Abstract
Objectives
A patient-centered collaborative care program for depression and uncontrolled diabetes and/or coronary heart disease (CHD) demonstrated im proved clinical outcomes relative to usual care. We report clinicallys tratified analyses of patient outcomes to inform the duration and targeting of care management services for complex patients with multimorbidity.
Methods
A 12-month randomized controlled trial of amultimorbidity collaborative care program followed patients at 6, 12, 18, and 24 months for diabetes (glycated hemoglob in [A1C]), blood pressure (systolic; SBP), low-density lipoprote in (LDL) cholesterol, and depression (Symptoms Check List-20 score). Depressed patients with less favorable medical control (Patient Health Questionnaire-9 score >10, A1C >8.0 %, SBP >140 mm Hg, and LDL cholesterol >120 mg/dL) were compared with depressed patients with more favorable medical control to describe differential intervention benefits overtime.
Results
In contrast to patients with more favorable baseline control, patients with depression and unfavorable control of A1C, SBP, and LDL at baseline showed improved outcomes as early as the 6-month follow-up assessment. Clinical benefits in the intervention group were largely sustained over the 24-month follow-up, except for some deterioration of glycemic control in intervention patients and trends toward improvement among controls over time. Among patients with depression and more favorable medical control at baseline, there were minimal between-group differences in medical disease outcomes. Conclusions Clinical benefits of amultimorbidity collaborative care management program occurred early, and were only found among patients with poor control of baseline diabetes and CHD risk factors. Targeting may maximize reach and improve affordability of complex care management.
Patients with multiple chronic conditions are prevalent in primary care.1-3 The high prevalence of depression and psychological distress accompanying common physical conditions such as diabetes and coronary heart disease (CHD) magnifies the complexity of care and intensifies resource utilization.4-6 About two-thirds of total healthcare spending in the United States is directed toward the one-fourth of patients with multimorbidity (defined as having more than 1 chronic condition).7 To better serve patients with complex healthcare needs, the Agency for Health Care Research and Quality recommended reorganizing primary care to include care managers, clinical decision support, and other resources.8 However, a comparative effectiveness review of care/case management found limited improvement in outcomes and quality of care, and little change in resource utilization among patients receiving complex care management.9
A recent randomized trial of a collaborative care intervention for patients with depression as well as uncontrolled diabetes and/or CHD demonstrated improved outcomes for diabetes, hypertension, hyper-lipidemia, and depression relative to patients receiving enhanced usual care (UC).10 In addition to better clinical outcomes, intervention patients reported higher functioning, quality of life, patient satisfaction, and self-efficacy in disease management after the 12-month intervention.11,12 Improved outcomes were achieved through a team-based, patient-centered, collaborative chronic care program targeting both physical and mental health goals.13 At the 2-year follow-up, cost-effectiveness analyses suggested outpatient cost savings; depression continued to be significantly improved in the intervention relative to enhanced UC.14 Benefit for control of hyperglycemia, hypertension, and hyperlipidemia had diminished between intervention and UC groups in the year after intervention cessation.14
We report analyses from this trial stratified by baseline status of disease-control parameters [glycated hemoglobin (A1C), systolic blood pressure (SBP), and low-density lipoprotein (LDL)] to shed light on ways this innovative and integrated intervention can be refined to achieve the “triple aim” of better care experience and outcomes at a lower cost.15 Specifically, this paper addresses the following questions: 1) Which patients should be targeted for care management? and 2) How long should care management be sustained?9,16 Analyses describe clinical outcomes over a 24-month period for the following subgroups: 1) depressed patients with less favorable medical control of diabetes, hypertension, or hyperlipidemia; versus 2) depressed patients with more favorable medical control of diabetes, hypertension, or hyperlipidemia.
METHOD
Setting and Participants
Participants with depression and uncontrolled diabetes and/or CHD were recruited from 14 Group Health primary care clinics from May 2007 to October 2009. An epidemiologic study at Group Health found a 12% prevalence of major depression among a large cohort of patients with diabetes.17 Electronic medical records identified patients with poor glycemic control (A1C ≥8.5%), systolic blood pressure (SBP ≥140/90 mm/Hg), or lipid control (LDL >120 mg/dL) for a 2-stage depression screen. Eligibility also required a depression score >10 on the 9-item Patient Health Questionnaire (PHQ-9).18 Exclusion criteria included terminal illness; pregnancy; planned disenrollment; limited English proficiency; bipolar disorder or schizophrenia; and mental confusion suggesting dementia.
Randomization and Intervention
Patients were assigned to treatment groups using a permuted block design with randomly selected block sizes of 4, 6, and 8 patients. After randomization, a study nurse contacted patients assigned to the intervention to initiate treatment. U C patients received enhanced routine care as they were advised to consult with their primary care physician (PCP) to receive care for depression, diabetes, and/or CHD. Their PCPs also received baseline 6-, 12-, 18-, and 24-month assessments of depression and blood pressure, as well as laboratory test results. Please see prior publications for additional method details.13,19 Based on sample distribution that was confirmed by clinical consensus, patients with depression (overall sample) were divided into the following subgroups: more versus less favorable glycemic control (baseline A1C ≥8.0%); more versus less favorable blood pressure (BP) control (SBP ≥140 mm Hg); and more versus less favorable lipid control (LDL ≥120 mg/dL). There were no patients with more favorable control of depression at baseline, as a PHQ-9 score ≥10 was an inclusion criterion.
Intervention: Multimorbidity Collaborative Care (TEAMcare)
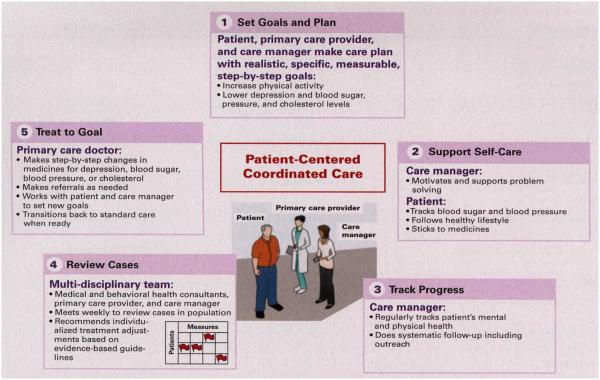
This intervention distilled elements from collaborative care for depression,20,21 the chronic care model,22,23 and treat-to-target strategies initially developed for diabetes. 24 This integrated program was applied systematically across 3 chronic illnesses (diabetes, depression, and CHD) for 12 m onths.13 Figure 1 illustrates core elements of a patient-centered, collaborative care program for patients with multiple chronic illnesses (Treatment, Enhancement, Activation, and Motivation care [TEAMcare]). The team consisted of our patients, the TEAMcare nurse care managers, the patient’s PCP and care team, and the medical and psychiatric consultants. Nurse care managers conducted in-person and telephone follow-up visits in a structured manner, and met the patient at their primary care clinic for the in-person visits.
Figure 1.
Integrated Collaborative Care for Multimorbidity: HowTEAMcare Works
Intervention began with a comprehensive face-to-face biopsychosocial assessment and included goal-setting and formulating “my health plan,” self-management support, monitoring of disease indicators, and pharma cotherapy with frequent treatment adjustments to control depression, hyperglycemia, hypertension, and hyperlipidemia. Patients collaborated with nurse care managers and PCPs to create individualized clinical and self-management goals and care plans. Nurses followed patients proactively to monitor clinical progress, and used motivational and problem-solving approaches to support medication adherence, healthy eating, and physical activity.25 An electronic registry supported tracking of PHQ-9 scores and A1C, LDL, and BP levels, and flagged patients who were not making good progress. Weekly case reviews were conducted as face-to-face interdisciplinary meetings with nurse care manager presentations on the new patients and focused updates on patients who were not making adequate progress. Care managers received medical and psychiatric consultation with a family medicine or internal medicine physician (EHBL or BY) and a psychiatrist (WK or PC) and psychologist (EJL). Treatment protocols employing commonly used medicines guided consultant recommendations, and medication changes were tailored to patient history and clinical response. The nurse communicated treatment change recommendations to the patient’s PCP, who was responsible for medication management. Once patients achieved targeted levels for relevant measures, the nurse and patient developed a relapse prevention and maintenance plan. Patients whose disease control had worsened were offered follow-up and protocol-based intensification of treatment regimens.13,25
Outcomes and Follow-up
At baseline and at 6, 12, 18, and 24 months, telephone interviewers assessed depression symptoms, according to the Symptoms Check List-20 score (37). Blood pressure and A1C were measured in person at baseline, 6 months, and 12 months; fasting LDL was measured at baseline and 12 months.
Study Oversight
The TEAMcare Data Safety Monitoring Board reviewed methods initially and outcomes every 6 months thereafter. The trial was approved by the Institutional Review Boards of Group Health and the University of Washington.
Statistical Analyses
We compared intervention versus UC trends over the 24-month follow-up period for subgroups with more versus less favorable control at baseline for glycemic control, BP control, and lipid control. We describe glycemic, BP, and lipid control differences with means and confidence intervals. Since these post hoc comparisons have substandaily smaller sample size th a n the unstratified analyses originally reported for the randomized controlled trial, the contrasts are necessarily underpowered to detect potentially clinically meaningful differences. The intent of these analyses is to describe the baseline clinical characteristics of patients who benefitted from TEAMcare, and the duration of observed benefits. Analyses adjusting for baseline levels were conducted using linear regression. All analyses were performed on those with complete followup data and were carried out using STATA 12.0 (Stata-Corp, College Station, Texas).
RESULTS
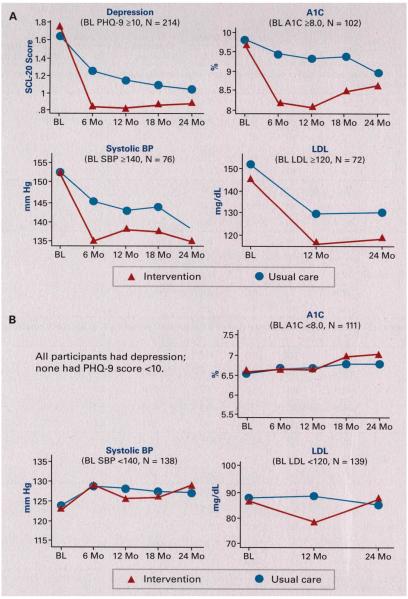
Table 1 shows demographic an d depression characteristics of the overall intervention and UC groups and contra sting disease control in the subgroups (more vs less favorable control) for BP, pressure, and lipid measures. All study patients met criteria for probable major depression (PHQ-9 >10). Figures 2a and 2b show the clinical course for diabetes, hypertension, and hyperlipidemia for subgroups with more versus less favorable medical control for A1C, SBP, and LDL cholesterol over the 2-year study period. Among the patients with comorbid depression, and less favorable glycemic, BP, and lipid control at baseline, both intervention and UC patients improved on all 4 outcomes. Yet, starting with the first follow-up assessment, patients with depression and less favorable clinical control who received the TEAMcare intervention demonstrated greater improvements in depression, glycemic, BP, and lipid outcomes than UC patients. After cessation of the yearlong intervention, clinical benefits were largely sustained over the 24-month follow-up for cardiovascular risk factors while glycemic control deteriorated slightly. In contrast, as shown in Figure 2b, among patients with comorbid depression and more favorable glycemic, BP, and lipid control at baseline, there were no apparent trends toward improvement (or deterioration) of medical control in either intervention or control patients. And, with 1 exception, there were no apparent benefits of receiving TEAMcare for improving control of A1C, SBP, or LDL cholesterol either initially or long-term among depressed patients with more favorable glycemic, blood pressure and lipid control at baseline. The single exception was lower LDL at 12 months among TEAMcare patients relative to UC patients in the subgroup with more favorable LDL control at baseline. This difference was not sustained at 24 months. Table 2 provides means and standard deviations for the contrasts depicted in Figures 2a and 2b.
Table 1.
Sociodemographic and Clinical Characteristics of Patients With Multimorbidity (stratified by more vs less favorable control at baseline)a
| Characteristic | Intervention N = 106 | Usual Care N = 108 |
|---|---|---|
| Age (y): mean (SD) | 57.4 (10.5) | 56.3 (12.1) |
| Female | 48% | 56% |
| ≥1 year of college | 61% | 56% |
| Minority (nonwhite or Hispanic) | 25% | 22% |
| Depression for ≥2 years | 72% | 76% |
| SCL-20: mean (SD), range | 1.7 (0.6), 0.16-3.25 | 1.7 (0.6), 0.30-2.95 |
| PHQ-9: mean (SD), range | 14.7 (3.8), 10-26 | 13.9 (3.1), 10-23 |
| BMI (%) | 36.9 (8.3) | 36.6 (8.5) |
| A1C | ||
| A1C ≥8.0%: mean (SD), range | 9.6% (1.8), 8-15.2 N = 53 |
9.8% (1.2), 8.2-13.1 N = 49 |
| A1C <8.0%: mean (SD), range | 6.6% (0.7), 5.1-79 N = 53 |
6.6% (0.7), 5.2-79 N = 58 |
| LDL | ||
| LDL ≥120 mg/dL: mean (SD), range | 144.9 (24.3), 120-203 N = 37 |
151.4 (26.6), 122-234 N = 35 |
| LDL <120 mg/dL: mean (SD), range | 86.0 (19.4), 31-119 N = 69 |
87.7 (16.9), 50-118 N = 70 |
| Systolic BP | ||
| ≥140 mm Hg: mean (SD), range | 152.5 (10.7), 140-185 N = 46 |
152.5 (12.0), 140-195.5 N = 30 |
| <140 mm Hg: mean (SD), range | 123.0 (11.4), 94.5-139.5 N = 60 |
123.4 (11.0), 93.5-139.5 N = 78 |
BMI indicates body mass index; BR blood pressure; LDL, low-density lipoprotein; PHQ-9, Patient Health Questionnaire-9; SCL20, Symptom Checklist 20.
Less favorable control: A1C >8.0%; systolic BP >140 mm Hg; LDL >120 mg/dL.
Figure 2.
Two-Year Comparisons for Glycemic, Blood Pressure, and Lipid Control
Table 2.
Subgroup Comparisons for Glycemic, Blood Pressure, and Lipid Control Over 24 Months
| BL Adjusted |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Means |
Between Group Difference (UC minus 1) |
95% CI |
Effect Size, Regression (t statistic) |
|||||||
| Outcome | N | I | UC | LCI | UCI | P | ||||
|
Unfavorable control at baseline subset: Linear regression of intervention (vs usual care) predicting 6- to 24-month outcomes, each
controlling for baseline levels | ||||||||||
|
| ||||||||||
|
>8.0% at
baseline |
A1C | BL | 92 | 9.6 | 9.8 | |||||
| 6 mo | 92 | 8.2 | 9.4 | −1.19 | −1.75 | −0.62 | −4.19 | .001 | ||
| 12 mo | 93 | 8.1 | 9.3 | −1.20 | −1.76 | −0.63 | −4.22 | <.001 | ||
| 18 mo | 81 | 8.5 | 9.4 | −0.86 | −1.62 | −0.09 | −2.24 | .028 | ||
| 24 mo | 82 | 8.6 | 8.9 | −0.30 | −0.99 | 0.38 | −0.88 | .382 | ||
|
| ||||||||||
|
>120 mg/dL at
baseline |
LDL | BL | 72 | 144.9 | 151.4 | |||||
| 12 mo | 63 | 116.1 | 129.6 | −9.9 | −28.8 | 9.0 | −1.05 | .298 | ||
| 24 mo | 56 | 118.7 | 130 | −9.1 | −28.7 | 10.5 | −0.93 | .355 | ||
|
| ||||||||||
|
>140 mm Hg at
baseline |
Systolic BP | BL | 76 | 9.6 | 9.8 | |||||
| 6 mo | 76 | 8.2 | 9.4 | −10.2 | −18.1 | −2.3 | −2.57 | .012 | ||
| 12 mo | 73 | 8.1 | 9.3 | −4.8 | −13.2 | 3.5 | −1.15 | .255 | ||
| 18 mo | 72 | 8.5 | 9.4 | −5.9 | −14.5 | 2.8 | −1.36 | .179 | ||
| 24 mo | 65 | 8.6 | 8.9 | −3.1 | −11.9 | 5.7 | −0.70 | .484 | ||
|
| ||||||||||
|
Fair control at baseline subset: Linear regression of intervention (vs usual care) predicting 6-to 24-month outcomes, each controlling
for baseline levels | ||||||||||
|
| ||||||||||
|
<8.0% at
baseline |
A1C | BL | 112 | 6.6 | 6.6 | |||||
| 6 mo | 101 | 6.7 | 6.7 | −0.06 | −0.34 | 0.21 | −0.46 | .648 | ||
| 12 mo | 105 | 6.6 | 6.7 | −0.06 | −0.36 | 0.24 | −0.40 | .693 | ||
| 18 mo | 90 | 7.0 | 6.8 | 0.18 | −0.21 | 0.56 | −0.91 | .366 | ||
| 24 mo | 93 | 7.0 | 6.8 | 0.27 | −0.16 | 0.70 | −1.26 | .210 | ||
|
| ||||||||||
|
<120 mg/dL at
baseline |
LDL | BL | 124 | 86.0 | 87.7 | |||||
| 12 mo | 124 | 78.4 | 88.1 | −8.8 | −17.2 | −0.3 | −2.05 | .042 | ||
| 24 mo | 112 | 87.3 | 84.6 | 3.7 | −6.2 | 13.6 | 0.73 | .464 | ||
|
| ||||||||||
|
<140 mm Hg at
baseline |
Systolic BP | BL | 138 | 123.0 | 123.5 | |||||
| 6 mo | 129 | 125.4 | 128.7 | 1.1 | −4.3 | 6.4 | 0.40 | .693 | ||
| 12 mo | 129 | 129.2 | 128.0 | −2.4 | −7.4 | 2.5 | −0.99 | .326 | ||
| 18 mo | 120 | 126.2 | 127.3 | −0.6 | −6.5 | 5.2 | −0.22 | .830 | ||
| 24 mo | 124 | 129.0 | 127.2 | 2.1 | −3.4 | 7.7 | 0.76 | .451 | ||
BL indicates baseline: BF) blood pressure; I, intervention; LCI, lower limit of 95% confidence interval; LDL, low-density lipoprotein; mo, month; UC, usual care; UCI, upper limit of 95% confidence interval.
DISCUSSION
We stratified patients with multimorbidity by baseline medical control to assess differential benefits of a teambased, integrated collaborative care program (TEAMcare). Durability of clinical benefits of this yearlong intervention was followed for 2 years. The TEAMcare intervention provided significant clinical improvement relative to UC exclusively among patients with less favorable medical control at baseline. Starting with the first follow-up assessment, glycemic, BP, and lipid control, in addition to depression, were enhanced relative to UC patients in this less favorable medical control group. Except for a slight deterioration in glycemic control after cessation of the 12-month intervention, these clinical benefits were largely sustained. Interestingly, UC patients with less favorable control also showed gradual improvement from baseline throughout the 24-month period. In contrast, among patients with more favorable medical control, there were no differences in clinical outcomes between TEAMcare and UC patients in glycemic, BP, or lipid control during the intervention period, or thereafter.
A significant limitation of the descriptive analyses reported here is the lack of statistical power in the subgroups comparisons. For example, the small sample size in the subgroup with less favorable control of hyperlipidemia at baseline likely accounted for the lack of statistical significance at long-term follow-up, whereas the between-group difference was clinically significant. The gradual improvement among UC patients in diabetes, BP, low-density lipoprotein, and depression is noteworthy. UC patients actually received enhanced routine care, as PCPs responded to feedback of trial-ordered test results for UC patients provided during the 2-year follow-up. There is also potential spillover effect of the intervention. A lthough considered a methodological limitation at times, spillover of intervention effect is in fact a desirable quality improvement outcome. Concurrent quality improvement efforts in primary care by the health plan used some core elements of this program and may have reduced disparities between intervention and UC patients. The common phenomenon of regression to the mean after baseline assessment can also contribute to the gradual clinical improvement in the UC group.
There is a paucity of evidence-based interventions for patients with multimorbidity.26 This is the first randomized trial that integrated collaborative care for depression with systematic chronic illness care for diabetes and cardiovascular risk factors. Encouraging results such as benefits in diabetes, hypertension, and hyperlipidemia control beyond depression improvement; enhanced quality of life; better functioning; and projected outpatient savings all contrast with prior studies.10,11,13,14 Randomized controlled trials of collaborative care focusing only on depression treatment among patients with co-existing diabetes consistently show the lack of improvement in glycemic control in spite of improved depression outcomes.27-29 The need for integrating biomedical and psychological or psychiatric treatments to address both depression and chronic medical illnesses is fur the runder scored.30 Findings of a large Medicare demonstration trial of complex case management showing minimal clinical, quality, or cost benefits31 highlight the necessity for patient-centered interventions that achieve the “triple aim” of improved health outcomes, patient experience, and cost-effectiveness.15
Study findings clarifying which patients benefit most from enhanced care management offer significant health services implications. The targeting of depressed patients with less favorable glycemic, BP, and lipid control to receive this integrated and efficacious care management would expand the reach of the program and maximize the number of patients most likely to benefit. Matching patients ’ clinical needs for additional care management resources would reduce unnecessary clinical encounters and improve cost-effectiveness. A recent Cochrane review on interventions for patients with multimorbidity also recommended targeting interventions to improve clinical and functionaloutcomes, implying the need to selectively provide services to those with less favorable clinical status at initial assessment.26,32
HHS issued a strategic framework to improve care for patients with multiple chronic conditions33 that described strategies used in this integrated collaborative care program for multimorbidity. The TEAMcare intervention has been adapted and implemented in a busy patient-centered medical home setting. Routine-care nurses, PCPs, and consultants received training and informatics support with weekly interdisciplinary caseload review. Results of this pilot dissemination project demonstrated more appropriate use of healthcare services as well as clinical improvements in depression and in glycemic and blood pressure control in the 1-year program with no significant cost increase.34
Efforts are currently under way to adapt, disseminate, and implement core elements o f this program (Figure I) across a wide range o f practice settings for diverse populations. Experiences with disseminating TEAMcare suggest that adaptation of core elements (eg, goal-setting in care plans, self-care support, progress monitoring, systematic case reviews, treat-to-goal) to meet unique needs and resources of individual practice settings is necessary in implementing this intervention.
Results indicating durability of this program help to shed light on another important challenge in the care of patients with complex healthcare needs: how long should care management continue in order to sustain clinical benefits? It’s encouraging that clinically significant and robust improvements in diabetes, hypertension, and depression outcomes were demonstrated in the first 6 months among th e patients with less favorable control at baseline. These differences were sustained over the 12-month intervention, and benefits, somewhat diminished, continued post intervention. Early improvement in glycemic control can have enduring benefits, as findings showing 12-month improvement in glycemic control in the United Kingdom Prospective Diabetes Study trial were associated with decreased risk for macrovascular and microvascular complications and overall mortality at the 10-year follow-up even though glycemic control differences did not persist for more than 1 year beyond trial completion.35
Two key questions remain for future research: 1) Would continued efforts in systematic follow-up and treatment adjustment sustain more robust clinical differences between intervention and UC groups? and 2) How might UC be enhanced for patients with poor medical control so UC patients could achieve improved outcomes as quickly as the patients benefiting from the care management intervention evaluated in this research?
CONCLUSION
Results showed that clinical benefits of a multimorbidity collaborative care management program occurred early, and were predominantly found among patients with unfavorable control of diabetes and CHD risk factors. These have important implications for improving clinical outcomes of complex patients and healthcare affordability.
Take-Away Points.
This paper addresses targeting and duration of care management among complex patients with multimorbidity. A patient-centered multimorbidity collaborative care program demonstrated better outcomes in depression, coexisting diabetes, and cardiovascular risk factors only among the subgroup of patients with unfavorable medical control at baseline (Patient Health Questionnaire-9 score >10, glycated hemoglobin >8.0%, systolic blood pressure >140 mm Hg, and low-density lipoprotein cholesterol >120 mg/dL). Improvements occurred early and continued over 2 years. In contrast, patients with depression and more favorable medical control showed no clinical benefit beyond reduced depression.
Implications for complex care management:
■ Target patients with unfavorable medical outcomes at baseline.
■ Targeting may maximize reach and improve affordability.
Acknowledgments
Source of Funding: National Institute of Mental Health grants (MH041739 and MH069741).
Footnotes
Author Disclosures: Dr Von Korff received a grant from Pfizer (subcontract from Geisinger) and he advises on approaches to prediction of healthcare costs and costs related to back pain. Dr Von KorfF is also the principal investigator of a grant to the Group Health Research Institute from Pfizer to study use of natural language processing methods for identifying misuse and abuse of opioid medications. The remaining authors report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article.
REFERENCES
- 1.Schneider KM, O'Donnell BE, Dean D. Prevalence of m ultiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7:82. doi: 10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodell S, Bodenheimer TS, Berry-Millett R. Care Management of Patients with Complex Health Care Needs: The Synthesis Project. Robert Wood Johnson Foundation; Princeton, NJ: 2009. [PubMed] [Google Scholar]
- 3.Stange KC. In this issue: challenges of managing multimorbidity. Ann Fam Med. 2012;10(1):2–3. doi: 10.1370/afm.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RW, Ashburner JM, Hong CS, Chang Y, Barry MJ, Atlas SJ. Defining patient complexity from the primary care physician's perspective: a cohort study. Ann Intern Med. 2011;155(12):797–804. doi: 10.7326/0003-4819-155-12-201112200-00001. [DOI] [PubMed] [Google Scholar]
- 5.Fortin M, Bravo G, Hudon C, Lapointe L, Dubois MF, Almirall J. Psychological distress and m ultimorbidity in primary care. Ann Fam Med. 2006;4(5):417–422. doi: 10.1370/afm.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzelnick DJ, Simon GE, Pearson SD, et al. Randomized trial of a depression management program in high utilizers of medical care. Arch Fam Med. 2000;9(4):345–351. doi: 10.1001/archfami.9.4.345. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe KE, Ogden LL, Galactionova K. Chronic conditions account for rise in Medicare spending from 1987 to 2006. Health Aff (Millwood) 2010;29(4):718–724. doi: 10.1377/hlthaff.2009.0474. [DOI] [PubMed] [Google Scholar]
- 8.Rich EC, Lipson D, Libersky J, Peikes DN, Parchman ML. Organizing care for complex patients in the patient-centered medical home. Ann Fam Med. 2012;10(1):60–62. doi: 10.1370/afm.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality Comparative Effectiveness of Case Management for Adults with Medical Illness and Complex Care Needs. Effective Health Care Program. 2013 www.effectivehealthcare.ahrq.gov/ehc/products/240/733/CER99_OutpatientCaseManagement_FinalReport_20130102.pdf.
- 10.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Korff M, Katon WJ, Lin EHB, et al. Functional outcomes of m ulti-condition collaborative care and successful ageing: results of randomised trial. BMJ. 2011;343:d6612. doi: 10.1136/bmj.d6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludman EJ, Peterson D, Katon WJ, et al. Improving confidence for self care in patients with depression and chronic illnesses. Behav Med. 2013;39(1):1–6. doi: 10.1080/08964289.2012.708682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin EH, Von Korff M, Ciechanowski P, et al. Treatment adjustment and medication adherence for complex patients with diabetes, heart disease, and depression: a randomized controlled trial. Ann Fam Med. 2012;10(1):6–14. doi: 10.1370/afm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a m ulticondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69(5):506–514. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Mill wood) 2008;27(3):759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 16.Bayliss EA. Sim plifying care for complex patients. Ann Fam Med. 2012;10(1):3–5. doi: 10.1370/afm.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katon W, Von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27(4):914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katon W, Von Korff M, Lin E, et al. Collaborative management to achieve depression treatment guidelines. J Clin Psychiatry. 1997;58(suppl 1):20–23. [PubMed] [Google Scholar]
- 20.Katon W, Von Korff M, Lin E, et al. Collaborative management to achieve treatment guidelines, impact on depression in primary care. JA/WA. 1995;273(13):1026–1031. [PubMed] [Google Scholar]
- 21.Unutzer J, Katon W, Callahan CM, et al. IMPACT Investigators Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 22.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 23.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann Intern Med. 1997;127(12):1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine Integrated Multimorbidity Collaborative Care or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 25.McGregor M, Lin EH, Katon WJ. TEAMcare: an integrated m ulticondition collaborative care program for chronic illnesses and depression. J Ambul Care Manage. 2011;34(2):152–162. doi: 10.1097/JAC.0b013e31820ef6a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SM, Soubhi H, Fortin M, Hudon C, O'Dowd T. Managing patients with m ultimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205. doi: 10.1136/bmj.e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 28.Williams JW, Jr, Katon W, Lin EH, et al. IMPACT Investigators The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med. 2004;140(12):1015–1024. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]
- 29.Ell K, Katon W, Xie B, et al. Collaborative care management of major depression among low-income, predominantly Hispanic subjects with diabetes: a randomized controlled trial. Diabetes Care. 2010;33(4):706–713. doi: 10.2337/dc09-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 31.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(61):603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Soubhi H, Fortin M, Hudon C, O'Dowd T. Interventions for improving outcomes in patients with m ultimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560. doi: 10.1002/14651858.CD006560.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Multiple Chronic Conditions: A Strategic Framework. HHS; Washington, DC: 2010. [Google Scholar]
- 34.Trehearne B, Fishman P, Lin EHB. Role of the nurse in chronic illness management: making the medical home more effective. Nursing Economics. 2014;32(4):178–184. [Google Scholar]
- 35.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]