Abstract
Glucagon-like peptide-1 (GLP-1) is an incretin hormone whose glucose-dependent insulinotropic actions have been harnessed as a novel therapy for glycaemic control in type 2 diabetes. Although it has been known for some time that the GLP-1 receptor is expressed in the CVS where it mediates important physiological actions, it is only recently that specific cardiovascular effects of GLP-1 in the setting of diabetes have been described. GLP-1 confers indirect benefits in cardiovascular disease (CVD) under both normal and hyperglycaemic conditions via reducing established risk factors, such as hypertension, dyslipidaemia and obesity, which are markedly increased in diabetes. Emerging evidence indicates that GLP-1 also exerts direct effects on specific aspects of diabetic CVD, such as endothelial dysfunction, inflammation, angiogenesis and adverse cardiac remodelling. However, the majority of studies have employed experimental models of diabetic CVD and information on the effects of GLP-1 in the clinical setting is limited, although several large-scale trials are ongoing. It is clearly important to gain a detailed knowledge of the cardiovascular actions of GLP-1 in diabetes given the large number of patients currently receiving GLP-1-based therapies. This review will therefore discuss current understanding of the effects of GLP-1 on both cardiovascular risk factors in diabetes and direct actions on the heart and vasculature in this setting and the evidence implicating specific targeting of GLP-1 as a novel therapy for CVD in diabetes.
Tables of Links
| LIGANDS | |
|---|---|
| ACh | Insulin |
| Adiponectin | Leptin |
| Alogliptin | Linagliptin |
| ANP | Liraglutide |
| Byetta | Lixisenatide |
| cAMP | Metformin |
| Exenatide | Nitric oxide (NO) |
| Exendin-4 | Rosiglitazone |
| Glimepiride | Saxagliptin |
| Glipizide | Sitagliptin |
| GLP-1 | Stromal cell-derived factor-1α |
| GLP-1(9-36) | VCAM-1 |
| Glyburide | VEGF-A |
| ICAM-1 | Victoza |
| IL-6 | Vildagliptin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d,eAlexander et al., 2013a,b,c,d,e,,,,).
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is increasing alarmingly with the 2013 figure of 382 million estimated to rise to 592 million by 2035 (International Diabetes Federation, 2014). A change in lifestyle coupled with an increase in obesity has led to a global epidemic, with diabetics typically carrying a fivefold greater mortality risk as a result of cardiovascular disease (CVD) compared with non-diabetics (Stamler et al., 1993), and coronary artery disease (CAD) being the leading underlying cause (Bertoni et al., 2004). It is well established that hyperglycaemia plays a central role in development and progression of CVD associated with diabetes (Nathan, 1996). Indeed, two long-term clinical trials, the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study and the United Kingdom Prospective Diabetes Study (UKPDS), have demonstrated that intensive glucose-lowering strategies are effective in markedly reducing the incidence of microvascular (e.g. retinopathy, nephropathy) and macrovascular (e.g. CAD, stroke) complications in both type 1 diabetes mellitus (T1DM) and T2DM (Holman et al., 2008), although several similar large-scale trials have reported limited benefits (Action to Control Cardiovascular Risk in Diabetes Study Group, 2008; Duckworth et al., 2009; Ginsberg, 2011). Nonetheless, there remains a significant incidence of CVD even in optimally treated diabetic patients, so it is clear that more effective strategies are required. In this regard, the incretin peptide hormone, glucagon-like peptide-1 (GLP-1), has received considerable recent attention.
The incretin effect is responsible for augmenting insulin secretion following nutrient ingestion and GLP-1 together with its sister hormone, gastrointestinal peptide, account for up to 60% of post-prandial insulin secretion, leading to rapid blood glucose reduction (Nauck et al., 1986; Drucker et al., 1987). Furthermore, they possess an inherent ability to reduce glucagon secretion (Kreymann et al., 1987), delay gastric emptying (Näslund et al., 1998a) and promote satiety (Flint et al., 1998). The metabolic actions of GLP-1 are mediated by GLP-1 receptor activation and stimulation of cAMP and several downstream kinases, including ERK1/2, PI3K and PKA. Under physiological conditions, GLP-1 has a short half-life (∼2 min) as it is rapidly degraded by its endogenous inhibitor, dipeptidyl peptidase-4 (DPP-4) (Deacon et al., 1995), resulting in cleavage of two amino acids from native GLP-1(7-36) to produce GLP-1(9-36), which acts as a weak GLP-1 receptor antagonist lacking insulinotropic activity (Green et al., 2004). However, emerging evidence suggests that ‘metabolically inactive’ GLP-1(9-36) may itself be an important signalling molecule (Ban et al., 2010; Gardiner et al., 2010). More detailed information on GLP-1 biology and signalling is provided by recent review articles (Grieve et al., 2009; Donnelly, 2012; Pabreja et al., 2013).
The unique ability of GLP-1 to promote insulin secretion in a glucose-dependent manner has been harnessed for treatment of T2DM, with GLP-1 receptor agonists resistant to DPP-4 (exenatide, Byetta®; liraglutide, Victoza®), and DPP-4 inhibitors (e.g. sitagliptin, vildagliptin) now widely used for effective glycaemic control. Interestingly, it is well recognized that GLP-1 exerts wide-ranging extra-pancreatic actions occurring independently of its established metabolic effects. Indeed, GLP-1 signalling is reported to play several important roles in the CVS in both health and disease (Grieve et al., 2009), although it appears that the GLP-1 receptor may not be as widely expressed as previously thought. For example, recent work suggests that cardiac GLP-1 receptor expression may be localized to atrial tissue, sinoatrial node and vasculature, with some species variation (Kim et al., 2013; Pyke et al., 2014; Richards et al., 2014), and that earlier reports of more ubiquitous expression may be questionable because of poor antibody selectivity and sensitivity (Panjwani et al., 2012). Nonetheless, it is clear that GLP-1 exerts important cardiovascular actions, although it is only recently that its effects in the setting of diabetes, a condition synonymous with micro/macrovascular complications, have been explored. This is clearly important because of the large number of patients receiving GLP-1-based therapies, in which its cardiovascular actions are largely unknown. This review will therefore discuss the current understanding of the effects of GLP-1 on both cardiovascular risk factors in diabetes and direct actions on the heart/vasculature in this setting and the evidence implicating specific targeting of GLP-1 as a novel therapy for CVD in diabetes, with a primary focus on the role of GLP-1 receptor agonists. More detailed discussion of the pleiotropic actions of DPP-4 inhibitors in this setting is provided by recent review articles specifically focused on this important aspect of cardiovascular GLP-1 signalling (Scheen, 2013; Aroor et al., 2014).
Influence of GLP-1 on cardiovascular risk factors in diabetes
BP and hypertension
Increased BP is an established risk factor for CVD in both normoglycaemia and T2DM (Turner et al., 1998; Vasan et al., 2001). Notably, therapeutic reductions in BP and circulating glucose have an additive effect in decreasing cardiovascular complications in T2DM patients, as highlighted by the UKPDS (Stratton et al., 2006). Indeed, in an experimental setting, chronic GLP-1 infusion inhibits development of hypertension in Dahl salt-sensitive rats, as well as reducing cardiac fibrosis and hypertrophy, effects which appear to occur via a natriuretic/diuretic mechanism independently of blood glucose (Yu et al., 2003), suggesting that GLP-1 may confer additional benefits which could be harnessed for the treatment of hypertension associated with T2DM. Consistent with an indirect BP-lowering effect, it was recently reported that liraglutide-stimulated reduction of angiotensin II-induced hypertension in mice was blocked by the natriuretic peptide receptor antagonist, anantin, in a GLP-1 receptor-dependent manner, but unaltered by the NOS inhibitor, NG-monomethyl-L-arginine, and that liraglutide induced rapid increases in atrial natriuretic peptide (ANP) secretion both in vivo and in isolated perfused hearts, suggesting that observed BP reduction occurred at least partly via direct activation of cardiac ANP (Kim et al., 2013). Importantly, in the context of diabetes, the GLP-1 mimetic, exendin-4, inhibited development of both spontaneous and high salt-induced hypertension in obese db/db mice via beneficial actions on renal sodium handling (Hirata et al., 2009). Furthermore, it was recently reported that treatment of insulin-resistant Zucker rats with the DPP-4 inhibitor, linagliptin, for 8 weeks reduced BP and improved diastolic function (Aroor et al., 2013).
Interestingly, although chronic administration of GLP-1 may prevent development of hypertension, it is widely reported that acute GLP-1 exposure is associated with increased BP and heart rate, which predisposes to CVD. For example, acute infusion of GLP-1(7-36) increased systolic/diastolic BP and heart rate in both normal and insulin-deficient streptozotocin (STZ)-induced T1DM rats (Barragán et al., 1994), with the same group reporting that exendin-4-induced increases in BP and heart rate were reversed by the GLP-1 receptor antagonist, exendin(9-39) (Barragán et al., 1996), suggesting that these effects occurred via an insulin-independent mechanism but involving GLP-1 receptor activation. Although similar increases in BP and heart rate after short-term GLP-1 administration have been reported in various experimental models (Grieve et al., 2009), the data from clinical studies are less clear. For example, GLP-1 infusion in a small number of T2DM patients with or without CAD for 105 min and 48 h, respectively, had no effect on heart rate or systolic/diastolic BP (Toft-Nielsen et al., 1999; Nyström et al., 2004), whereas 48 h GLP-1 infusion in patients with ischaemic heart failure (Halbirk et al., 2010) and treatment of T2DM patients with an exendin-transferrin fusion protein or exenatide for 7 or 10 days, respectively (Kothare et al., 2008; Gustavson et al., 2011), resulted in elevated heart rate and diastolic BP. However, the data from longer-term GLP-1 clinical trials are more consistent, with the majority reporting decreased BP and minimal effects on heart rate. For example, the Liraglutide Effect and Action in Diabetes (LEAD)-4 study, investigating 26 week liraglutide treatment in combination with metformin in T2DM patients, reported a modest reduction in systolic BP of 6 mmHg compared with placebo (1.1 mmHg) (Zinman et al., 2009). Similarly, the LEAD-2 study, assessing liraglutide combination therapy, reported a 2–3 mmHg decrease in systolic BP versus a small increase (0.4 mmHg) in the glimepiride control group (Nauck et al., 2009). Furthermore, a 30 week trial comparing once-weekly versus twice-daily exenatide injection in drug-naïve T2DM patients observed a significant decrease in systolic/diastolic BP compared with baseline (Drucker et al., 2008), while another 20 week trial in obese patients reported reduced systolic/diastolic BP in response to liraglutide, which persisted for the 2 year follow-up period (Astrup et al., 2012). Interestingly, meta-analysis of six clinical trials comprising 2171 T2DM patients found that exenatide treatment for 6 months produced maximal systolic BP reduction in individuals with abnormally high baseline levels, whereas no effects were observed in normotensive subjects (Okerson et al., 2010). It should be noted that BP reduction is positively correlated with weight loss (Neter et al., 2003), so it is possible that the observed changes after chronic GLP-1 treatment may occur secondary to its metabolic effects. However, although beneficial effects of GLP-1 on body weight are associated with improved hypertension, it is clear that this cannot solely account for its vascular effects as several studies have reported a BP reduction prior to weight loss. For example, a combined meta-analysis of three 26 week liraglutide trials reported decreased BP after only 2 weeks, while maximal weight loss did not occur until 8 weeks (Gallwitz et al., 2010). Indeed, heart rate, which is not linked to body weight, was increased by chronic administration of both liraglutide and exenatide in T2DM patients in parallel with reduced systolic BP (Garber et al., 2009; Gill et al., 2010). Interestingly, it was recently reported that GLP-1 secretory function increases with age and is negatively correlated with systolic BP, suggesting that this may represent an adaptive response (Yoshihara et al., 2013).
Dyslipidaemia
The pathophysiology of diabetes is commonly considered largely in terms of associated hyperglycaemia. However, it is increasingly apparent that dyslipidaemia is equally important and represents a significant risk factor for CVD in diabetic patients (Reiner et al., 2011). It is likely that impaired insulin sensitivity contributes to dyslipidaemia in T2DM, which is associated with reduced GLP-1 secretion. Indeed, in addition to their established glycaemic actions, GLP-1 receptor activation and DPP-4 inhibition are reported to improve lipid profiles in both experimental and clinical diabetes. For example, short-term infusion of GLP-1 in normoglycaemic Syrian golden hamsters decreased lipid absorption and triglyceride levels, an effect potentiated by oral glucose (Hein et al., 2013), suggesting that its incretin action may inhibit intestinal production of chylomicrons, which are strongly linked to atherosclerosis (Nakano et al., 2008). Similarly, circulating triglycerides and fat pad mass in rats with diet-induced obesity were reduced after 4 week treatment with liraglutide (Madsen et al., 2010), while 40 day administration of exendin-4 ameliorated systemic and cardiac insulin resistance and dyslipidaemia in both genetic KKAy and diet-induced T2DM mouse models (Monji et al., 2013). Furthermore, chronic GLP-1 receptor activation with both the GLP-1 analogue, CNTO3649, and exendin-4 in apolipoprotein E3-Leiden transgenic mice, which develop severe hypercholesterolaemia after high-fat feeding, resulted in reduced very-low-density lipoprotein (VLDL)-triglyceride and apolipoprotein B synthesis in parallel with decreased hepatic triglyceride, cholesterol and phospholipids and lipogenesis gene expression (Parlevliet et al., 2012). The long-acting DPP-4 inhibitor, teneligliptin, is also reported to decrease circulating triglyceride and free fatty acid levels in insulin-resistant Zucker fatty rats after 2 week treatment (Fukuda-Tsuru et al., 2012).
Importantly, the majority of clinical studies have also demonstrated beneficial outcomes of GLP-1 administration on lipid metabolism in T2DM, such as reduced circulating triglycerides and low-density lipoprotein (LDL) cholesterol (Flock et al., 2007; Drucker et al., 2008; Tremblay et al., 2011), although one study in which patients were treated with exenatide for 24 weeks reported a similar plasma lipid profile versus controls (Moretto et al., 2008). For example, decreased circulating levels of atherogenic triglyceride-rich lipoproteins were observed following 4 week vildagliptin monotherapy in drug-naïve T2DM patients, characterized by specific reductions in total plasma and chylomicron triglycerides, together with apolipoprotein B-48 and cholesterol in the chylomicron subfraction (Matikainen et al., 2006). Furthermore, the LEAD-4 study found that 26 week liraglutide treatment in combination with metformin and rosiglitazone decreased circulating LDL cholesterol, triglycerides and free fatty acids in T2DM patients compared with placebo controls, although it is interesting to note that these changes were greater in response to low-dose treatment (Zinman et al., 2009). Indeed, similar results are reported for DPP-4 inhibitors, which produce much lower circulating levels of GLP-1. For example, twice-daily sitagliptin led to a significant reduction in circulating triglycerides and free fatty acids in a large number of T2DM patients compared with placebo and glipizide control groups, despite similar decreases in fasting plasma glucose and HbA1c levels (Scott et al., 2007). Interestingly, a single injection of exenatide was shown to attenuate postprandial increases in triglycerides, apolipoprotein B-48 and CIII/remnant lipoprotein cholesterol for up to 8 h in patients with impaired glucose tolerance and recent-onset T2DM (Schwartz et al., 2010), suggesting that such lipid profile benefits may not be explained solely by chronic changes in body weight, glucose levels and insulin resistance. Indeed, it was recently reported that exendin-4 completely reverses hepatic steatosis in mice fed a high-fat diet via a GLP-1 receptor-dependent mechanism resulting in reduced numbers/size of circulating VLDL-triglyceride and VLDL-apolipoprotein B particles (Parlevliet et al., 2012), suggesting that GLP-1 may exert direct effects on dyslipidaemia in diabetes.
Obesity
Although it is well known that obesity significantly increases the risk of T2DM (Willett et al., 1999), and both are independent risk factors for CVD (Hubert et al., 1983), many established diabetes therapies, including sulfonylureas and thiazolidinediones, may increase body weight. However, GLP-1 reduces body weight because of beneficial effects on glucagon secretion, gastric emptying and satiety (Kreymann et al., 1987; Flint et al., 1998; Näslund et al., 1998a), so it seems likely that impaired GLP-1 secretion observed in non-diabetic obese individuals (Holst et al., 1982; Näslund et al., 1998b) may at least partly account for their increased body weight. Indeed, weight loss improves the postprandial GLP-1 response in severely obese patients (Verdich et al., 2001), suggesting that the two are interlinked. Furthermore, a 20 week treatment with liraglutide was reported to cause significant weight loss in obese individuals and to reduce the incidence of prediabetes (Astrup et al., 2009), confirming an apparent role for GLP-1 in weight control which may be harnessed for therapeutic benefit. This assertion is supported by the LEAD trials which have consistently reported a reduction in body weight in T2DM patients following liraglutide treatment (Moretto et al., 2008; Buse et al., 2009; Garber et al., 2009; Nauck et al., 2009; 2013,; Zinman et al., 2009). For example, in the LEAD-2 trial, 26 week combination therapy of liraglutide with metformin in T2DM patients resulted in increased weight loss compared with metformin alone (Nauck et al., 2013). Importantly, weight loss associated with both liraglutide and exenatide treatment is reported to be linked to improved cardiovascular risk factors, such as HbA1c and BP, and to persist for at least 2 years (Klonoff et al., 2007; Astrup et al., 2009; 2012,), highlighting important benefits of GLP-1 which may not be related to its insulinotropic actions. The long-term effects of GLP-1 on weight loss may be particularly important as conventional weight loss is typically poorly maintained in T2DM patients. Despite GLP-1 receptor agonists promoting weight loss in both diabetic and non-diabetic obese subjects, DPP-4 inhibitors appear to be weight-neutral (Amori et al., 2007), suggesting that GLP-1 receptor agonists may exert direct gastrointestinal effects in addition to improving insulin resistance (Rask et al., 2001), although this could simply be due to differences in circulating GLP-1 levels. However, postprandial GLP-1 levels are reported to be increased immediately after gastric bypass surgery, despite patients remaining obese, indicating that GLP-1 may regulate appetite and food intake directly (Morinigo et al., 2006). Indeed, in T2DM patients, GLP-1 promotes satiety, thereby reducing energy consumption (Gutzwiller et al., 1999), while in healthy individuals i.v. administration of GLP-1(7-36) slows gastric emptying in a dose-dependent manner (Nauck et al., 1997).
GLP-1 and vascular disease
Vascular function
Impaired endothelial and vascular function are established as key initiating factors underlying the development of microvascular and macrovascular complications associated with diabetes. Indeed, it has been known for some time that native GLP-1(7-36) induces ex vivo dose-dependent vasodilatation in a number of isolated rodent vessels, including aorta (Golpon et al., 2001; Green et al., 2008), pulmonary artery (Richter et al., 1993; Golpon et al., 2001), femoral artery (Nyström et al., 2005) and mesenteric artery (Ban et al., 2008), although several different mechanisms have been proposed. For example, some studies indicate that the vasorelaxant actions of GLP-1 are dependent upon endothelium-derived NO (Golpon et al., 2001; Ban et al., 2008; Gaspari et al., 2011), whereas others have proposed endothelium-independent mechanisms involving mediators such as KATP channels, cAMP and β2-adrenoceptor activation (Nyström et al., 2005; Gardiner et al., 2008; Green et al., 2008). Interestingly, although several studies suggest that the vascular actions of GLP-1 are dependent upon the GLP-1 receptor (Gaspari et al., 2011; Chai et al., 2012), it appears that they may also be mediated, at least partly, by its truncated metabolite, GLP-1(9-36), which induces dose-dependent relaxation in both isolated mouse mesenteric artery (Ban et al., 2008) and rat aorta (Green et al., 2008). It should be noted that although the synthetic GLP-1 mimetic, exendin-4, exerts similar actions in rat aorta (Golpon et al., 2001; Green et al., 2008), they are of reduced magnitude compared with GLP-1(7-36) and are absent in mouse mesenteric artery (Ban et al., 2008). Importantly, the vasorelaxant actions of GLP-1 are also reported in vivo. For example, systemic administration of GLP-1(7-36) by both bolus dose and short-term infusion in rats induced hindquarters vasodilatation (Gardiner et al., 2010). Interestingly, however, GLP-1 promoted vasoconstriction in both mesenteric and renal arteries, while exendin-4 exerted similar vasoconstriction in mesenteric artery but induced vasodilatation in both hindquarters and renal artery (Gardiner et al., 2008), suggesting differential vascular effects. Indeed, a similar study demonstrated that GLP-1(7-36) infusion acutely increased muscle microvascular blood volume in the absence of changes in microvascular blood flow velocity or femoral blood flow, in association with increased plasma NO, muscle insulin clearance/uptake, hindlimb glucose extraction and muscle interstitial oxygen saturation (Chai et al., 2012). It should be noted that in contrast to the ex vivo studies, GLP-1(9-36) failed to modulate vascular function in rats in vivo when given as either a bolus dose or via short-term infusion, which together with the fact that DPP-4 inhibitors prolonged the vascular actions of native GLP-1(7-36) in this setting (Gardiner et al., 2010), suggest that the actions of this ‘inactive’ metabolite may not be significant in vivo.
Importantly, it appears that the vascular effects of GLP-1 are also evident in the setting of diabetes, where they are reported to promote beneficial actions. Chronic treatment of STZ/nicotinamide T1DM rats with either GLP-1(7-36) or exendin-4 was shown to prevent endothelial dysfunction in parallel with reduction of blood glucose (Özyazgan et al., 2005), effects which may be mediated via activation of endothelial NOS (eNOS) (Goyal et al., 2010). Although similar studies have not been performed in the setting of overt T2DM, it was recently reported that chronic treatment of insulin-resistant Zucker rats with the DPP-4 inhibitor, linagliptin, resulted in reduced hypertension in parallel with increased expression of total/phosphorylated eNOS (Aroor et al., 2013). Furthermore, high-fat fed apolipoprotein E-deficient mice treated with a different DPP-4 inhibitor, des-fluoro-sitagliptin, demonstrated attenuation of endothelial dysfunction in parallel with eNOS activation and improved glucose tolerance (Matsubara et al., 2012). Interestingly, however, endothelial dysfunction in rat femoral artery induced by short-term triglyceride exposure was not affected by exendin-4 (Nathanson et al., 2009), suggesting that the reported in vivo protective actions may occur via indirect mechanisms. In this regard, it is important to note that the vascular actions of GLP-1 in diabetes are likely to occur, at least partly, secondary to stimulation of insulin, which induces vascular relaxation via Ca2+-dependent activation of eNOS (Han et al., 1995; Kahn et al., 1998).
In addition to the data supporting important vascular actions of GLP-1 in experimental diabetes, several studies have reported beneficial functional effects in the clinical setting. For example, in T2DM patients with stable CAD, acute GLP-1 administration improved brachial artery flow-mediated vasodilatation, an effect not observed in healthy individuals (Nyström et al., 2004). Comparable effects were observed in insulin-resistant patients with obesity-related metabolic syndrome, where acute treatment with GLP-1(7-36) enhanced insulin-mediated forearm blood flow responses to both ACh and sodium nitroprusside in the absence of changes in forearm glucose extraction/uptake, while GLP-1(9-36) did not affect vascular function (Tesauro et al., 2013). Similarly, in patients with T1DM, brachial artery endothelial dysfunction induced by acute blood glucose modulation was counteracted by simultaneous infusion of GLP-1 (Ceriello et al., 2013). Interestingly, GLP-1-induced enhancement of endothelium-dependent peripheral vasodilatation observed in non-diabetic individuals is differentially modulated by sulphonylureas, with glyburide abolishing GLP-1-induced ACh-mediated responses which are unaltered by glimepiride (Basu et al., 2007). Furthermore exenatide, which is commonly used for hyperglycaemic control in T2DM, also increased postprandial endothelial function assessed by peripheral arterial tonometry in patients with recent-onset disease when given as a single dose, largely secondary to a reduction in circulating triglycerides (Koska et al., 2010), although chronic treatment for 3 months in obese prediabetic patients had no additional effect when compared with those receiving metformin (Kelly et al., 2012). While it appears that clinical GLP-1 administration exerts acute vascular effects in T2DM, data on its chronic actions in this setting are variable. T2DM patients who received exenatide for a period of 4 months as an adjunct to standard metformin therapy demonstrated improved brachial artery flow-mediated dilatation, indicated by elevated peak dilatation and shear rate which are reflective of improved macrovascular and microvascular function respectively (Irace et al., 2013). Notably, enhanced vasodilatation in exenatide-treated patients in this study was significantly greater than that observed in those receiving glimepiride as an add-on to metformin therapy. Furthermore, liraglutide treatment for 12 weeks in T2DM patients well controlled on metformin monotherapy resulted in an improvement in both circulating markers of vascular function [asymmetric dimethylarginine, plasminogen activator inhibitor-1 (PAI-1), E-selectin] and retinal microvascular endothelial function (Forst et al., 2012). However, a similar study in a small number of severely obese T2DM patients chronically treated with GLP-1 receptor agonists for 6 months reported that neither exenatide or liraglutide had any effect on brachial artery endothelial-dependent flow-mediated dilation (Hopkins et al., 2013), indicating potential for other confounding factors in this setting which may need to be considered. Furthermore, 6 week treatment with sitagliptin or alogliptin significantly reduced flow-mediated dilatation in male T2DM patients (Ayaori et al., 2013), suggesting that chronically increased physiological levels of GLP-1 may exert unfavourable vascular actions, although it is possible that this could be a class-specific effect of DPP-4 inhibitors warranting further investigation.
Inflammation and atherosclerosis
It is well known that the incidence and progression of endothelial dysfunction is exacerbated in T2DM, secondary to established risk factors, such as insulin resistance, dyslipidaemia and hyperglycaemia. Endothelial dysfunction in this setting is characterized by an elevation in circulating adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), and an increased propensity to develop atherosclerosis which is typified by inflammatory cell infiltration and plaque formation (Van Gaal et al., 2006). Interestingly, several recent clinical and experimental studies appear to indicate that GLP-1 exerts both anti-inflammatory and anti-atherogenic actions. For example, GLP-1 treatment in T2DM patients is associated with beneficial effects on a number of established CVD biomarkers, including high sensitivity C-reactive protein (hs-CRP) and PAI-1, which are important in atherosclerosis development (Haffner, 2006). Similarly, 14 week treatment of T2DM patients with liraglutide resulted in significantly reduced PAI-1 levels, and a dose-dependent decrease in plasma hs-CRP levels (Vilsbøll et al., 2007; Courrèges et al., 2008), an effect that was also observed after 26 week treatment with exenatide (Bergenstal et al., 2010) and was over and above that seen in patients treated with insulin glargine (Diamant et al., 2010). Importantly, the beneficial effects of exenatide on circulating hs-CRP appear to persist at 1 year treatment in T2DM patients receiving standard metformin therapy, resulting in reduced levels of both hs-CRP and leptin (Bunck et al., 2010). DPP-4 inhibitors seem to exert similar effects as T2DM patients receiving sitagliptin for 6 months also demonstrated significant reductions in plasma hs-CRP, together with VCAM-1 and associated albuminuria, which may attenuate glucose excursion and inhibit vascular inflammation (Horváth et al., 2009; Hattori, 2010). Interestingly, a recent study demonstrated that cessation of exenatide treatment resulted in the reversal of benefits on circulating hs-CRP within 6 months (Varanasi et al., 2011). It should be noted that although these studies support the suggestion that GLP-1 may protect against inflammation and atherosclerosis in the clinical setting, it is not possible to draw clear conclusions because of the absence of longer-term studies specifically assessing effects on disease development. Interestingly, a recent study has reported a positive correlation between circulating GLP-1 levels and CAD in both diabetic and non-diabetic patients undergoing angiography because of typical or atypical chest pain, highlighting the possibility that GLP-1 may exert detrimental effects in this setting (Piotrowski et al., 2013).
Nonetheless, the majority of clinical data are broadly supportive of the anti-inflammatory actions of GLP-1, which persist for up to 12 weeks in obese T2DM patients after a single exenatide injection (Chaudhuri et al., 2011). Indeed, in this study, GLP-1 was associated with a specific reduction of several inflammatory mediators, including TNF-α, toll-like receptor-2 (TLR-2) and TLR-4, in parallel with suppression of NF-κB signalling and MMP-9 activity, which are key initiating factors of atherosclerosis. Furthermore, in obese T2DM patients, 8 week liraglutide treatment is reported to decrease levels of the inflammatory macrophage activation molecule, sCD163, and pro-inflammatory cytokines, TNF-α and IL-6, while increasing levels of the anti-inflammatory adipokine, adiponectin (Hogan et al., 2014). Importantly, these clinical observations are supported by a number of experimental studies which have specifically assessed the effects of GLP-1 on development and progression of atherosclerosis. For example, continuous infusion of exendin-4 in both wild-type and apolipoprotein E-deficient normoglycaemic mice was reported to decrease monocyte adhesion and development of atherosclerotic lesions in thoracic aorta, effects proposed to occur via cAMP/PKA-dependent suppression of inflammation (Arakawa et al., 2010). These findings were confirmed by a different group who demonstrated reduced aortic macrophage recruitment, foam cell formation and atherosclerotic lesion development in apolipoprotein E-deficient mice after GLP-1 infusion (Nagashima et al., 2011). Furthermore, chemokine-induced migration of CD4+ lymphocytes is inhibited by both GLP-1(7-36) and exendin-4 in a GLP-1 receptor-dependent manner (Marx et al., 2010), while liraglutide suppresses NF-κB signalling in HUVECs and THP-1 monocyte adhesion in human aortic endothelial cells via downstream activation of several proinflammatory and cell adhesion molecules, including TNF-α, VCAM-1 and E-selectin (Shiraki et al., 2012; Krasner et al., 2014). Indeed, liraglutide inhibits TNF-α in human vascular endothelial cells and reduces hyperglycaemia-mediated PAI-1, ICAM-1 and VCAM-1 activation, which is associated with endothelial dysfunction and accelerated atherogenesis in T2DM (Liu et al., 2009). Furthermore, the DPP-4 inhibitor, des-fluoro-sitagliptin, is reported to exert cAMP-dependent anti-inflammatory actions in cultured human macrophages by increasing GLP-1 levels and to reduce atherosclerotic lesion formation in apolipoprotein E-deficient mice (Matsubara et al., 2012), while alogliptin inhibits vascular monocyte/macrophage recruitment and reduces atherosclerotic burden in high-fat diet-fed, LDL receptor-deficient mice in association with improvement of metabolic indices (Shah et al., 2011). Taken together, these experimental data clearly support an important role for GLP-1 in protecting against vascular inflammation and atherogenesis, effects which are borne out by the reported clinical benefits of GLP-1 treatment on circulating inflammatory mediators and CVD biomarkers. However, it is evident that long-term studies specifically investigating effects on atherosclerotic disease development and progression are required to ascertain whether the apparent protective effects of GLP-1 under both normoglycaemic and diabetic conditions may translate to the clinical setting.
Angiogenesis
Abnormal angiogenesis is a hallmark of CVD which is exacerbated by diabetes, with impaired neovascularization contributing significantly to progression of ischaemic disease associated with peripheral and coronary arteries. Interestingly, it is becoming apparent that GLP-1 may modulate angiogenesis suggesting that such actions may underlie some of its reported beneficial cardiovascular effects. For example, exendin-4 stimulates proliferation of human coronary artery endothelial cells in a GLP-1 receptor-dependent manner via downstream activation of eNOS, PKA and PI3K/Akt signalling (Erdogdu et al., 2010) and promotes in vitro HUVEC migration, ex vivo aortic sprouting angiogenesis and in vivo blood vessel formation in Matrigel plugs (Kang et al., 2013), while native GLP-1(7-36) stimulates in vitro angiogenesis in HUVECs via Akt, Src and PKC-dependent pathways (Aronis et al., 2013), suggesting that GLP-1 may directly modulate neovascularization. Importantly, these effects appear to translate to the pathological situation, with several recent studies reporting that GLP-1 can promote the pro-angiogenic actions of mesenchymal stem cells in different disease settings. Intracoronary artery delivery of GLP-1 eluting encapsulated human mesenchymal stem cells in a porcine model of experimental myocardial infarction (MI) resulted in improved left ventricular function and remodelling which was associated with increased infarct zone angiogenesis (Wright et al., 2012), while peri-adventitial treatment of porcine vein grafts with these cells inhibited neointima formation in parallel with accelerated adventitial angiogenesis (Huang et al., 2013). Furthermore, the addition of GLP-1 to encapsulated human mesenchymal cells significantly improved blood flow recovery and foot salvage in a mouse model of hindlimb ischaemia via increased capillary and arteriole formation secondary to paracrine activation of VEGF-A (Katare et al., 2013). Although the majority of work investigating the pro-angiogenic actions of GLP-1 has been performed in normoglycaemic models, a recent study reported similar beneficial effects in the setting of diabetes. Impaired myocardial angiogenesis in STZ-treated T1DM rats and associated fibrosis and diastolic dysfunction were reversed by genetic deletion of DPP-4 or pharmacological inhibition with vildagliptin (Shigeta et al., 2012). Interestingly, this study identified DPP-4 as being membrane-bound and localized to the cardiac capillary endothelium with increased expression in diabetes, which together with a report of increased binding affinity of GLP-1 to the coronary endothelium but not cardiomyocytes in isolated perfused T1DM rat hearts (Barakat et al., 2011), supports a key endothelial-specific role of GLP-1 in this setting. Although these data provide supportive evidence for pro-angiogenic actions of GLP-1 in diabetes, it is clear that additional mechanistic studies are required using different CVD models in order to define its precise role. Furthermore, it is important to assess the effects of GLP-1 therapy in diabetic patients in order to investigate whether the apparent pro-angiogenic effects of GLP-1 translate to the clinical setting and are of functional relevance.
GLP-1 and the diabetic myocardium
The heart is one of the major organ targets of GLP-1 and an increasing number of studies have investigated the actions of native GLP-1(7-36), GLP-1 receptor agonists and DPP-4 inhibitors in the context of cardioprotection. The majority of experimental studies have focused on the effects of GLP-1 in cardiac ischaemia and its apparent ability to protect against acute myocardial damage. Indeed, it is well established that GLP-1 pretreatment and chronic DPP-4 inhibition reduce infarct size after experimental ischaemia in both small and large animal models, which is associated with increased survival and improved cardiac function (Bose et al., 2005; Ban et al., 2008; 2010,; Timmers et al., 2009). Interestingly, a recent study employing a rabbit model of ischaemia/reperfusion injury reported protective actions of transferrin-stabilized GLP-1, when given both 12 h prior to ischaemia and immediately upon reperfusion, suggesting that GLP-1 may limit infarct size and contractile dysfunction directly, rather than by preconditioning the heart against ischaemia, as suggested by previous reports (Matsubara et al., 2011). In addition to its established beneficial actions against acute ischaemic myocardial damage, GLP-1 also confers protection against contractile dysfunction associated with experimental chronic post-MI remodelling, dilated cardiomyopathy and hypertensive heart failure (Nikolaidis et al., 2004a; Poornima et al., 2008; Liu et al., 2010), with similar results reported in the clinical setting in response to short-term GLP-1 treatment (Nikolaidis et al., 2004b; Sokos et al., 2006).
Until recently, only limited data were available on the cardiac actions of GLP-1 in diabetes, but it is becoming increasingly apparent that GLP-1 also plays a key cardioprotective role in this setting. This is important as it is well known that hyperglycaemia is associated with increased susceptibility to cardiac disease and poor outcomes in both humans and experimental models (Shiomi et al., 2003; Liu et al., 2005; Greer et al., 2006; Vergès et al., 2007). Chronic DPP-4 inhibition with linagliptin improves obesity-related diastolic dysfunction in insulin-resistant Zucker rats, but has no effect on cardiomyocyte hypertrophy and fibrosis (Aroor et al., 2013). Indeed, exendin-4 has been reported to directly protect isolated rat cardiomyocytes from high glucose-induced apoptosis via inhibition of endoplasmic reticulum stress and activation of sarcoplasmic reticulum Ca2+ ATPase 2a (Younce et al., 2013). GLP-1 also appears to protect against diabetic cardiomyopathy, which is defined as cardiac dysfunction in the absence of, or disproportionate to, associated hypertension and CAD and is characterized by marked collagen accumulation and impaired diastolic function (Bugger and Abel, 2014). Both GLP-1 receptor activation and DPP-4 inhibition attenuate development of cardiac dysfunction, extracellular matrix remodelling, cardiomyocyte hypertrophy and apoptosis in experimental models of T1DM and T2DM, with various mechanisms proposed including reduction of lipid accumulation, oxidative stress and myocardial inflammation, and modulation of the MMP-2/tissue inhibitor of MMP-2 axis, endoplasmic reticulum stress and microvascular barrier function (Shigeta et al., 2012; Liu et al., 2013; Monji et al., 2013; Picatoste et al., 2013; Wang et al., 2013). Furthermore, it appears that GLP-1 also confers infarct-reducing actions in diabetes, which is associated with increased susceptibility to myocardial ischaemia. For example, mice made diabetic by a combination of STZ injection and high-fat feeding and treated with the GLP-1 receptor agonist, liraglutide, prior to coronary artery ligation, demonstrated reduced infarct development and improved survival compared with those treated with the glucose-lowering drug, metformin, suggesting that the observed effects occurred via direct actions on the heart and not secondary to reduced blood glucose (Noyan-Ashraf et al., 2009). Similar cardioprotective effects have been reported with DPP-4 inhibition in experimental diet-induced obesity (Huisamen et al., 2013), while the infarct-limiting effects of exendin-4 in mice with T2DM were shown to be mediated by cAMP-induced PKA activation (Ye et al., 2013). Interestingly, it has recently been suggested that the infarct-reducing actions of DPP-4 inhibitors may be glucose-dependent, as both sitagliptin and vildagliptin were found to only decrease infarct size in isolated rat hearts subjected to ischaemia-reperfusion injury when they were perfused with elevated glucose concentrations ≥7 mmol L−1, with similar results observed in vivo in diabetic, but not normoglycaemic rats (Hausenloy et al., 2013). This raises the intriguing possibility that glucose-lowering may counteract the cardioprotective actions of GLP-1 and explain why several large-scale clinical trials focused on intensive glucose control in T2DM have failed to demonstrate significant cardiovascular benefits (Giorgino et al., 2013). Furthermore, it appears that at least part of the observed beneficial actions of DPP-4 inhibitors against ischaemia-reperfusion injury may be mediated by the chemokine, stromal cell-derived factor 1α in a GLP-1-independent manner (Bromage et al., 2014).
In addition to the experimental data highlighting a protective role for GLP-1 in the diabetic heart, importantly, a small number of studies have assessed its cardiac actions in patients with diabetes. It has been known for some time that short-term GLP-1 treatment exerts beneficial effects in clinical heart failure in both normoglycaemic and diabetic patients. For example, in a small number of heart failure patients (New York Heart Association class III/IV), 5 week infusion with GLP-1 plus standard therapy improved left ventricular ejection fraction and myocardial oxygen consumption compared with those receiving standard therapy alone, effects that were seen in both diabetic and non-diabetic patients (Sokos et al., 2006). Furthermore, a small non-randomized trial of 72 h GLP-1 infusion following primary angioplasty after acute MI led to improved cardiac function in both non-diabetic and diabetic patients which was still evident upon 120 day follow-up (Nikolaidis et al., 2004b). More recently, a larger randomized trial in patients presenting with ST-segment elevation MI reported that exenatide infusion for 15 min prior to primary angioplasty continued until 6 h post-reperfusion resulted in improved myocardial salvage at 3 months although no functional benefits were observed (Lønborg et al., 2012). Indeed, two current clinical trials are assessing the potential of using exenatide as a post-conditioning agent to reduce reperfusion injury following percutaneous coronary intervention (Effect of Additional Treatment With EXenatide in Patients With an Acute Myocardial Infarction, the EXAMI trial, NCT01254123; Pharmacological Postconditioning to Reduce Infarct Size Following Primary PCI, POSTCON II, NCT00835848). Interestingly, in patients with left ventricular diastolic dysfunction, DPP-4 activity in the coronary sinus and peripheral circulation is reported to be negatively correlated with diastolic function and increased by co-morbid diabetes (Shigeta et al., 2012), suggesting that reduced GLP-1 levels in diabetes may underlie the associated cardiac dysfunction. Exenatide has also been found to modulate myocardial glucose transport and uptake in T2DM patients dependent upon the degree of insulin resistance (Gejl et al., 2012), although a similar study reported that GLP-1-induced increases in resting myocardial glucose uptake in lean individuals were absent in obese T2DM patients, with parallel studies in pigs suggesting that this was due to impaired p38-MAPK signalling (Moberly et al., 2013). Interestingly, a recent experimental study found that exendin-4 reduced contractile function and was unable to stimulate glucose utilization in normal rat hearts in the presence of fatty acids (Nguyen et al., 2013), despite previous reports of increased myocardial glucose uptake in response to GLP-1 in experimental myocardial ischaemia and dilated cardiomyopathy (Nikolaidis et al., 2005; Zhao et al., 2006; Bhashyam et al., 2010). Such findings highlight the need for detailed investigation of the effects of GLP-1 on altered myocardial metabolism in diabetic patients both with and without cardiac complications, in which the effects of GLP-1 may diverge.
Although these clinical and experimental data are clearly supportive of an important role for GLP-1 signalling in the diabetic heart, they are largely descriptive with limited focus on underlying mechanisms. Previous studies in experimental models of heart failure have highlighted several pathways which may mediate the cardioprotective effects of GLP-1, including cAMP/PKA, PI3K/Akt, p44/p42MAPK, ERK1/2 (Bose et al., 2005; Timmers et al., 2009; Ravassa et al., 2011), together with suggestions of GLP-1 receptor-independent signalling (Nikolaidis et al., 2005; Sonne et al., 2008). However, the precise mechanisms underlying the apparent protective actions of GLP-1 in the diabetic heart, in which GLP-1 signalling is likely to be different, remain unknown and clearly need to be defined in order to fully assess the therapeutic potential of GLP-1 in this setting.
Summary and future perspective
Over recent years, it has become clear that GLP-1 exerts important actions on the CVS in both health and disease in addition to its prototypic effects on glycaemic control (Grieve et al., 2009) and also confers beneficial effects on the cardiovascular risk profile in diabetic patients. However, emerging evidence now strongly suggests that GLP-1 exerts specific cardiovascular actions in diabetes and may attenuate the development and progression of associated cardiovascular complications (summarized in Figure 1), although the precise mechanisms are yet to be established with several candidate pathways proposed (see Figure 2). Nonetheless, it is important to note that the majority of data pointing towards such beneficial effects are largely experimental and that equivalent information on the cardiovascular actions of GLP-1 in the clinical setting of diabetes is somewhat lacking, particularly in relation to its chronic effects. In this regard, the first large-scale GLP-1 clinical trials to assess cardiovascular outcomes after long-term treatment (SAVOR-TIMI 53, EXAMINE) have recently reported that chronic DPP-4 inhibition with either saxagliptin or apogliptin had no significant effects on their primary composite end points of cardiovascular death, or non-fatal MI/stroke (Scirica et al., 2013; White et al., 2013), although it should be noted that SAVOR-TIMI 53 reported a 27% increase in hospitalization for heart failure. However, until the results of several ongoing clinical trials investigating the chronic cardiovascular effects of GLP-1 receptor agonists are known (summarized in Table 1 together with further DPP-4 inhibitor trials), in which circulating GLP-1 levels will be much higher than those achieved using DPP-4 inhibitors, no conclusions can be drawn on either the potential clinical cardiovascular benefits or safety of GLP-1-based therapies in diabetes. Nonetheless, the apparent complexity of cardiovascular GLP-1 signalling under both normal and diabetic conditions clearly suggests that it is likely that selective targeting of specific aspects of CVD may be required in order to realize the optimal benefits of GLP-1 targeting in this setting.
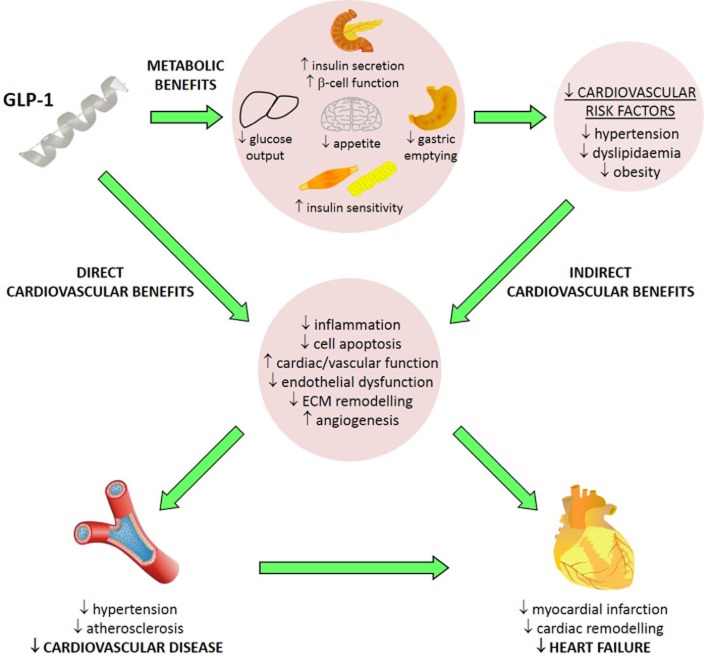
Figure 1.
Summary of the cardiovascular actions of GLP-1 in diabetes. GLP-1 exerts indirect cardiovascular benefits in diabetes secondary to its established metabolic actions and subsequent reduction of cardiovascular risk factors. In addition, GLP-1 promotes direct cardiovascular benefits which confer protection against CVD and heart failure, the latter of which may occur via direct myocardial actions or secondary to reduced hypertension and coronary atherosclerosis.
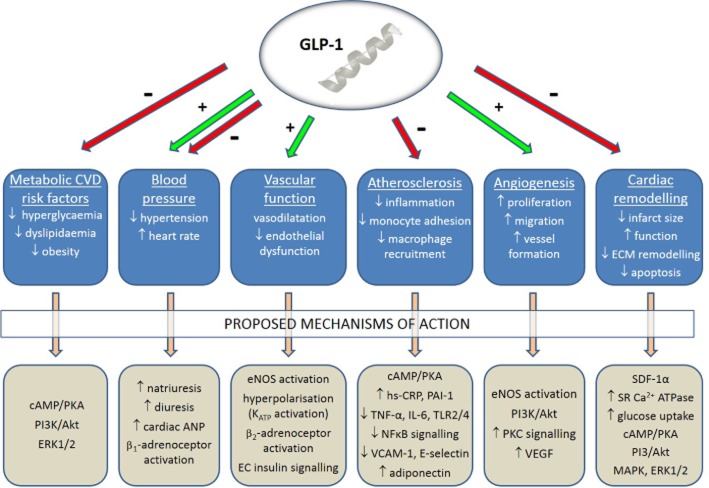
Figure 2.
Proposed mechanisms underlying the reported cardiovascular actions of GLP-1. Although it is well established that GLP-1 exerts several beneficial effects on the CVS relevant to diabetes, such as reduction of metabolic CVD risk factors, BP modulation, improved vascular function, decreased atherosclerosis, promotion of angiogenesis and attenuation of adverse cardiac remodelling, the precise mechanisms are yet to be established, although several pathways have been proposed which are the focus of further investigation. EC, endothelial cell; ECM, extracellular matrix; SDF, stromal cell-derived factor 1α; SR, sarcoplasmic reticulum.
Table 1.
Current long-term clinical trials of GLP-1-based therapies on cardiovascular outcomes
| Study name | Drug | Administration | Estimated patient enrollment | Primary outcome(s) | Expected end date | NCT identifier |
|---|---|---|---|---|---|---|
| EXSCEL | Exenatide | s.c. injection, 2 mg, once weekly | 14 000 | Rate of CV death, non-fatal MI or non-fatal stroke | December 2017 | NCT01144338 |
| LEADER | Liraglutide | s.c. injection, 1.8 mg, once daily | 9 340 | Time to CV death, non-fatal MI or non-fatal stroke | October 2015 | NCT01179048 |
| ELIXA | Lixisenatide | s.c. injection, 20 μg, once daily | 6 000 | Time to first primary CV event | January 2015 | NCT01147250 |
| REWIND | Dulaglutide | s.c. injection, 1.5 mg, once weekly | 9622 | Time to CV death, non-fatal MI or non-fatal stroke | August 2019 | NCT01394952 |
| TECOS | Sitagliptin | p.o., 50 or 100 mg, once daily | 14 000 | Time to first CV event | December 2014 | NCT00790205 |
| CAROLINA | Linagliptin | Not specified | 8 300 | Time to CV death, non-fatal MI, non-fatal stroke and hospitalization for unstable angina pectoris | January 2018 | NCT01897532 |
| CARMELINA | Linagliptin | p.o., 5 mg, once daily | 6 000 | Time to CV death, non-fatal MI, non-fatal stroke and hospitalization for unstable angina pectoris | September 2018 | NCT01243424 |
CV, cardiovascular; MI, myocardial infarction; NCT, National Clinical Trial.
Acknowledgments
The authors' work is supported by the British Heart Foundation, Diabetes UK and the Medical Research Council.
Glossary
- ANP
atrial natriuretic peptide
- CAD
coronary artery disease
- CVD
cardiovascular disease
- DPP-4
dipeptidyl peptidase-4
- eNOS
endothelial NOS
- GLP-1
glucagon-like peptide-1
- hs-CRP
high sensitivity C-reactive protein
- ICAM-1
intercellular adhesion molecule-1
- MI
myocardial infarction
- PAI-1
plasminogen activator inhibitor-1
- STZ
streptozotocin
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TLR
toll-like receptor
- UKPDS
United Kingdom Prospective Diabetes Study
- VCAM-1
vascular cell adhesion molecule-1
Conflict of interest
None.
References
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14:Transporters. Br J Pharmacol. 2013d;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013e;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronis KN, Chamberland JP, Mantzoros CS. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism. 2013;62:1279–1286. doi: 10.1016/j.metabol.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154:2501–2513. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroor AR, Sowers JR, Jia G, DeMarco VG. Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am J Physiol Heart Circ Physiol. 2014;307:H477–H492. doi: 10.1152/ajpheart.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean M, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaori M, Iwakami N, Uto-Kondo H, Sato H, Sasaki M, Komatsu T, et al. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc. 2013;2:e003277. doi: 10.1161/JAHA.112.003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz S, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- Ban K, Kim K, Cho C, Sauvé M, Diamandis EP, Backx PH, et al. Glucagon-like peptide (GLP)-1 (9-36) amide-mediated cytoprotection is blocked by exendin (9-39) yet does not require the known GLP-1 receptor. Endocrinology. 2010;151:1520–1531. doi: 10.1210/en.2009-1197. [DOI] [PubMed] [Google Scholar]
- Barakat GM, Nuwayri-Salti N, Kadi LN, Bitar KM, Al-Jaroudi WA, Bikhazi AB. Role of glucagon-like peptide-1 and its agonists on early prevention of cardiac remodeling in type 1 diabetic rat hearts. Gen Physiol Biophys. 2011;30:34–44. doi: 10.4149/gpb_2011_01_34. [DOI] [PubMed] [Google Scholar]
- Barragán J, Rodríguez R, Blázquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7-36) amide in rats. Am J Physiol Endocrinol Metab. 1994;266:E459–E466. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- Barragán JM, Rodriguez RE, Eng J, Blázquez E. Interactions of exendin-(9-39) with the effects of glucagon-like peptide-1-(7-36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67:63–68. doi: 10.1016/s0167-0115(96)00113-9. [DOI] [PubMed] [Google Scholar]
- Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- Bergenstal RM, Wysham C, MacConell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen Y, et al. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–521. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- Bromage DI, Davidson SM, Yellon DM. Stromal derived factor 1α: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther. 2014;143:305–315. doi: 10.1016/j.pharmthera.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care. 2010;33:1734–1737. doi: 10.2337/dc09-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–2350. doi: 10.2337/dc12-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Ghanim H, Vora M, Sia CL, Korzeniewski K, Dhindsa S, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2011;97:198–207. doi: 10.1210/jc.2011-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courrèges J, Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with Type 2 diabetes. Diabet Med. 2008;25:1129–1131. doi: 10.1111/j.1464-5491.2008.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–2243. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol. 2012;166:27–41. doi: 10.1111/j.1476-5381.2011.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- Erdogdu Ö, Nathanson D, Sjöholm Å, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006–3013. doi: 10.2337/db07-0697. [DOI] [PubMed] [Google Scholar]
- Forst T, Michelson G, Ratter F, Weber M, Anders S, Mitry M, et al. Addition of liraglutide in patients with Type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet Med. 2012;29:1115–1118. doi: 10.1111/j.1464-5491.2012.03589.x. [DOI] [PubMed] [Google Scholar]
- Fukuda-Tsuru S, Anabuki J, Abe Y, Yoshida K, Ishii S. A novel, potent, and long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, improves postprandial hyperglycemia and dyslipidemia after single and repeated administrations. Eur J Pharmacol. 2012;696:194–202. doi: 10.1016/j.ejphar.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Gallwitz B, Vaag A, Falahati A, Madsbad S. Adding liraglutide to oral antidiabetic drug therapy: onset of treatment effects over time. Int J Clin Pract. 2010;64:267–276. doi: 10.1111/j.1742-1241.2009.02265.x. [DOI] [PubMed] [Google Scholar]
- Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- Gardiner S, March J, Kemp P, Bennett T. Autonomic nervous system-dependent and -independent cardiovascular effects of exendin-4 infusion in conscious rats. Br J Pharmacol. 2008;154:60–71. doi: 10.1038/bjp.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S, March J, Kemp P, Bennett T, Baker D. Possible involvement of GLP-1 (9-36) in the regional haemodynamic effects of GLP-1 (7-36) in conscious rats. Br J Pharmacol. 2010;161:92–102. doi: 10.1111/j.1476-5381.2010.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari T, Liu H, Welungoda I, Hu Y, Widdop RE, Knudsen LB, et al. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE-/-mouse model. Diab Vasc Dis Res. 2011;8:117–124. doi: 10.1177/1479164111404257. [DOI] [PubMed] [Google Scholar]
- Gejl M, Søndergaard H, Stecher C, Bibby BM, Møller N, Bøtker H, et al. Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E1165–E1169. doi: 10.1210/jc.2011-3456. [DOI] [PubMed] [Google Scholar]
- Gill A, Hoogwerf BJ, Burger J, Bruce S, MacConell L, Yan P, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6. doi: 10.1186/1475-2840-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) Lipid trial: what we learn from subgroup analyses. Diabetes Care. 2011;34(Suppl. 2):S107–S108. doi: 10.2337/dc11-s203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgino F, Leonardini A, Laviola L. Cardiovascular disease and glycemic control in type 2 diabetes: now that the dust is settling from large clinical trials. Ann N Y Acad Sci. 2013;1281:36–50. doi: 10.1111/nyas.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7-36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- Goyal S, Kumar S, Bijjem KV, Singh M. Role of glucagon-like peptide-1 in vascular endothelial dysfunction. Indian J Exp Biol. 2010;48:61–69. [PubMed] [Google Scholar]
- Green B, Mooney M, Gault V, Irwin N, Bailey C, Harriott P, et al. Lys9 for Glu9 substitution in glucagon-like peptide-1 (7-36) amide confers dipeptidyl peptidase IV resistance with cellular and metabolic actions similar to those of established antagonists glucagon-like peptide-1 (9-36) amide and exendin (9-39) Metabolism. 2004;53:252–259. doi: 10.1016/j.metabol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys. 2008;478:136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290:H146–H153. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- Grieve DJ, Cassidy RS, Green BD. Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br J Pharmacol. 2009;157:1340–1351. doi: 10.1111/j.1476-5381.2009.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson S, Chen D, Somayaji V, Hudson K, Baltrukonis D, Singh J, et al. Effects of a long-acting GLP-1 mimetic (PF-04603629) on pulse rate and diastolic blood pressure in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13:1056–1058. doi: 10.1111/j.1463-1326.2011.01479.x. [DOI] [PubMed] [Google Scholar]
- Gutzwiller J, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3–11. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Halbirk M, Nørrelund H, Møller N, Holst JJ, Schmitz O, Nielsen R, et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1096–H1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- Han SZ, Ouchi Y, Karaki H, Orimo H. Inhibitory effects of insulin on cytosolic Ca2+ level and contraction in the rat aorta endothelium-dependent and -independent mechanisms. Circ Res. 1995;77:673–678. doi: 10.1161/01.res.77.4.673. [DOI] [PubMed] [Google Scholar]
- Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J. 2010;58:69–73. doi: 10.1507/endocrj.k10e-382. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Whittington HJ, Wynne AM, Begum SS, Theodorou L, Riksen N, et al. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol. 2013;12:154. doi: 10.1186/1475-2840-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 2013;62:373–381. doi: 10.2337/db12-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, et al. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380:44–49. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Hogan AE, Gaoatswe G, Lynch L, Corrigan MA, Woods C, O'Connell J, et al. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57:781–784. doi: 10.1007/s00125-013-3145-0. [DOI] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Holst J, Schwartz T, Lovgreen N, Pedersen O, Beck-Nielsen H. Diurnal profile of pancreatic polypeptide, pancreatic glucagon, gut glucagon and insulin in human morbid obesity. Int J Obes. 1982;7:529–538. [PubMed] [Google Scholar]
- Hopkins N, Cuthbertson D, Kemp G, Pugh C, Green D, Cable N, et al. Effects of 6 months glucagon-like peptide-1 receptor agonist treatment on endothelial function in type 2 diabetes mellitus patients. Diabetes Obes Metab. 2013;15:770–773. doi: 10.1111/dom.12089. [DOI] [PubMed] [Google Scholar]
- Horváth E, Benko R, Kiss L, Murányi M, Pék T, Fekete K, et al. Rapid ‘glycaemic swings’ induce nitrosative stress, activate poly (ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009;52:952–961. doi: 10.1007/s00125-009-1304-0. [DOI] [PubMed] [Google Scholar]
- Huang W, Newby GB, Lewis AL, Stratford PW, Rogers CA, Newby AC, et al. Periadventitial human stem cell treatment reduces vein graft intimal thickening in pig vein-into-artery interposition grafts. J Surg Res. 2013;183:33–39. doi: 10.1016/j.jss.2012.11.060. [DOI] [PubMed] [Google Scholar]
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- Huisamen B, George C, Dietrich D, Genade S. Cardioprotective and anti-hypertensive effects of Prosopis glandulosa in rat models of pre-diabetes: cardiovascular topics. Cardiovasc J Afr. 2013;24:10–16. doi: 10.5830/CVJA-2012-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation. 2014. IDF Diabetes Atlas Sixth Edition. Available at: http://www.idf.org/diabetesatlas (accessed 5/25/2014)
- Irace C, De Luca S, Shehaj E, Carallo C, Loprete A, Scavelli F, et al. Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: results from an observational research. Diab Vasc Dis Res. 2013;10:72–77. doi: 10.1177/1479164112449562. [DOI] [PubMed] [Google Scholar]
- Kahn AM, Husid A, Odebunmi T, Allen JC, Seidel CL, Song T. Insulin inhibits vascular smooth muscle contraction at a site distal to intracellular Ca2+ concentration. Am J Physiol Endocrinol Metab. 1998;274:E885–E892. doi: 10.1152/ajpendo.1998.274.5.E885. [DOI] [PubMed] [Google Scholar]
- Kang H, Kang Y, Chun HJ, Jeong J, Park C. Evaluation of the in vitro and in vivo angiogenic effects of exendin-4. Biochem Biophys Res Commun. 2013;434:150–154. doi: 10.1016/j.bbrc.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Katare R, Riu F, Rowlinson J, Lewis A, Holden R, Meloni M, et al. Perivascular delivery of encapsulated mesenchymal stem cells improves postischemic angiogenesis via paracrine activation of VEGF-A. Arterioscler Thromb Vasc Biol. 2013;33:1872–1880. doi: 10.1161/ATVBAHA.113.301217. [DOI] [PubMed] [Google Scholar]
- Kelly AS, Bergenstal RM, Gonzalez-Campoy JM, Katz H, Bank AJ. Effects of exenatide vs. metformin on endothelial function in obese patients with pre-diabetes: a randomized trial. Cardiovasc Diabetol. 2012;11:64. doi: 10.1186/1475-2840-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2007;24:275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- Koska J, Schwartz EA, Mullin MP, Schwenke DC, Reaven PD. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care. 2010;33:1028–1030. doi: 10.2337/dc09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothare PA, Linnebjerg H, Isaka Y, Uenaka K, Yamamura A, Yeo KP, et al. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with type 2 diabetes mellitus. J Clin Pharmacol. 2008;48:1389–1399. doi: 10.1177/0091270008323750. [DOI] [PubMed] [Google Scholar]
- Krasner NM, Ido Y, Ruderman NB, Cacicedo JM. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE. 2014;9:e97554. doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymann B, Ghatei M, Williams G, Bloom S. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;330:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- Liu H, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol. 2009;201:59–66. doi: 10.1677/JOE-08-0468. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Chen L, Wang Y, Li J. Glucagon-like peptide-1 analog liraglutide protects against diabetic cardiomyopathy by the inhibition of the endoplasmic reticulum stress pathway. J Diabetes Res. 2013;2013:630537. doi: 10.1155/2013/630537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Anderson C, Broyde A, Polizzi C, Fernandez R, Baron A, et al. Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol. 2010;9:76. doi: 10.1186/1475-2840-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54:778–784. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206:287–296. doi: 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- Marx N, Burgmaier M, Heinz P, Ostertag M, Hausauer A, Bach H, et al. Glucagon-like peptide-1 (1-37) inhibits chemokine-induced migration of human CD4-positive lymphocytes. Cell Mol Life Sci. 2010;67:3549–3555. doi: 10.1007/s00018-010-0396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen N, Mänttäri S, Schweizer A, Ulvestad A, Mills D, Dunning B, et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–2057. doi: 10.1007/s00125-006-0340-2. [DOI] [PubMed] [Google Scholar]
- Matsubara J, Sugiyama S, Sugamura K, Nakamura T, Fujiwara Y, Akiyama E, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59:265–276. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Kanemoto S, Leshnower BG, Albone EF, Hinmon R, Plappert T, et al. Single dose GLP-1-Tf ameliorates myocardial ischemia/reperfusion injury. J Surg Res. 2011;165:38–45. doi: 10.1016/j.jss.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly SP, Mather KJ, Berwick ZC, Owen MK, Goodwill AG, Casalini ED, et al. Impaired cardiometabolic responses to glucagon-like peptide 1 in obesity and type 2 diabetes mellitus. Basic Res Cardiol. 2013;108:365. doi: 10.1007/s00395-013-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Mitsui T, Bando YK, Aoyama M, Shigeta T, Murohara T. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;305:H295–H304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- Moretto TJ, Milton DR, Ridge TD, MacConell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–1460. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Morinigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Nakajima K, Niimi M, Fujita MQ, Nakajima Y, Takeichi S, et al. Detection of apolipoproteins B-48 and B-100 carrying particles in lipoprotein fractions extracted from human aortic atherosclerotic plaques in sudden cardiac death cases. Clin Chim Acta. 2008;390:38–43. doi: 10.1016/j.cca.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Nathan DM. The pathophysiology of diabetic complications: how much does the glucose hypothesis explain? Ann Intern Med. 1996;124:86–89. doi: 10.7326/0003-4819-124-1_part_2-199601011-00002. [DOI] [PubMed] [Google Scholar]
- Nathanson D, Erdogdu Ö, Pernow J, Zhang Q, Nyström T. Endothelial dysfunction induced by triglycerides is not restored by exenatide in rat conduit arteries ex vivo. Regul Pept. 2009;157:8–13. doi: 10.1016/j.regpep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M, Frid A, Hermansen K, Thomsen A, During M, Shah N, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15:204–212. doi: 10.1111/dom.12012. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and c-peptide responses. J Clin Endocrinol Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol Endocrinol Metab. 1997;273:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998a;68:525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- Näslund E, Grybäck P, Backman L, Jacobsson H, Juul J, Theodorsson HE, et al. Distal small bowel hormones: correlation with fasting antroduodenal motility and gastric emptying. Dig Dis Sci. 1998b;43:945–952. doi: 10.1023/a:1018806129102. [DOI] [PubMed] [Google Scholar]
- Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Shingu Y, Amorim PA, Schwarzer M, Doenst T. Glucagon-like peptide-1 reduces contractile function and fails to boost glucose utilization in normal hearts in the presence of fatty acids. Int J Cardiol. 2013;168:4085–4092. doi: 10.1016/j.ijcard.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004a;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004b;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Shen Y-T, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- Noyan-Ashraf MH, Momen MA, Ban K, Sadi A, Zhou Y, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- Nyström T, Gonon AT, Sjöholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23:334–339. doi: 10.1038/ajh.2009.245. [DOI] [PubMed] [Google Scholar]
- Özyazgan S, Kutluata N, Afsar S, Özdas S, Akkan A. Effect of glucagon-like peptide-1 (7-36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology. 2005;74:119–126. doi: 10.1159/000084277. [DOI] [PubMed] [Google Scholar]
- Pabreja K, Mohd M, Koole C, Wootten D, Furness S. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br J Pharmacol. 2013;171:1114–1128. doi: 10.1111/bph.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology. 2012;154:127–139. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]
- Parlevliet ET, Wang Y, Geerling JJ, Schröder-Van der Elst JP, Picha K, O'Neil K, et al. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS ONE. 2012;7:e49152. doi: 10.1371/journal.pone.0049152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picatoste B, Ramirez E, Caro-Vadillo A, Iborra C, Egido J, Tuñón J, et al. Sitagliptin reduces cardiac apoptosis, hypertrophy and fibrosis primarily by insulin-dependent mechanisms in experimental type-II diabetes. Potential roles of GLP-1 isoforms. PLoS ONE. 2013;8:e78330. doi: 10.1371/journal.pone.0078330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski K, Becker M, Zugwurst J, Biller-Friedmann I, Spoettl G, Greif M, et al. Circulating concentrations of GLP-1 are associated with coronary atherosclerosis in humans. Cardiovasc Diabetol. 2013;12:117. doi: 10.1186/1475-2840-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- Rask E, Olsson T, Söderberg S, Johnson O, Seckl J, Holst JJ, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24:1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- Ravassa S, Zudaire A, Carr RD, Diez J. Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am J Physiol Heart Circ Physiol. 2011;300:H1361–H1372. doi: 10.1152/ajpheart.00885.2010. [DOI] [PubMed] [Google Scholar]
- Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen M, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63:1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter G, Feddersen O, Wagner U, Barth P, Goke R, Goke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 1993;265:L374–L381. doi: 10.1152/ajplung.1993.265.4.L374. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. Cardiovascular effects of gliptins. Nat Rev Cardiol. 2013;10:73–84. doi: 10.1038/nrcardio.2012.183. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis. 2010;212:217–222. doi: 10.1016/j.atherosclerosis.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171–180. doi: 10.1111/j.1742-1241.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, et al. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta T, Aoyama M, Bando YK, Monji A, Mitsui T, Takatsu M, et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126:1838–1851. doi: 10.1161/CIRCULATIONAHA.112.096479. [DOI] [PubMed] [Google Scholar]
- Shiomi T, Tsutsui H, Ikeuchi M, Matsusaka H, Hayashidani S, Suematsu N, et al. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J Am Coll Cardiol. 2003;42:165–172. doi: 10.1016/s0735-1097(03)00509-6. [DOI] [PubMed] [Google Scholar]
- Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- Sonne DP, Engstrøm T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1 (9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- Stratton I, Cull C, Adler A, Matthews D, Neil H, Holman R. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75) Diabetologia. 2006;49:1761–1769. doi: 10.1007/s00125-006-0297-1. [DOI] [PubMed] [Google Scholar]
- Tesauro M, Schinzari F, Adamo A, Rovella V, Martini F, Mores N, et al. Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care. 2013;36:683–689. doi: 10.2337/dc12-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers L, Henriques JP, de Kleijn DP, DeVries JH, Kemperman H, Steendijk P, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen M, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22:1137–1143. doi: 10.2337/diacare.22.7.1137. [DOI] [PubMed] [Google Scholar]
- Tremblay A, Lamarche B, Deacon CF, Weisnagel S, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:366–373. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- Turner R, Millns H, Neil H, Stratton I, Manley S, Matthews D, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Varanasi A, Chaudhuri A, Dhindsa S, Arora A, Lohano T, Vora MR, et al. Durability of effects of exenatide treatment on glycemic control, body weight, systolic blood pressure, C-reactive protein, and triglyceride concentrations. Endocr Pract. 2011;17:192–200. doi: 10.4158/EP10199.OR. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety – effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- Vergès B, Zeller M, Dentan G, Beer J-C, Laurent Y, Janin-Manificat L, et al. Impact of fasting glycemia on short-term prognosis after acute myocardial infarction. J Clin Endocrinol Metab. 2007;92:2136–2140. doi: 10.1210/jc.2006-2584. [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges J-P, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- Wang D, Luo P, Wang Y, Li W, Wang C, Sun D, et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes. 2013;62:1697–1708. doi: 10.2337/db12-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- Wright EJ, Farrell KA, Malik N, Kassem M, Lewis AL, Wallrapp C, et al. Encapsulated glucagon-like peptide-1-producing mesenchymal stem cells have a beneficial effect on failing pig hearts. Stem Cells Transl Med. 2012;1:759–769. doi: 10.5966/sctm.2012-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Qian J, Castillo AC, Ling S, Ye H, Perez-Polo JR, et al. Phosphodiesterase-3 inhibition augments the myocardial infarct size-limiting effects of exenatide in mice with type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;304:H131–H141. doi: 10.1152/ajpheart.00609.2012. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Akasaka H, Ohnishi H, Miki T, Furukawa T, Yuda S, et al. Glucagon-like peptide-1 secretory function as an independent determinant of blood pressure: analysis in the Tanno-Sobetsu study. PLoS ONE. 2013;8:e67578. doi: 10.1371/journal.pone.0067578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younce CW, Burmeister MA, Ayala JE. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am J Physiol Cell Physiol. 2013;304:C508–C518. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]
- Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen Y-T, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+ TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]