.5) | 93.5% (91.7–95.1) |
| Specificity (95% CI) | 99.6% (99.5–99.6) | 79.4% (77.2–81.4) | 98.6% (98.4–98.9) | 92.7% (90.5–94.5) |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
CI = confidence interval; PCR = polymerase chain reaction.
3. Discussion
Sputum smear microscopy is the most widely available diagnostic test for pulmonary TB. A recent meta-analysis of over eight studies showed that pooled sensitivity and pooled specificity were 64% and 98%, respectively, for smear microscopy [3]. However, our results showed lower sensitivity and higher specificity than these previously reported values. It is unclear why low sensitivity values were obtained in this study. The possible explanations for low sensitivity are laboratory workload, differences in experimenter's skill, and capacity variation between laboratories. The exact reason for such a low sensitivity can be identified by collecting data over many years and analyzing them.
Although there are differences in the number of samples tested every month, the M. tuberculosis culture-positive rate was relatively constant. Therefore, it is obvious that the prevalence rate of TB is constant and that the various seasons do not have an impact on the prevalence of TB. When comparing the total number of samples tested regionwise, the number of samples tested was relatively higher for densely populated areas; however, in this case, the culture-positive rate differed slightly, with rates lower than the average reported in some areas. The reason of high culture-positive rate in Osong is Korea institute of Tuberculosis as central center located there. They conducted epidemiological samples suspected be infected with TB. Therefore, it is necessary to survey multilateral conditions such as quality inspection, medical referral level, and the sample state to identify these causes.
The liquid culture system showed good sensitivity and specificity in detecting the pathogen. In combination with solid culture, the sensitivity of detection is increased [4,5]. Our results also confirm the advantage of using liquid culture to detect M. tuberculosis isolates. Specimens of patients registered in the epidemiological and health centers were tested with the liquid culture. The smear microscopy and real-time PCR results show that the specificity and sensitivity of the liquid culture were higher than those of the solid culture. However, further improvements are required with regard to isolation of mycobacteria and decreasing culture contamination rate by introducing necessary steps such as modifying sample pretreatment conditions before culture.
Because of the good sensitivity of real-time PCR for smear cultures, the technique can be used as a supplement for identifying positive and negative smear cultures. In a previous meta-analysis, 125 separate studies from 105 articles that reported real-time PCR results from respiratory specimens were included. The pooled sensitivity and pooled specificity in that study were found to be 85.0% and 97.0%, respectively [1]. Another study compared three molecular assays for the detection of rifampin resistance in M. tuberculosis and reported sensitivity for the assays to be between 84.0% and 94.0% and specificity to be 100% [2]. In this study, the specificity of real-time PCR based on solid culture was 79.4%. Therefore, there is an urgent need to identify additional techniques to decrease the number of false-positive cases detected for efficient diagnosis of TB.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by grants from Korea Centers for Disease Control and Prevention, Osong, Korea (Grant No. 4800-4844-300).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Ling D.I., Flores L.L., Riley L.W. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-egression. PLoS One. 2008 Feb;3(2):e1536. doi: 10.1371/journal.pone.0001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon H.W., Hur M., Kim J.Y. Comparison of three molecular assays for the detection of rifampin resistance in Mycobacterium tuberculosis. J Clin Lab Anal. 2014 May doi: 10.1002/jcla.21742. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis J.L., Cattamanchi A., Cuevas L.E. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013 Feb;13(2):147–154. doi: 10.1016/S1473-3099(12)70232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien H.P., Yu M.C., Wu M.H. Comparison of the BACTEC MGIT 960 with Löwenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Int J Tuberc Lung Dis. 2000 Sep;4(9):866–870. [PubMed] [Google Scholar]
- 5.Cruciani M., Scarparo C., Malena M. Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J Clin Microbiol. 2004 May;42(5):2321–2325. doi: 10.1128/JCM.42.5.2321-2325.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


