Abstract
Background and Purpose
Long-term intake of dietary fatty acids is known to predispose to chronic inflammation, but their effects on acute intestinal ischaemia/reperfusion (I/R) injury is unknown. The aim of this study was to determine the consequences of a diet rich in n-3 or n-6 polyunsaturated fatty acids (PUFA) on intestinal I/R-induced damage.
Experimental Approach
Mice were fed three different isocaloric diets: a balanced diet used as a control and two different PUFA-enriched diets, providing either high levels of n-3 or of n-6 PUFA. Intestinal injury was evaluated after intestinal I/R. PUFA metabolites were quantitated in intestinal tissues by LC-MS/MS.
Key Results
In control diet-fed mice, intestinal I/R caused inflammation and increased COX and lipoxygenase-derived metabolites compared with sham-operated animals. Lipoxin A4 (LxA4) was significantly and selectively increased after ischaemia. Animals fed a high n-3 diet did not display a different inflammatory profile following intestinal I/R compared with control diet-fed animals. In contrast, intestinal inflammation was decreased in the I/R group fed with high n-6 diet and level of LxA4 was increased post-ischaemia compared with control diet-fed mice. Blockade of the LxA4 receptor (Fpr2), prevented the anti-inflammatory effects associated with the n-6 rich diet.
Conclusions and Implications
This study indicates that high levels of dietary n-6, but not n-3, PUFAs provides significant protection against intestinal I/R-induced damage and demonstrates that the endogenous production of LxA4 can be influenced by diet.
Tables of Links
| LiGANDS | |
|---|---|
| 5-HETE | CXCL1 |
| 8-HETE | DHA, docosahexaenoic acid |
| 12-HETE | LTB4 |
| 15-HETE | LxA4 |
| AA, arachidonic acid | PGE2 |
| CCL2 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b,).
Introduction
There is growing evidence, which indicates bioactive lipid mediators derived from n-3 or n-6 polyunsaturated fatty acids (PUFA) play a pivotal role in initiating and sustaining the inflammatory response or engaging proresolving/anti-inflammatory pathways in several cardiovascular and inflammatory pathologies. These include atherosclerosis, thrombosis, arrhythmia and ischaemia reperfusion (I/R) injury (De Caterina, 2011). The recent development of new techniques of high sensitivity LC-MS/MS allows a better comprehension of disease-associated lipidic metaboloma detecting simultaneously the presence of a number of PUFA metabolites in tissues. In our previous study, we characterized, by LC-MS/MS, the tissue profile of n-6 and n-3 PUFA metabolites in an experimental model of murine intestinal I/R (Gobbetti et al., 2013). We identified temporal and quantitative differences in n-3 and n-6 PUFA metabolite production, which correlated with inflammatory damage. However, it is unclear whether modulating the n-3/n-6 ratio composition in membranes could be used as therapeutic tool to modify the resistance of the gut to I/R injury.
Acute mesenteric ischaemia is a life-threatening pathological event associated with a number of diseases including vessel occlusion, hernias, septic shock, major cardiovascular surgery, necrotizing enterocolitis and small bowel transplantation (Cerqueira et al., 2005; Eltzschig and Eckle, 2011; Vollmar and Menger, 2011). Intestinal ischaemia remains a clinical challenge because of difficulty in diagnosis and especially to the lack of established pharmacological treatments. Therefore, the need for new therapeutic strategies is urgent. The intestinal mucosa is particularly prone to I/R injury because of the anatomical and physiological characteristic of the villus microcirculation (Vollmar and Menger, 2011). The temporary interruption of the blood leads to a lack of oxygen supply to the tissue (ischaemia), which in turn causes cellular dysfunction, protease and phospholipases activation (Otamiri et al., 1987; Vollmar and Menger, 2011; Gobbetti et al., 2012). Paradoxically, the restoration of blood flow and the consequent tissue re-oxygenation (reperfusion) aggravates the local (epithelial/endothelial damage) and systemic inflammatory response, leading to bacterial translocation and multiple organ failure (Cerqueira et al., 2005).
The main objective of this study was to identify if the modification of n-6/n-3 PUFA status induced by the modification of PUFA metabolite biosynthesis associated with diet changes might improve intestinal resistance to I/R injury. We performed a dietary intervention by exposing mice to a diet rich in n-3, n-6 PUFA or to a balanced diet (Kelavkar et al., 2006; Ducheix et al., 2013) and we evaluated the importance of n-3 and n-6 PUFA metabolites on intestinal I/R injury. Dietary intervention significantly changed the abundance of hepatic PUFA and intestinal PUFA metabolites. Intestinal inflammation was decreased in the group fed with a high n-6 diet and the level of lipoxin A4 (LxA4) after ischaemia was increased compared with the group fed with a balanced diet. Early systemic pharmacological blockade of LxA4 receptor, formyl peptide receptor (Fpr)2, prevented the anti-inflammatory effects attained by the n-6-rich diet. This study indicates that early mobilization of LxA4 leads to significant protection against intestinal I/R-induced damage.
Methods
Animals
All animal care and experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the European Council and were approved by the Animal Care and Ethics Committee of US006/CREFE (CEEA-122) with permit No. MP/01/64/09/12. All animal experiments were reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010a,b,; McGrath et al., 2010). A total of 160 mice were included in this study. C57 BL/6 male mice (3 weeks old) were purchased from Janvier (Le Genest Saint Isle, France). Animals were maintained in ventilated cages (four mice per cage) in a specific pathogen-free room at 20–24°C and relative humidity (40–70%) with a 12 h light/dark cycle and given free access to food and water.
Study design and surgical procedure
One hundred and twenty mice were randomly assigned to one of three isocaloric diets containing 5% fat (w/w) for 9 weeks. A balanced diet providing both n-3 and n-6 fatty acid (FA) was used as a control, against two different PUFA-enriched diets, providing either high n-3 or high n-6 intake. Mice were weighed once a week during the diet and the weight gain determined. After 9 weeks, a randomized complete block design has been used to split the experiment into a number of five ‘mini-experiments’. The days of the surgery, each diet group were split in four different experimental groups: (i) sham ischaemia, (ii) ischaemia, (iii) sham I/R and (iv) I/R (n = 10 per group). For each surgery day, two animals of the three different diet conditions have been subject to the surgical protocol (24 animals per day for 5 days).
Mice were anaesthetized by an i.p. injection of sodium pentobarbital (50 mg·kg−1). Animals were kept under anaesthesia during all periods of surgery. The adequacy of anaesthesia was monitored with a toe pinch testing to check the animal's reaction. Following abdominal laparotomy, the small bowel was retracted to the left and the superior mesenteric artery (SMA) was temporarily occluded using a microvascular clip (Harvard Apparatus, Les Ulis, France) to cause ischaemia. Small bowel and SMA were replaced in mice abdomen and the incision was protected with a gauze soak in saline solution (NaCl 0.9%). After 50 min, the clip was gently removed allowing reperfusion. Mice of the ischaemia groups were killed immediately after the ischaemic period. Mice of I/R groups were killed 5 h after reperfusion. Sham-operated animals, in which abdominal laparotomy and artery isolation were performed without occlusion of the vessel, served as controls and were killed just after the corresponding ischaemic (sham ischaemia) or I/R (sham I/R) time. After the surgical procedure, two-layer sutures closed the midline incision of the abdominal wall. During the surgical procedure, mice were placed on a warming plate (32°C). An independent experiment was performed to determine the effect of Fpr2 endogenous activation during intestinal I/R. Eighty mice were fed with the diet providing high n-6 intake. After 9 weeks, animals were randomized in four different subgroups of 20 animals: sham ischaemia, sham I/R, ischaemia and I/R, as previously described. Ten mice per group were treated by i.v. injection of the tail vein with Boc2 (500 μg kg−1). Animals were killed at the end of the study for the purpose of sample collection by a lethal overdose of pentobarbital i.p. followed by cervical dislocation.
Fatty composition of diets
The three diets were isocaloric and contained 5% fat (w/w) and were designed as previously described (Ducheix et al., 2013). Pellets were prepared by UPAE (unité de préparation des aliments expérimentaux) (INRA, Jouy en Josas, France). Oils used for experimental diets preparation were grape seed and colza oils (50/50) for the Control diet (Ctrl), grape seed oil (n-6) and colza oil/fish oils (80/20) for a long-chain n-3 FA-enriched diet (n-3). The eicosapentaenoic acid (EPA)-enriched fish oil that was used in this study was obtained from Polaris (Quimper, France). The full composition of the diet is given in Table 1. The FA composition expressed in percentage of total PUFA is provided in Table 2. Pellets were stored under vacuum at −20°C. The diets were changed twice a week in each animal cage to avoid oxidative degradation of lipids.
Table 1.
Composition in percentage (%) of the three isocaloric diets
| Control | n-6 | n-3 | |
|---|---|---|---|
| Cellulose | 2 | 2 | 2 |
| Casein | 22 | 22 | 22 |
| Starch | 43.6 | 43.6 | 43.6 |
| Methionine | 0.2 | 0.2 | 0.2 |
| Sucrose | 21.8 | 21.8 | 21.8 |
| Minerals | 4.5 | 4.5 | 4.5 |
| Vitamins | 1.0 | 1.0 | 1.0 |
| Grape seed oil | 2.5 | 5 | 0 |
| Colza oil | 2.5 | 0 | 4 |
| Fish oil | 0 | 0 | 1 |
Table 2.
Composition in percentage (%) of PUFA of the three isocaloric diets containing 5% fat (w/w)
| Control | n-6 | n-3 | |
|---|---|---|---|
| C12:0 | 0.0 | 0.0 | 0.0 |
| C14:0 | 0.0 | 0.0 | 0.0 |
| C16:0 | 6.0 | 6.5 | 4.5 |
| C18:0 | 2.5 | 4.2 | 0.7 |
| C18:1n-9 | 38.8 | 18.4 | 47.4 |
| C18:2n-6 | 47.4 | 70.0 | 19.9 |
| C18:3n-3 | 5.2 | 1.0 | 7.6 |
| C20:5n-3 | 0.0 | 0.0 | 17.0 |
| C22:6n-3 | 0.0 | 0.0 | 3.0 |
| n-6/n-3 ratio | 10 | 70 | 0.72 |
Assessment of inflammation
Specimens of the ileum were embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin. Microscopic histological damage was scored, without knowledge of the treatments and was based on a semi-quantitative scoring system in which the following features were graded: extent of destruction of normal mucosal architecture, presence and degree of cellular infiltration, extent of muscle thickening, presence or absence of crypt abscesses and presence or absence of goblet cell depletion. The scores for each feature were summed with a maximum possible score of 11 as previously described (Cattaruzza et al., 2006).
Myeloperoxidase (MPO) activity was assessed in the ileum tissue and was used as an index of granulocyte infiltration as previously described (Motta et al., 2012). The chemokines CXCL1 and CCL2 were measured in intestinal tissue by cytometric bead array (BD Biosciences, Le Pont de Claix, France) as previously described (Motta et al., 2012).
Immunohistochemistry
Intestines were cryoprotected, cut into 5 μm sections in a cryostat and mounted on a Superfrost slide (Thermo Fisher Scientific, Illkirch, France). Slides were washed in PBS, 0.5% Triton X-100 and 1% BSA solution (Sigma, Saint-Quentin Fallavier, France) and incubated overnight at 4°C with the primary antibody directed against Fpr2 (1/100, Santa Cruz, Heidelberg, Germany) along with anti-cytokeratin 18 (1/500, Santa Cruz), anti-CD45 (1/500, R&D Systems, Lille, France) or anti-Ly-6B.2 (1/400, Bio-Rad AbD Serotec GmbH, Colmar, France). After washes, slides were incubated with appropriate secondary antibody (Alexa Fluor 488 and 555; Invitrogen, Cergy Pontoise, France) for 2 h. Slides were mounted with Prolong® containing DAPI (Invitrogen). As control procedures, reactions were performed with omission of primary or secondary antibodies. Images were acquired using Zeiss LSM-710 confocal microscopes with 63X objective in the inverted configuration (Carl Zeiss MicroImaging, Inc., Göttingen, Germany). Images of stained and control slides were collected and processed identically. The quantification of fluorescence was determined using Metamorph software (Molecular Devices, Berkshire, UK).
Real-time PCR analysis
Total RNA from small intestine was isolated with TRIzol reagent (Invitrogen, Paisley, UK) and 1 μg of the total RNA was then reverse-transcribed with random hexamer oligonucleotides and SuperScript III (Invitrogen), according to the manufacturer's instructions. Amplification was performed with a LightCycler 480 using a SYBR Green I Master kit (Qiagen, Ltd., Manchester, UK) and one of the following mouse primer: fpr2 (QT00171514, Qiagen, Ltd.), alox12b (QT00173551), alox15 (QT00111034) or alox5 (QT00258622). Relative expression of the target gene was normalized to expression of the Hprt gene, using the ΔΔCt method (Boue et al., 2011).
Hepatic and intestinal FA profiling
Hepatic FAs were analysed as described previously (Zadravec et al., 2010). Briefly, following homogenization of tissue samples in methanol/5 mM EGTA (2:1, v/v), lipids corresponding to an equivalent of 2 mg of tissue were extracted in the presence of glyceryl triheptadecanoate as an internal standard. The lipid extract in 1 mL of heptane was transmethylated with 1 mL of boron trifluoride in methanol (MeOH, 1:20, v/v) for 150 min at 100°C, evaporated to dryness, and the fatty acid methyl esters (FAMEs) were extracted with hexane/water (3:1). The organic phase was evaporated to dryness and dissolved in 50 μL ethyl acetate. One microliter of FAME was analysed by gas-LC on a 5890 Hewlett-Packard system (Hewkett-Packard, Palo Alto, CA, USA) using a Famewax fused-silica capillary column (30.0 × 0.32 mm i.d., 0.25 μm film thickness; Restek, Belfast, UK). Oven temperature was programmed from 110 to 220°C at a rate of 2°C per min and the carrier gas was hydrogen (0.5 bar). The injector and the detector were at 225 and 245°C respectively.
Intestinal lipid extraction
Mouse small intestines were crushed with a FastPrep®-24 Instrument (MP Biomedical, Illkirch, France) in 500 μL of HBSS (Invitrogen) and 5 μL of internal standard mixture (LxA4-d5, LTB4-d4 and 5-HETE-d8 at 400 ng mL−1 in MeOH). After two crush cycles (6.5 m s−1, 30 s), 10 μL is withdrawn for protein quantification and 300 μL of cold methanol was added. Samples were centrifuged at 2000× g for 15 min at 4°C. Supernatants were collected, completed to 2 mL in H2O and submitted to solid-phase extraction using HRX-50 mg 96-well (Macherey Nagel, Hoerd, France). Briefly, after plate conditioning, the sample was loaded at flow rate of 0.1 mL min−1. After complete loading, the plate was washed with H2O/MeOH (90:10, 2 mL) and lipid mediators were eluted with MeOH (2 mL). Solvent was evaporated under nitrogen and samples were dissolved with MeOH and stored at −80°C for LC-MS/MS measurements.
LC-MS/MS measurements
By this technique, we performed the quantification of 6kPGF1α, TXB2, PGE2, PGA1, 8-isoPGA2, PGE3, LxA4, LxB4, RvD1, RvD2, 7-MaR1, LTB4, LTB5, PDx, 18-HEPE, 5,6-DiHETE, 15-HETE, 12-HETE, 8-HETE, 5-HETE, 17-HDoHE, 14-HDoHE, 14,15-EET, 11,12-EET, 8,9-EET, 5,6-EET and 5-oxo-ETE in mouse intestinal tissue (Le Faouder et al., 2013). To simultaneously separate 27 lipids of interest and three deuterated internal standards, LC-MS/MS analysis was performed on ultra HPLC system (UHPLC, Agilent LC1290 Infinity) coupled to Agilent 6460 triple quadrupole MS (Agilent Technologies, Les Ulis, France) equipped with electrospray ionization operating in negative mode. Reverse-phase UHPLC was performed using ZorBAX SB-C18 column (Agilent Technologies) with a gradient elution. The mobile phases consisted of water, acetonitrile (ACN) and formic acid (75:25:0.1; v/v/v) (A) and ACN, formic acid (100:0.1, v/v) (B). The linear gradient was as follows: 0% B at 0 min, 85% B at 8.5 min, 100% B at 9.5 min, 100% B at 10.5 min and 0% B at 12 min. The flow rate was 0.35 mL min−1. The autosampler was set at 5°C and the injection volume was 5 μL. Data were acquired in multiple reaction monitoring mode with optimized conditions. Peak detection, integration and quantitative analysis were performed using Mass Hunter Quantitative analysis software (Agilent Technologies). For each standard, calibration curves were built using 10 solutions at concentration ranging from 0.95 to 500 ng mL−1. A linear regression with a weight factor of 1/X was applied for each compound. The limit of detection (LOD) and the limit of quantification (LOQ) were determined for the 27 compounds using signal to noise ratio. The LOD corresponded to the lowest concentration leading to a signal to noise over 3 and LOQ corresponded to the lowest concentration leading to a signal to noise over 10. All values under the LOQ were not considered. Importantly, blank samples were evaluated and their injection showed no interference (no peak detected) during the analysis. Hierarchical clustering and heat-map were obtained with R (www.r-project.org). PUFA metabolite quantities were transformed to z-scores and clustered based on one Pearson correlation coefficient as distance and the Ward algorithm as agglomeration criterion.
Data analysis
Data are presented as mean ± SEM. Analyses were performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). All data were normally distributed. Between-group comparisons were performed by Student's unpaired two-tailed t-test. Multiple comparisons within groups I/R were performed by repeated-measures one-way anova followed by Tukey's procedure. Statistical significance was accepted at P ≤ 0.05.
Materials
N-tert-butoxycarbonyl-L-Phe-D-Leu-L-Phe-D-Leu-L-Phe (Boc2; Bachem, Bubendorf, Switzerland) was dissolved in dimethyl sulfoxide (1%)/sodium chloride (NaCl) 0.9%. 6-keto-prostaglandin F1α (6kPGF1α), thromboxane B2 (TXB2), PGE2, PGA1, 8-isoPGA2, PGE3, LxA4, lipoxin B4 (LxB4), lipoxin A4 deuterated (LxA4-d5), resolvin D1 (RvD1), resolvin D2 (RvD2), 7(S)-maresin (7-MaR1), leukotriene B4 (LTB4), leukotriene B5 (LTB5), leukotriene B4 deuterated (LTB4-d4), 10(S),17(S)-protectin (PDx), 18-hydroxyeicosapentaenoic acid (18-HEPE), dihydroxy-eicosatetraenoic acid (5,6-DiHETE), 15-hydroxyeicosatetraenoic acid (15-HETE) and 12-HETE, 8-HETE, 5-HETE, 5-HETE-d8, 17-hydroxy-docosahexaenoic acid (17-HDoHE) and 14-HDoHE, 14,15-epoxyeicosatrienoic acid (14,15-EET) and 11,12-EET, 8,9-EET, 5,6-EET, 5-oxoeicosatetraenoic acid (5-oxo-ETE) were purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Results
Effect of dietary intervention on the abundance of FAs
In order to investigate the effect of the dietary intervention on the lipid composition, intestinal and hepatic FAs were profiled by gas chromatography analysis. Compared with the control group, the ratio between arachidonic (AA) and docosahexaenoic (DHA) acids increased threefold in the liver of animals receiving the n-6 diet (Figure 1). Conversely, animals receiving the n-3 diet had a significantly decreased ratio. Even if the total intestinal FA content was the same, the dietary intervention had a major impact on the relative FA composition of the gut (Table 3). In the intestine of mice fed with n-6 diet, linoleic acid (LA, C18:2 n-6), C20:2 n-6 and AA (C20:4 n-6) were significantly increased compared with the control diet group (Table 3). In the intestine of mice fed on n-3 diet, α-LA (C18:3 n-3), EPA (C20:5 n-3), C22:5 n-3 and DHA (C22:6 n-3) were significantly increased compared with the control diet. On the other hand, in this group, C20:2 n-6, C20:3 n-6 and AA were significantly decreased. The ratio between AA and DHA mirrored the changes in the relative abundance of intestinal PUFA. During dietary intervention, weight gain was weekly assessed and it was found to be similar regardless of diet (Supporting Information Fig. S1).
Figure 1.
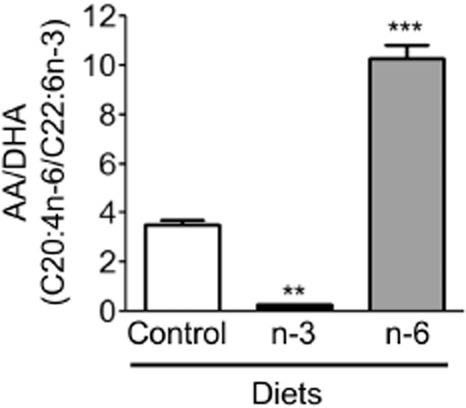
Effect of dietary intervention on the hepatic FAs composition. Total AA (C20:4 n-6) and DHA (C22:6 n-3) ratio in liver of mice submitted to control, n-3 or n-6 PUFA diet for 9 weeks. Values are mean ± SEM, n = 6–8 per group. **P < 0.01, ***P < 0.001, significantly different from control diet group.
Table 3.
Concentration of FA (nmol·mg−1 of protein) in mouse intestine
| Diet | |||
|---|---|---|---|
| Control | n-3 | n-6 | |
| 10:0 | 0.00 | 0.00 | 0.00 |
| 12:0 | 0.00 | 0.00 | 0.00 |
| 14:0 | 0.00 | 5.39 ± 2.57 | 1.02 ± 1.02 |
| 15:0 | 0.00 | 0.00 | 0.00 |
| 16:0 | 286.85 ± 43.95 | 349.08 ± 75.12 | 329 ± 79.41 |
| 18:0 | 185.97 ± 23.68 | 147.24 ± 21.21 | 127.53 ± 42.45 |
| 20:0 | 0.00 | 0.00 | 0.00 |
| 21:0 | 0.00 | 0.00 | 0.00 |
| 22:0 | 0.00 | 0.00 | 0.00 |
| 14:1 n-5 | 0.00 | 0.00 | 0.00 |
| 15:1 n-5 | 0.00 | 0.00 | 0.00 |
| 16:1 n-9 | 0.00 | 0.00 | 0.00 |
| 16:1 n-7 | 150.83 ± 13.09 | 157.22 ± 38.09 | 146.05 ± 14.37 |
| 17:1 n-7 | 0.00 | 0.00 | 0.00 |
| 18:1 n-9 | 413.22 ± 68.93 | 450.11 ± 49.71 | 445.18 ± 65.70 |
| 18:1 n-7 | 100.73 ± 17.17 | 93.00 ± 11.58 | 94.35 ± 19.73 |
| 20:1 n-9 | 0.00 | 0.00 | 0.00 |
| 22:1 n-9 | 0.00 | 0.00 | 0.00 |
| 24:1 n-9 | 0.00 | 0.00 | 0.00 |
| 18:2 n-6 (LA) | 84.56 ± 18.38 | 69.76 ± 10.07 | 209.05 ± 23.64* |
| 18:3 n-6 | 0.00 | 0.00 | 0.00 |
| 18:3 n-3 (ALA) | 0.00 | 15.65 ± 1.65* | 0.00 |
| 20:2 n-6 | 3.41 ± 1.50 | 0.00* | 6.97 ± 0.92* |
| 20:3 n-3 | 0.00 | 0.00 | 0.00 |
| 20:3 n-6 | 18.29 ± 1.03 | 8.56 ± 0.86* | 13.47 ± 1.34 |
| 20:4 n-6 (AA) | 39.95 ± 2.34 | 13.81 ± 1.19* | 60.37 ± 5.70* |
| 20:5 n-3 (EPA) | 0.00 | 10.78 ± 1.86* | 0.00 |
| 22:5 n-3 | 0.00 | 5.69 ± 0.78* | 0.00 |
| 22:2 n-6 | 0.00 | 0.00 | 0.00 |
| 22:4 n-6 | 0.00 | 0.00 | 0.00 |
| 22:5 n-6 | 0.00 | 0.00 | 0.00 |
| 22:6 n-3 (DHA) | 1.88 ± 1.21 | 19.87 ± 3.14* | 0.93 ± 0.52 |
| 20:4 n-6/22:6 n-3 | 9.51 ± 0.75 | 0.73 ± 0.07* | 44.09 ± 11.34* |
| Total SAFA | 472.82 ± 67.64 | 501.71 ± 98.91 | 457.98 ± 122.88 |
| Total MUFA | 664.78 ± 99.19 | 700.33 ± 99.38 | 685.58 ± 99.80 |
| Total PUFA | 148.08 ± 24.46 | 144.50 ± 19.93 | 290.78 ± 32.30* |
| Total FA | 1284 ± 151 | 1345 ± 162 | 1432 ± 114 |
Mice were submitted to 9 weeks of control, n-3 or n-6 diet before FA metabolite extraction. Data are express as mean ± SEM, n = 10. MUFA, monounsaturated fatty acid; SAFA, saturated fatty acid. *P < 0.05 versus control diet group.
Long-term n-6 diet prevents intestinal I/R injury
In animals fed with the control diet, 50 min of ischaemia followed by 5 h of reperfusion resulted in acute inflammation. This was characterized by microscopic damage: fragments of mucosa and red blood cells were found in the lumen. Villi were completely denuded or severely damaged, together with loss of crypt architecture. MPO activity and CXCL1/CCL2 expressions were also markedly increased in I/R versus sham operation in control diet animals (Figure 2). These inflammatory markers were unchanged in animals receiving a high n-3 diet and then subjected to I/R. Conversely, the microscopic damage score, MPO activity and CXCL1/CCL2 intestinal expression were significantly decreased in the I/R group fed with a high n-6 diet (Figure 2) compared with control diet group. Taken together, these data demonstrate that the administration of a long-term n-6 diet, but not of n-3 diet, protects the gut from the proinflammatory damaging consequences of I/R injury.
Figure 2.
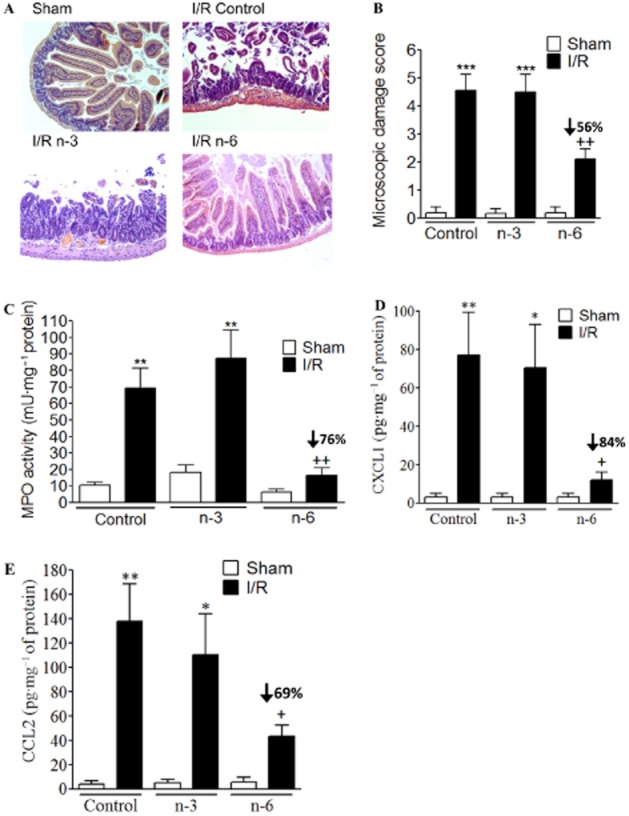
Inflammation of the intestine induced by I/R. (A) Representative hematoxylin and eosin-stained histological sections of small intestine from sham-operated mouse or mouse submitted to I/R and receiving control, n-3 or n-6 PUFA diet. Different inflammatory parameters were followed: microscopic damage scores (B), MPO activity (C) in sham or I/R mice exposed to control, n-3 or n-6 PUFA diet for 9 weeks. (D–E) CXCL1 and CCL2 expressions were quantified in I/R mice versus corresponding sham, exposed to control, n-3 or n-6 PUFA diet. Values are mean ± SEM, n = 6–8 per group. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from sham; +P < 0.05, ++P < 0.01, significantly different from control diet group.
Effects of diet on the production of intestinal n-6 and n-3 metabolites after ischaemia or ischaemia followed by reperfusion
We questioned how much the dietary FAs might influence the eicosanoid and docosanoid profiles likely to influence I/R-induced damage. RvD1, RvD2, 7-MaR1, 14,15-EET, 11,12-EET, 8,9-EET and 5,6-EET were not detectable and PGA1, 8-isoPGA2 and 5,6-DiHETE were not quantifiable in intestinal tissue. PUFA metabolites hierarchical clustering was used to reveal the main differences (Figure 3A). The columns represented the average of PUFA metabolite quantity (pg·mg−1 of proteins) of the different groups of mice fed Ctrl, n-3 or n-6 diet. PUFA metabolites formed four different clusters. The first cluster was composed by product of AA metabolism by COX- (PGE2, 6kPGF1α, TXB2) and lipoxygenase (LOX)-derived metabolites (LTB4, LxB4, 5/8/15/12-HETE). The principal characteristic of this cluster was that the quantity of eicosanoids was maximal following I/R in groups of mice submitted to control diet (Figure 3A). 8/15/12-HETE, PGE2, 6kPGF1α, TXB2, LTB4 and LxB4 were significantly increased only in control diet group submitted to I/R compared with sham I/R (Supporting Information Fig. S2). 5-HETE was significantly increased in control diet group submitted to ischaemia compared with sham ischaemia (Supporting Information Fig. S2). The second cluster was represented only by LxA4; its quantity was maximal in the group of mice submitted to n-6 diet and to ischaemia (Figure 3A). 5-oxo-ETE and 15dPGj2 formed the third cluster characterized by a decrease of these two metabolites by ischaemia independently of the diet. The last cluster regrouped all n-3 PUFA metabolites. They were increased in animals submitted to n-3 diet, independently of ischaemia or I/R (Figure 3A).
Figure 3.
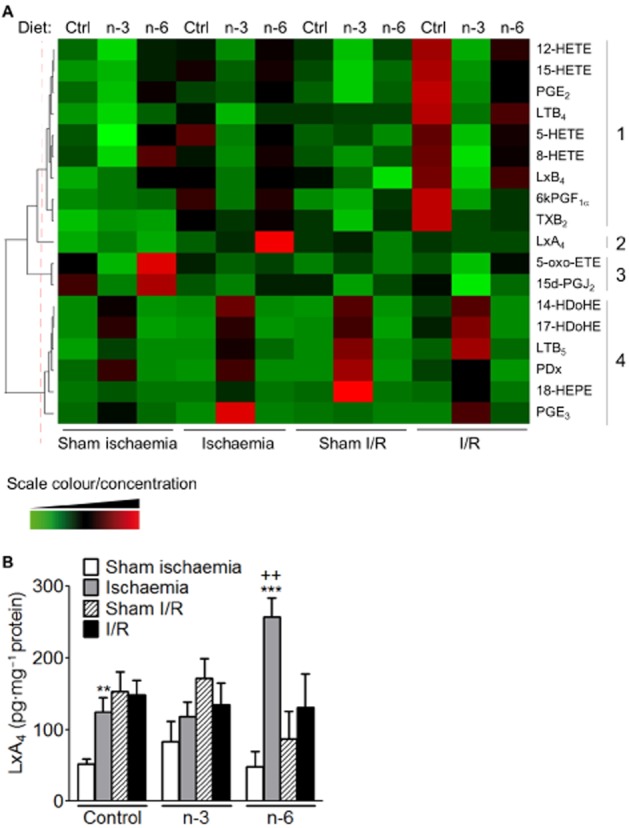
Quantification of PUFA metabolites in intestine of mice exposed to I/R and to the three different diets. (A) Heat-map and clustering of quantified PUFA metabolites by LC-MS/MS. The heat-map represents a color coding of average z-scores obtained for the different PUFA metabolites (in row) and the groups of mice (in column). A red colour indicates a quantity greater than the mean of the row, a green colour indicates a quantity lower than the mean and a black colour indicates a quantity close to the mean. The dendrogram reflects the correlation of the PUFA metabolites. The dotted red line represents the clusters. (B) Synthesis of LxA4 was measured by LC-MS/MS in sham ischaemia, sham I/R animals and following 50 min of ischaemia or 50 min of ischaemia and 5 h of reperfusion (I/R). Data are expressed in pg·mg−1 protein and represent means ± SEM of six to eight animals per group. **P < 0.01, ***P < 0.001, significantly different from corresponding sham; ++P < 0.01, significantly different from ischaemia control diet group.
Because of the protective effect of n-6 diet on I/R injury, we focused our attention on LxA4. In mice receiving the control diet, the anti-inflammatory mediator LxA4 was significantly increased compared with sham after ischaemia (Figure 3B). In mice submitted to n-6 diet, after ischaemia, LxA4 was significantly increased compared with sham and also compared with mice submitted to control diet and ischaemia (Figure 3B). Moreover, in this group of mice, we quantified a significant increase in alox5, alox12 and alox15 mRNA expression compared with mice receiving the control diet after ischaemia (Supporting Information Fig. S3). These findings suggest that diet supplementation is able to change the PUFA metabolites quantity in colonic tissue after ischaemia or I/R by an increase of LOX expression. Interestingly, after ischaemia, the increased LxA4 in n-6 diet versus control diet suggests a possible protective role for this anti-inflammatory lipid in the protection offered by this diet.
Fpr2 is expressed in mouse small intestine
Because the tissue levels of LxA4 were modified in some of the groups studied, we also investigated the expression of the LxA4 receptor Fpr2 in the same tissues. Ischaemia did not modify fpr2 expression compared with sham (Figure 4A). We observed an increase of fpr2 mRNA after I/R in control and n-3 diet groups compared with corresponding sham (Figure 4A). Following I/R, the expression of fpr2 mRNA was not different in mice that received the n-6 diet compared with sham (Figure 4A). By immunohistochemistry, we observed that Fpr2 was expressed in the mucosa of murine small intestine (Figure 4B). Fpr2-immunoreactivity colocalized with cytokeratin 18, Ly-6B.2 and partially with CD45-immunoreactivity meaning that Fpr2 was expressed on epithelial cells, neutrophils and on 44% of nucleated hematopoietic cells respectively (Figure 4B). In those different cell types, no difference of Fpr2 fluorescence intensity was observed (Supporting Information Fig. S4A) between the different groups or diets. In correlation with MPO activity (Figure 2B), Ly-6B.2 expression was increased following I/R in control and n-3 diet groups compared with I/R n-6 diet group (Supporting Information Fig. S4B). Thus, in these two groups, the overexpression of fpr2 could be the consequence of the infiltration of granulocytes expressing Fpr2. Pictures of immunochemistry control procedures are shown in Supporting Information Fig. S4C.
Figure 4.
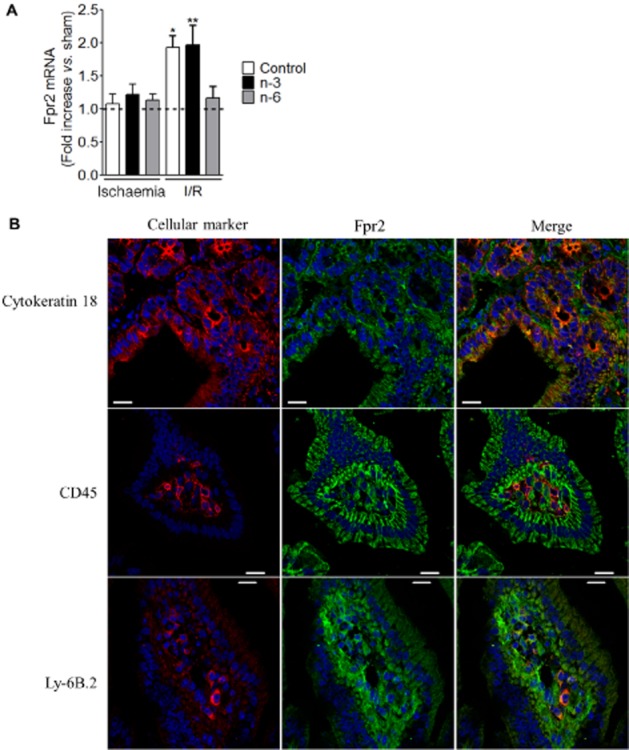
Expression of Fpr2 in mouse small intestine. (A) Fpr2 mRNA expression was quantified in I/R mice versus corresponding sham exposed to control, n-3 or n-6 PUFA diet. (B) Pictures of Fpr2, cytokeratin 18, CD45 and Ly-6B.2 immunoreactivity detected in the small intestine of mice exposed to sham and to n-6 PUFA diet (scale: 50 μm). Values are mean ± SEM, n = 6–8 per group. *P < 0.05, **P < 0.01, significantly different from corresponding ischaemia group.
Pharmacological blockade of Fpr2 prevented the anti-inflammatory effects obtained by the n-6 rich diet
LxA4 is an effector of endogenous anti-inflammation acting via its receptor, Fpr2. In mice receiving a high n-6 diet, administration of the Fpr pan antagonist, Boc2 (500 μg kg−1 before ischaemia i.v.) abolished the inhibition of microscopic damage score, MPO activity, CXCL1 and CCL2 expression induced by n-6 diet following I/R (Figure 5A). Administration of the Fpr2 antagonist also prevented the inhibition of 15-HETE, PGE2, 6kPGF1α and TXB2 induced by the n-6-enriched diet (Figure 5B). Administration of Boc2 had no effect on the increase of LxA4 induced by ischaemia in n-6 diet group (Figure 5C). These findings show that the blockade of the LxA4 receptor (Fpr2) annulled the anti-inflammatory effects obtained by the n-6-rich diet and that n-6 supplementation-induced protection after I/R is indeed mediated by Fpr2 activation.
Figure 5.
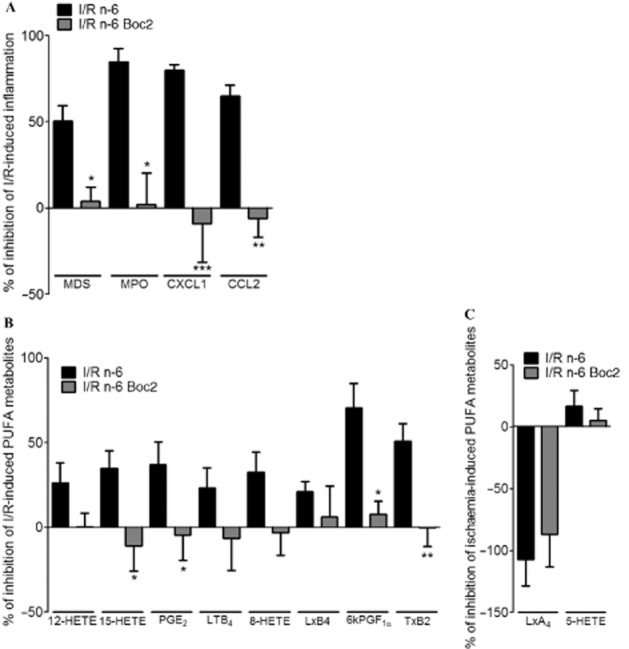
Role of Fpr2 antagonist in n-6 PUFA diet anti-inflammatory effects. (A) Different inflammatory parameters were followed: MPO activity, microscopic damage scores, CXCL1 expression and CCL2 expression, in sham or I/R mice treated with the Fpr2 antagonist (Boc2, 500 μg kg−1 before ischaemia i.v.) and exposed to n-6 PUFA diet for 9 weeks. (B) n-6 PUFA-derived metabolites in sham or I/R mice treated with the Fpr2 antagonist (Boc2, 500 μg kg−1 before ischaemia i.v.) and exposed to n-6 PUFA diet for 9 weeks. Data are expressed as percentage of inhibition of I/R-induced effect in control diet group. Values are mean ± SEM, n = 6–8 per group. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from I/R n-6 vehicle group.
Discussion
In the present study, we evaluated the effect of dietary intervention based on different n-6 and n-3 PUFA intake, on injury associated with intestinal I/R. Our data indicate that n-6 PUFA afforded significant protection against I/R-induced tissue injuries, while n-3 PUFA diet had no effect on intestinal damage as determined in these settings. Specifically, in mice subjected to intestinal I/R, n-6 PUFA supplementation in diet decreased mucosal damage, granulocyte recruitment and chemokine expression compared with animals fed with a control balanced diet. Protection against I/R injury was associated with an increase of alox5, alox12 and alox15 mRNA expression and consequently of LxA4 production following ischaemia in intestinal tissues from n-6-enriched FA-treated group compared with control diet-fed group. In vivo experiments demonstrated that the LxA4 receptor Fpr2 was involved in the protective effects associated with n-6 PUFA-enriched diet. Collectively, these data make us propose that early during ischaemia, an endogenous protective pathway mediated by LxA4 and its receptor could be triggered by changes in dietary FA.
Our results further confirmed the essential role that AA-derived lipid mediators could play in resolving inflammatory response in vivo (Serhan and Samuelsson, 1988). This notion has been well developed by a number of studies and AA is now considered not only as a precursor of proinflammatory bioactive lipids, but also of potent anti-inflammatory mediators named lipoxins (Morris et al., 2006; Schwab and Serhan, 2006; Wallace, 2006; Dufton and Perretti, 2010). Indeed, in our experimental conditions, we observed that ischaemia and ischaemia followed by reperfusion led to an increase of proinflammatory bioactive lipids or pathway marker derived from AA metabolism by COX (PGE2, 6kPGF1α TxB2) and LOX (5-, 8-, 12-, 15-HETE, LTB4). Simultaneously, potent anti-inflammatory mediators derived from the AA metabolism, LxA4 and LxB4, were also increased. These two lipoxins are differentially expressed in intestinal tissues over time, LxA4 being produced from the ischaemic period and LxB4 (its positional isomer) increased only after 5 h reperfusion. In our study, increased level of LxA4 was significantly higher in n-6 diet group after ischaemia compared with control diet group. However, levels of LxB4 [whose receptor(s) is yet to be discovered] were the same in both groups. We have recently reported a mechanism activated by neutrophil/platelet aggregates to increased circulating levels of LxA4 at the end of 30 min mesenteric ischaemia (Brancaleone et al., 2013). This is in agreement with our current data and supports the notion that LxA4 is an early metabolite of ischaemic conditions. The effect of LXA4/Fpr2 system in the protective effects of n-6 diet suggests a minor role for LxB4 in our experimental conditions.
LxA4 was identified by Serhan et al. in 1984 as a short-lived lipid produced principally by transcellular synthesis (Serhan et al., 1984). LxA4 is described as a potent endogenous anti-inflammatory bioactive lipid mediator (Serhan and Savill, 2005). Acting through its receptor, Fpr2 (Lavigne et al., 2002; Perretti et al., 2002; Ye et al., 2009), a GPCR belonging to the Fpr family, LxA4 is able to halt leukocyte migration (Chiang et al., 2006; Perretti and D'Acquisto, 2009) and promote macrophage phagocytosis of infectious agents and apoptotic neutrophils (Godson et al., 2000; Maderna et al., 2005). In agreement with our results, several studies have demonstrated a protective role for pharmacologically delivered lipoxins or stable lipoxin analogues in I/R (Bannenberg et al., 2004; Sun et al., 2009; Wu et al., 2012).
Cell–cell interaction is one of the routes by which lipoxins are endogenously biosynthesized by cooperation between 15- and 5-LOX (Serhan et al., 2008). Primarily described in granulocytes and later in airway epithelial cells and dendritic cells, metabolism of AA by 15-LOX produces 15S-hydroperoxyeicosatetraenoic acid or the reduced alcohol form 15S-hydroxyeicosatetraenoic acid (15S-HETE) (Serhan, 1989; Levy et al., 1993; Serhan et al., 2008). These metabolites are further transformed into epoxytetraene intermediates by the action of leukocyte 5-LOX yielding LxA4 and LxB4 (Serhan, 1989; Levy et al., 1993; Serhan et al., 2008). In the intestine, resident tissue cells such as intestinal epithelial cells expressed 15-LOX and could further contribute to LxA4 generation by the production of 15S-H(p)ETE (McMahon et al., 2001). Indeed, LxA4 is synthesized in the human gut by colonic mucosa (Mangino et al., 2006). Interestingly, in pathological conditions such as ulcerative colitis, LxA4 endogenous production is significantly reduced compared with control patients (Mangino et al., 2006). This decrease was coupled to a lower level of 15-LOX in the mucosa of ulcerative colitis patients compared with tissues from healthy controls (Mangino et al., 2006). In our experimental conditions, where alox5 and alox15 expression and LxA4 were increased after ischaemia before granulocyte infiltration, epithelial cells could be the principal source of 15S-H(p)ETE or 15S-HETE metabolized into LxA4 by 5-LOX-bearing cells (polymorphonuclear leukocytes, macrophages and mast cells that physiologically populate the gut). LxA4 acts on a specific target receptor termed Fpr2/lipoxin A4 receptor (FPR2/ALX) in humans and Fpr2/3 in mice (Perretti et al., 2002; Ye et al., 2009). FPR2 not only transduces the proresolving properties of LXA4, but also of the glucocorticoid regulated protein Annexin A1 and humanin (Dufton and Perretti, 2010). Increasing evidence suggests that members of the FPR family and in particular FPR2 might have an important role in the pathophysiology of I/R injury (Gavins, 2010). In a recent study, the use of fpr2/3-deficient mice highlighted the importance of this receptor in I/R; in the absence of Fpr2/3, an aberrant inflammatory response characterized by prolonged neutrophil adhesion and infiltration was observed (Brancaleone et al., 2013). Furthermore, exogenous delivery of LxA4 attenuated I/R-mediated inflammation in wild-type but not in Fpr2/3-deficient animals (Brancaleone et al., 2013). In mice receiving a high n-6 diet, administration of the Fpr2 antagonist before ischaemia reverted the protective anti-inflammatory effect of this diet. Our data indicate that the protective association between n-6 supplementation and the prevention of post-ischaemic damage was due, at least in part, to the interaction between LxA4 (produced in higher amount in the n-6 group) and its receptor, Fpr2. Human FPR2/ALX was first identified on neutrophils and monocytes (Fiore et al., 1992; Takano et al., 1997). Here, we observed that the murine receptor was expressed also in intestinal epithelial cells, confirming a previous in vitro study that had identified Fpr2 in intestinal epithelial cell lines (Goh et al., 2001). Interestingly, in vitro studies have identified Fpr2 expression preferentially on the basolateral side of intestinal epithelial cells (Kucharzik et al., 2003). LxA4 produced on the serosal side of the epithelium by leukocyte–epithelial cell interaction could rapidly signal by activating the receptor on the basolateral side, resulting in potential attenuation of inflammation. LxA4 promotes anti-inflammatory signals not only by regulating innate immune cells, but also by acting on intestinal epithelial cells. Upon stimulation with bacteria (Salmonella typhimurium) (Gewirtz et al., 1998), TNF-α (Gronert et al., 1998; Goh et al., 2001) or LPS (Kure et al., 2010), human intestinal epithelial cells release the proinflammatory chemokine IL-8: this response could be inhibited by cell exposure to LxA4. In accordance with these data, we observed that after I/R the n-6-enriched diet was associated with a decrease in CXCL1, the murine equivalent of human IL-8. Other proinflammatory signals, such as the transcription factor NF-κB can be modulated by LxA4 in epithelial cells (Gewirtz et al., 1998). Such inhibition is also downstream of Fpr2 engagement by LxA4 (Gewirtz et al., 2002). There is conflicting data regarding how much n-6 PUFA (i.e. LA) should be consumed in a healthy diet. Some claim that high LA intake promotes inflammation through accumulation of tissue AA and subsequent production of proinflammatory lipid mediators. Human studies indicate that high LA in the diet or circulation is not associated with higher in vivo or ex vivo proinflammatory responses. Several studies have shown that individuals consuming the highest level of LA have the lowest inflammatory status (Fritsche, 2008). In our experimental animal study, we did not detect any ‘proinflamamtory phenotype’ of the n-6 diet group that underwent a sham operation compared with the n-3 or control diet. Conversely, the n-6 but not the n-3-enriched diet displayed anti-inflammatory properties indicating that the consumption of high n-6 diets may work in specific intestinal scenarios. Exploiting this n-6-dependent endogenous anti-inflammatory loop described here could be of therapeutic impact in diseases in which intestinal ischaemia contributes to the pathogenesis, such as neonatal necrotizing enterocolitis (NEC) (Berman and Moss, 2011). It has been shown that the type and composition of infant diet can regulate the outcome of this pathology. In a clinical study, Carlson et al. found a lower incidence of NEC in infants fed formula containing higher amounts of PUFA compared with controls (Carlson et al., 1998). In the rat NEC model, supplementation of the formula with AA has been shown to reduce the degree of intestinal injury (Lu et al., 2007). Thus, in this pathology, delivery of LxA4, shown to be the most important AA metabolite in our model, could be even more effective than AA-based treatment.
In conclusion, these data prompt us to propose that the endogenous anti-inflammatory circuit activated by a selective Fpr2 agonist, LxA4, could be harnessed by boosting the n-6 PUFA content of the diet, as a viable therapeutic approach for the prevention of intestinal I/R injuries.
Acknowledgments
The authors thank the microscope core facility, INSERM UMR1043, Toulouse, the animal core facility and the histology core facility, Genetoul, anexplo, US006/INSERM, Toulouse. Authors thank Dr. Pascal Martin for heat-map construction and analyses. This work was supported by the Agence Nationale de la Recherche (to N. V., ANR-12-BSV1-0030-01 and N. C., ANR-12-JSV1-0001-01), the INSERM (to N. V.), two grants from the Region Midi-Pyrénées (to P. L. F. and N. C.), the European Research Council (to N. V., ERC-2012-StG-20111109) and the Wellcome Trust (program 086867/Z/08/Z, to T. G. and M. P.). Ambiotis SAS is supported by grants from the Region Midi-Pyrénées and the European Union.
Glossary
- 6kPGF1α
6-keto-prostaglandin F1α
- 7-MaR1
7(S)-maresin
- AA
arachidonic acid
- ACN
acetonitrile
- DHA
docosahexaenoic acid
- DiHETE
dihydroxy-eicosatetraenoic acid
- EET
epoxyeicosatrienoic acid
- EPA
eicosapentaenoic acid
- FA
fatty acid
- Fpr2
LxA4 receptor
- HDoHE
hydroxy docosahexaenoic acid
- HEPE
hydroxyeicosapentaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- I/R
ischaemia/reperfusion
- LTB4-d4
deuterated LTB4
- LOD
limit of detection
- LOQ
limit of quantification
- LxA4
lipoxin A4
- LxA4-d5
deuterated lipoxin A4
- LxB4
lipoxin B4
- LOX
lipoxygenase
- MeOH
methanol
- MPO
myeloperoxidase
- NEC
neonatal necrotizing enterocolitis
- oxo-ETE
oxoeicosatetraenoic acid
- PDx
10(S),17(S)-protectin
- PUFA
polyunsaturated fatty acids
- RvD1
resolvin D1
- RvD2
resolvin D2
- SMA
superior mesenteric artery
- UHPLC
ultra HPLC
Author contributions
T. G. contributed in the acquisition, analysis and interpretation of data and in the redaction of the manuscript. S. D. contributed in the acquisition and analysis of data. P. L. F. contributed in the acquisition and analysis of data and in the redaction of the manuscript. J. B. contributed in the acquisition and analysis of data. J. B.-M. contributed in the analysis and interpretation of data. M. D. contributed in the interpretation of data and in the redaction of the manuscript. H. G. contributed in the conception and design of the study, interpretation of data and in the redaction of the manuscript. M. P. contributed in the conception and design of the study and in the redaction of the manuscript. N. V. contributed in the conception and design of the study and in the redaction of the manuscript. N. C. contributed in the conception and design of the study, acquisition, analysis and interpretation of data and in the redaction of the manuscript. T. G. and N. C. take responsibility for the integrity of the work as a whole, from inception to the published article.
Conflict of interest
The authors have no potential conflicts (financial, professional or personal) that are relevant to the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Effect of different PUFA diets on weight gain. Body weight gain in male C57BL/6J mice given n-3, n-6 or control diet for 9 weeks. Values are mean ± SEM, n = 6–8 per group.
Figure S2 Concentration of PUFA metabolites in intestine of mice exposed to sham, ischaemia or I/R and to the three different diets. PUFA concentration in sham ischaemia (white bar), sham I/R animals (striped bar) and following 50 min of ischaemia (gray bar) or 50 min of ischaemia and 5 h of reperfusion (I/R, black bar). Data are expressed in pg·mg−1 protein and represented as mean ± SEM of six to eight animals per group. *P < 0.05, **P < 0.0l, ***P < 0.00l, significantly different from corresponding sham.
Figure S3 Expression of LOX isoforms in mouse small intestine. Arachidonate 15 1ipoxygenase (ALOX15; A), arachidonate 12 lipoxygenase (ALOX12; B) and arachidonate 5 lipoxygenase (ALOX5; C) mRNA expression were quantified in ischaemia and I/R mice versus corresponding sham, exposed to control (white bar), n-6 (black bar) or n-3 (gray bar) PUFA diet. Values are mean ± SEM, n = 6–8 per group. **P < 0.0l, significantly different from control diet/ischaemia group.
Figure S4 Quantification of FPR2 and Ly-6B.2 expression in mouse small intestine. (A) Quantification of FPR2 fluorescence intensity in the different cell types of the small intestine of mice exposed to sham, ischaemia, sham I/R and I/R and to the control, n-3 or n-6 diet. (B) quantification of the area positive for Ly-6B.2 immunoreactivity in the small intestine of mice exposed to sham, ischaemia, sham I/R and I/R and to the control, n-3 or n-6 diet. Values are mean ± SEM, n = 5–6 per group. *P < 0.05, **P < 0.0l, significantly different from n-6 diet and I/R group. (C) Pictures of immunochemistry control procedures: reaction performed with omission of primary (488 donkey anti-rabbit Ig + 555 donkey anti-goat IgG or 488 donkey anti-rabbit IgG + 555 donkey anti-rat IgG) or secondary antibodies (rabbit anti-FPR2 + rat anti-LY-6B.2 + goat anti-CD45 + goat anti-CK18).
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013a;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med. 2011;16:145–150. doi: 10.1016/j.siny.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Boue J, Blanpied C, Brousset P, Vergnolle N, Dietrich G. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J Immunol. 2011;186:5078–5084. doi: 10.4049/jimmunol.1003335. [DOI] [PubMed] [Google Scholar]
- Brancaleone V, Gobbetti T, Cenac N, le Faouder P, Colom B, Flower RJ, et al. A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood. 2013;122:608–617. doi: 10.1182/blood-2013-04-496661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SE, Montalto MB, Ponder DL, Werkman SH, Korones SB. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr Res. 1998;44:491–498. doi: 10.1203/00006450-199810000-00005. [DOI] [PubMed] [Google Scholar]
- Cattaruzza F, Cenac N, Barocelli E, Impicciatore M, Hyun E, Vergnolle N, et al. Protective effect of proteinase-activated receptor 2 activation on motility impairment and tissue damage induced by intestinal ischemia/reperfusion in rodents. Am J Pathol. 2006;169:177–188. doi: 10.2353/ajpath.2006.051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336–343. doi: 10.1590/s0102-86502005000400013. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- Ducheix S, Montagner A, Polizzi A, Lasserre F, Marmugi A, Bertrand-Michel J, et al. Essential fatty acids deficiency promotes lipogenic gene expression and hepatic steatosis through the liver X receptor. J Hepatol. 2013;58:984–992. doi: 10.1016/j.jhep.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Dufton N, Perretti M. Therapeutic anti-inflammatory potential of formyl-peptide receptor agonists. Pharmacol Ther. 2010;127:175–188. doi: 10.1016/j.pharmthera.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Ryeom SW, Weller PF, Serhan CN. Lipoxin recognition sites. Specific binding of labeled lipoxin A4 with human neutrophils. J Biol Chem. 1992;267:16168–16176. [PubMed] [Google Scholar]
- Fritsche KL. Too much linoleic acid promotes inflammation-doesn't it? Prostaglandins Leukot Essent Fatty Acids. 2008;79:173–175. doi: 10.1016/j.plefa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Gavins FN. Are formyl peptide receptors novel targets for therapeutic intervention in ischaemia-reperfusion injury? Trends Pharmacol Sci. 2010;31:266–276. doi: 10.1016/j.tips.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, et al. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, et al. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Gobbetti T, Cenac N, Motta JP, Rolland C, Martin L, Andrade-Gordon P, et al. Serine protease inhibition reduces post-ischemic granulocyte recruitment in mouse intestine. Am J Pathol. 2012;180:141–152. doi: 10.1016/j.ajpath.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbetti T, Le Faouder P, Bertrand J, Dubourdeau M, Barocelli E, Cenac N, et al. Polyunsaturated fatty acid metabolism signature in ischemia differs from reperfusion in mouse intestine. PLoS ONE. 2013;8:e75581. doi: 10.1371/journal.pone.0075581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Goh J, Baird AW, O'Keane C, Watson RW, Cottell D, Bernasconi G, et al. Lipoxin A(4) and aspirin-triggered 15-epi-lipoxin A(4) antagonize TNF-alpha-stimulated neutrophil-enterocyte interactions in vitro and attenuate TNF-alpha-induced chemokine release and colonocyte apoptosis in human intestinal mucosa ex vivo. J Immunol. 2001;167:2772–2780. doi: 10.4049/jimmunol.167.5.2772. [DOI] [PubMed] [Google Scholar]
- Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelavkar UP, Hutzley J, Dhir R, Kim P, Allen KG, McHugh K. Prostate tumor growth and recurrence can be modulated by the omega-6: omega-3 ratio in diet: athymic mouse xenograft model simulating radical prostatectomy. Neoplasia. 2006;8:112–124. doi: 10.1593/neo.05637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010a;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010b;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Gewirtz AT, Merlin D, Madara JL, Williams IR. Lateral membrane LXA4 receptors mediate LXA4's anti-inflammatory actions on intestinal epithelium. Am J Physiol Cell Physiol. 2003;284:C888–C896. doi: 10.1152/ajpcell.00507.2001. [DOI] [PubMed] [Google Scholar]
- Kure I, Nishiumi S, Nishitani Y, Tanoue T, Ishida T, Mizuno M, et al. Lipoxin A(4) reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. J Pharmacol Exp Ther. 2010;332:541–548. doi: 10.1124/jpet.109.159046. [DOI] [PubMed] [Google Scholar]
- Lavigne MC, Murphy PM, Leto TL, Gao JL. The N-formylpeptide receptor (FPR) and a second G(i)-coupled receptor mediate fMet-Leu-Phe-stimulated activation of NADPH oxidase in murine neutrophils. Cell Immunol. 2002;218:7–12. doi: 10.1016/s0008-8749(02)00564-6. [DOI] [PubMed] [Google Scholar]
- Le Faouder P, Baillif V, Spreadbury I, Motta JP, Rousset P, Chene G, et al. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;932C:123–133. doi: 10.1016/j.jchromb.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572–1579. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Jilling T, Li D, Caplan MS. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2007;61:427–432. doi: 10.1203/pdr.0b013e3180332ca5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2–26) J Immunol. 2005;174:3727–3733. doi: 10.4049/jimmunol.174.6.3727. [DOI] [PubMed] [Google Scholar]
- Mangino MJ, Brounts L, Harms B, Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon B, Mitchell S, Brady HR, Godson C. Lipoxins: revelations on resolution. Trends Pharmacol Sci. 2001;22:391–395. doi: 10.1016/s0165-6147(00)01771-5. [DOI] [PubMed] [Google Scholar]
- Morris T, Rajakariar R, Stables M, Gilroy DW. Not all eicosanoids are bad. Trends Pharmacol Sci. 2006;27:609–611. doi: 10.1016/j.tips.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Motta JP, Bermudez-Humaran LG, Deraison C, Martin L, Rolland C, Rousset P, et al. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med. 2012;4:158ra144. doi: 10.1126/scitranslmed.3004212. [DOI] [PubMed] [Google Scholar]
- Otamiri T, Franzen L, Lindmark D, Tagesson C. Increased phospholipase A2 and decreased lysophospholipase activity in the small intestinal mucosa after ischaemia and revascularisation. Gut. 1987;28:1445–1453. doi: 10.1136/gut.28.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Serhan CN. On the relationship between leukotriene and lipoxin production by human neutrophils: evidence for differential metabolism of 15-HETE and 5-HETE. Biochim Biophys Acta. 1989;1004:158–168. doi: 10.1016/0005-2760(89)90264-6. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Samuelsson B. Lipoxins: a new series of eicosanoids (biosynthesis, stereochemistry, and biological activities) Adv Exp Med Biol. 1988;229:1–14. doi: 10.1007/978-1-4757-0937-7_1. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YP, Tjonahen E, Keledjian R, Zhu M, Yang R, Recchiuti A, et al. Anti-inflammatory and pro-resolving properties of benzo-lipoxin A(4) analogs. Prostaglandins Leukot Essent Fatty Acids. 2009;81:357–366. doi: 10.1016/j.plefa.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg. 2011;396:13–29. doi: 10.1007/s00423-010-0727-x. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Nitric oxide, aspirin-triggered lipoxins and NO-aspirin in gastric protection. Inflamm Allergy Drug Targets. 2006;5:133–137. doi: 10.2174/187152806776383116. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, et al. A lipoxin A4 analog ameliorates blood-brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. J Mol Neurosci. 2012;46:483–491. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, et al. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadravec D, Brolinson A, Fisher RM, Carneheim C, Csikasz RI, Bertrand-Michel J, et al. Ablation of the very-long-chain fatty acid elongase ELOVL3 in mice leads to constrained lipid storage and resistance to diet-induced obesity. FASEB J. 2010;24:4366–4377. doi: 10.1096/fj.09-152298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effect of different PUFA diets on weight gain. Body weight gain in male C57BL/6J mice given n-3, n-6 or control diet for 9 weeks. Values are mean ± SEM, n = 6–8 per group.
Figure S2 Concentration of PUFA metabolites in intestine of mice exposed to sham, ischaemia or I/R and to the three different diets. PUFA concentration in sham ischaemia (white bar), sham I/R animals (striped bar) and following 50 min of ischaemia (gray bar) or 50 min of ischaemia and 5 h of reperfusion (I/R, black bar). Data are expressed in pg·mg−1 protein and represented as mean ± SEM of six to eight animals per group. *P < 0.05, **P < 0.0l, ***P < 0.00l, significantly different from corresponding sham.
Figure S3 Expression of LOX isoforms in mouse small intestine. Arachidonate 15 1ipoxygenase (ALOX15; A), arachidonate 12 lipoxygenase (ALOX12; B) and arachidonate 5 lipoxygenase (ALOX5; C) mRNA expression were quantified in ischaemia and I/R mice versus corresponding sham, exposed to control (white bar), n-6 (black bar) or n-3 (gray bar) PUFA diet. Values are mean ± SEM, n = 6–8 per group. **P < 0.0l, significantly different from control diet/ischaemia group.
Figure S4 Quantification of FPR2 and Ly-6B.2 expression in mouse small intestine. (A) Quantification of FPR2 fluorescence intensity in the different cell types of the small intestine of mice exposed to sham, ischaemia, sham I/R and I/R and to the control, n-3 or n-6 diet. (B) quantification of the area positive for Ly-6B.2 immunoreactivity in the small intestine of mice exposed to sham, ischaemia, sham I/R and I/R and to the control, n-3 or n-6 diet. Values are mean ± SEM, n = 5–6 per group. *P < 0.05, **P < 0.0l, significantly different from n-6 diet and I/R group. (C) Pictures of immunochemistry control procedures: reaction performed with omission of primary (488 donkey anti-rabbit Ig + 555 donkey anti-goat IgG or 488 donkey anti-rabbit IgG + 555 donkey anti-rat IgG) or secondary antibodies (rabbit anti-FPR2 + rat anti-LY-6B.2 + goat anti-CD45 + goat anti-CK18).


