Abstract
Background and Purpose
Enrollment into acute stroke clinical trials is limited to experienced tertiary centers with emergency research infrastructure. Feasibility of remote enrollment via telemedicine into an acute thrombolytic clinical trial has never been demonstrated.
Methods
Using telemedicine, our hub stroke research center partnered with two spoke community hospitals to jointly participate in a randomized, phase III adjunctive thrombolysis clinical trial in the first 3 h after symptom onset to expand recruitment of the trial. Eligible patients were successfully identified, consented, randomized, and received therapy/placebo at the spoke hospitals under real-time direction by hub trialists via telemedicine.
Results
Ten patients were identified from May 2013 to July 2014, and six were enrolled via telemedicine. No study procedure delays, safety events, or major protocol violations occurred.
Conclusions
It is feasible to randomize and enroll stroke patients via remote telemedicine into an acute thrombolytic clinical trial. This novel approach could expand access and accelerate completion of clinical trials if widely implemented.
Introduction
Advances in acute stroke treatment development are dependent on timely completion of clinical trials; however, current recruitment into acute stroke trials is inefficient.1 A major barrier to enrollment is access to tertiary centers where most trials are conducted.2 Solutions, such as transfers from smaller community facilities, are not cost-effective or feasible for the majority of acute stroke trials testing time-dependent therapies in which patients have to be enrolled within the first few hours after symptom onset. Many acute ischemic stroke trials are testing adjunctive approaches with standard-of-care treatment which require initiation during the 1-h infusion of the only proven therapy, intravenous tissue plasminogen activator (IV t-PA).
A recent study demonstrated telemedicine transfer facilitation of patients from outside facilities to a tertiary center and subsequent enrollment into an acute stroke trial, but there was a significant delay in treatment due to transportation delays between the spoke and hub hospital.3 These delays precluded enrollment into acute ischemic stroke trials that tested novel or adjunctive reperfusion therapies. Further, faster stroke onset to thrombolysis time, in 15-min increments, is linked to reduction of in-hospital mortality and increased odds for independent ambulation at hospital discharge and hospital discharge home.4 We hypothesized that by establishing real-time audiovisual connections, telemedicine could enable stroke specialists to conduct acute stroke trials at community hospitals. To enhance enrollment into our ongoing acute ischemic stroke clinical trials, we expanded our clinical research sites to include two of our telemedicine spokes. In this report, we share our experience enrolling a variety of stroke patients using telemedicine.
Methods
Telemedicine network
The University of Texas Health Science Center Houston Telemedicine network (UTHEALTH) was established in 2005 and currently provides acute neurological coverage to 14 rural and community spoke hospitals in Southeast Texas. The hub, located at UTHSC-H, provides 24/7 acute neurological consultation using two-way, live audiovisual technology (see Fig.1), which includes access to CT images via remote PACS system. The spoke emergency department staff or physician initiates the consult. With the assistance of the spoke staff, a telemedicine neurologist evaluates the patient, reviews relevant laboratory and CT images, formulates a plan of care with the spoke care team, completes an electronic note for the chart, and in some instances arranges transfer to the hub if higher level of care is indicated.
Figure 1.
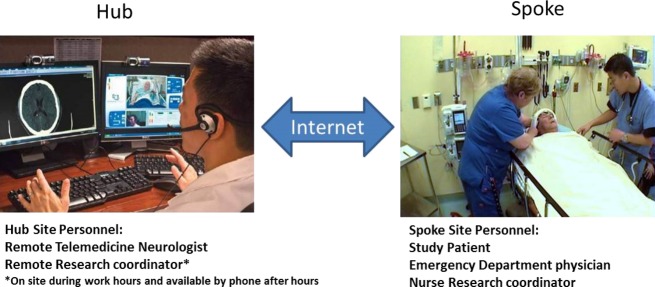
Telemedicine set-up.
We identified two spokes with sufficient acute ischemic stroke and IV t-PA volumes and staff to allow the telemedicine physician to conduct an emergent, randomized adjunctive t-PA clinical trial. The study principal investigator approached the spokes, reviewed the protocol with emergency department staff and stroke coordinator and assisted with review board submission. Baptist Beaumont and Memorial Hermann-Southwest hospitals were already accredited primary stroke centers and key staff was placed on the study FDA-1572 research form. Emergency department staff underwent in-servicing by the hub team, including study inclusion/exclusion criteria, protocol procedures, consent process, and placement of head-frame. The research team taking telemedicine call at UTHEALTH was also in-serviced regarding the logistics of enrolling trial patients via telemedicine. All physicians who participate in telemedicine are credentialed at the telemedicine spoke. Key personnel, including all remote telemedicine physicians, remote research coordinators, and designated local emergency department nurses and physicians were all trained in human subject protection.
Remote enrollment by telemedicine
During the process of evaluating for IV-t-PA eligibility, the telemedicine physician identifies the patient located at telemedicine spokes as a possible CLOTBUST-ER study candidate and pages the UTHSC-H CLOTBUST-ER research team notifying them of a possible eligible patient at the spoke. The CLOTBUST-ER team meets the telemedicine physician at their office during daytime enrollments or contacts them via telephone for after hour or weekend enrollments. Together they review the inclusion and exclusion criteria of the trial, and if eligible, the telemedicine physician proceed in obtaining informed consent (physical copies of informed consent are available in the spoke emergency department) via telemedicine from the patient or legally authorized representative. The patient or legally authorized representative signs the informed consent and the spoke emergency department nurse coordinator signs as the witness.
Once the informed consent is signed, the spoke nurse coordinator or emergency department physician places the head frame on the patient while the hub research member relays the blinded treatment assignment received (A or B) generated by the trial interactive web response system. Next, the spoke selects then initiates the “A” or “B” option on the device control box that connects by cable to the head frame.
The patient then receives the 2-h study procedure (ultrasound insonation vs. sham), and the telemedicine physician performs the follow-up National Institutes of Health Stroke Scale (NIHSS) and skin examination after 2 h per protocol. The spoke nurse coordinator makes copies of all the paperwork to be transferred with the patient to the hub. Transfer is arranged for transport to the hub where the research team continues the remainder of the protocol. The telemedicine physician who obtained the informed consent signs the original form after it arrives with the patient and a copy of the fully signed document is faxed back to the spoke hospital for chart incorporation.
Clinical trial
Independent meta-analyses of several small randomized studies have shown transcranial ultrasound (sonothrombolysis) safely increases tPA-mediated recanalization and appears to reduce 3-month death or dependency.5,6 The CLOTBUST-ER study (Combined Lysis of Thrombus with Ultrasound and Systemic Tissue Plasminogen Activator for Emergent Revascularization; Cerevast Therapeutics, Inc. Redmond, WA, NCT#01098981) is an ongoing, randomized, double-blind, phase III clinical trial to evaluate the efficacy of 2 MHz frequency pulsed wave transcranial Doppler ultrasound versus sham-ultrasound with a proprietary head frame6 as adjunctive therapy to IV t-PA in acute ischemic stroke. After randomization and head-frame placement, the ultrasound/sham therapy must begin within 30 min of the t-PA bolus (see Fig.2 for study flow).
Figure 2.
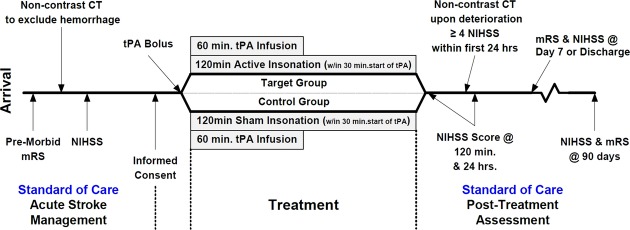
CLOTBUST-ER study flow.
Results
Safety and outcome
Between May 2013 and July 2014, 65 patients at the two spokes were screened, and 10 patients identified via telemedicine met study eligibility at the spoke hospitals. In one case, there was insufficient time (R<5 min) for consent and protocol procedures due to delay in study team activation. Six of the remaining nine patients (56%) agreed to participate (Table1). Three patients/family members declined participation due to lack of interest in participating in a clinical trial.
Table 1.
Patient characteristics, time metrics, and safety of telemedicine-enrolled subjects
| Patient characteristics | |
| N | 6 |
| Age, mean (range) | 65 (50–72) |
| Male gender, n | 4 |
| NIHSS, median (range) | |
| Baseline (pre t-PA) | 13 (10–17) |
| 2-H (end of sonothrombolysis/sham-ultrasound) | 13 (5–35) |
| Discharge or day 7 | 13 (0–23) |
| Interval time metrics in minutes, mean (range) | |
| Stroke symptom onset to t-PA bolus | 112 (89–126) |
| Emergency Department arrival to t-PA bolus | 60 (49–78) |
| t-PA bolus to signed informed consent | 4.8 (−6 to 26) |
| Signed informed consent to study website randomization | 6 (1–11) |
| Randomization to study head-frame placement | 5 (1–11) |
| Head-frame placement to start of sonothrombolysis/sham-ultrasound | 0.3 (0–1) |
| Informed consent to start of sonothrombolysis/sham-ultrasound | 9 (2–14) |
| t-PA and ultrasound/sham-ultrasound overlap | 43 (33–48) |
| Safety | |
| Adverse events attributed to the study | 0 |
| Clinical trial protocol violations | 1 |
NIHSS, National Institutes of Health Stroke Scale; t-PA, tissue plasminogen activator.
Family members were present for informed consent in each case. Enrollment into the trial did not interfere with standard treatments, including t-PA. Three of the five telemedicine enrolled patients received t-PA within the recommended 60 min door to t-PA window, and had comparable t-PA + sham/treatment overlap times. Treatment/sham insonation was initiated according to trial requirements of 30 min after t-PA bolus and no serious adverse events or major protocol deviations occurred. One minor protocol violation was captured which consisted of a patient receiving IV-tpa while on warfarin with a normal INR. This was deemed a protocol violation as the package insert for Alteplase (IV-tPA) states that regardless of INR, if a patient is on oral anticoagulants, it is a contraindication. All time metrics were comparable to four patients enrolled at the hub hospital during the same period of time.
Discussion
Telemedicine with real-time two way audio/video conferencing has transformed clinical care for stroke patients by assisting treating physicians with remote guidance by experts in the administration of t-PA treatment. We now demonstrate that telemedicine can be used not only to assist in the treatment of patients with acute stroke at spoke facilities but also can be used to facilitate enrollment of these patients into clinical trials testing adjunctive treatments with t-PA. The concept of using telemedicine to enhance recruitment into acute stroke trials was reported by Switzer et al.3; however, their model of recruitment, involved transferring patients to the hub prior to the study intervention. Transferring patients would not be feasible in most acute ischemic stroke trials that are linked with thrombolytics as those trials are time sensitive and need to be initiated with IV-tPA. In our approach, the entire process of clinical trial enrollment from screening, to informed consent, to actual study intervention, is feasible at spoke hospitals using telemedicine. Furthermore, enrollment by telemedicine did not delay administration of t-PA or contribute to any major protocol violations. We achieved all clinical trial requirements. Other studies have also explored enrollment of patients at remote sites using web-based systems. We now add another approach in which acute stroke research was conducted entirely via a remote encounter and may serve as a template for other emergent clinical trials in other disease entities that are time sensitive.
The success of remote clinical trial enrollment will likely depend on specific requirements of the trial. CLOTBUST-ER has a straightforward design and can be conducted with minimal training and experience beyond selecting and treating patients with IV t-PA. It does not use an experimental drug that would involve a remote investigational pharmacy, and the intervention lasts for only 2 h. Placement of the head frame can be performed by a bedside nurse following simple instructions and telemedicine guidance, if necessary. While all of our patients were transferred to the hub, this is not a requirement for enrollment.
This new use of telemedicine could substantially increase access and opportunities for stroke patients at rural and community hospitals to receive promising new therapies. Traditionally, complex reperfusion acute stroke trials have been primarily conducted in large academic centers with substantial experience. Although not every spoke or acute ischemic stroke clinical trial can be conducted using telemedicine, this paradigm offers tremendous potential to expedite testing of new therapies. Our experience warrants further expansion of this novel approach to other centers to confirm widespread feasibility and safety.
Conflict of Interest
Andrew D. Barreto is on Cerevast Therapeutic's Scientific Advisory Board and the U.S. national principal investigator for the phase III clinical trial, CLOTBUST-ER (NCT01098981). All other authors report no disclosures.
References
- Fisher M, Albers GW, Donnan GA, et al. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry Roundtable. Stroke. 2005;36:1808–1813. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- Leira EC, Ahmed A, Lamb DL, et al. Extending acute trials to remote populations: a pilot study during interhospital helicopter transfer. Stroke. 2009;40:895–901. doi: 10.1161/STROKEAHA.108.530204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer JA, Hall CE, Close B, et al. A telestroke network enhances recruitment into acute stroke clinical trials. Stroke. 2010;41:566–569. doi: 10.1161/STROKEAHA.109.566844. [DOI] [PubMed] [Google Scholar]
- Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- Barreto AD, Alexandrov AV, Shen L, et al. CLOTBUST-Hands Free: pilot safety study of a novel operator-independent ultrasound device in patients with acute ischemic stroke. Stroke. 2013;44:3376–3381. doi: 10.1161/STROKEAHA.113.002713. [DOI] [PubMed] [Google Scholar]


