Abstract
Background:
Population-based testing for BRCA1/2 mutations detects the high proportion of carriers not identified by cancer family history (FH)–based testing. We compared the cost-effectiveness of population-based BRCA testing with the standard FH-based approach in Ashkenazi Jewish (AJ) women.
Methods:
A decision-analytic model was developed to compare lifetime costs and effects amongst AJ women in the UK of BRCA founder-mutation testing amongst: 1) all women in the population age 30 years or older and 2) just those with a strong FH (≥10% mutation risk). The model assumes that BRCA carriers are offered risk-reducing salpingo-oophorectomy and annual MRI/mammography screening or risk-reducing mastectomy. Model probabilities utilize the Genetic Cancer Prediction through Population Screening trial/published literature to estimate total costs, effects in terms of quality-adjusted life-years (QALYs), cancer incidence, incremental cost-effectiveness ratio (ICER), and population impact. Costs are reported at 2010 prices. Costs/outcomes were discounted at 3.5%. We used deterministic/probabilistic sensitivity analysis (PSA) to evaluate model uncertainty.
Results:
Compared with FH-based testing, population-screening saved 0.090 more life-years and 0.101 more QALYs resulting in 33 days’ gain in life expectancy. Population screening was found to be cost saving with a baseline-discounted ICER of -£2079/QALY. Population-based screening lowered ovarian and breast cancer incidence by 0.34% and 0.62%. Assuming 71% testing uptake, this leads to 276 fewer ovarian and 508 fewer breast cancer cases. Overall, reduction in treatment costs led to a discounted cost savings of £3.7 million. Deterministic sensitivity analysis and 94% of simulations on PSA (threshold £20000) indicated that population screening is cost-effective, compared with current NHS policy.
Conclusion:
Population-based screening for BRCA mutations is highly cost-effective compared with an FH-based approach in AJ women age 30 years and older.
Genetic testing for high-penetrance BRCA mutations is currently restricted to individuals from high-risk families fulfilling stringent family history (FH)–based criteria. However, a large proportion of BRCA carriers do not fulfil the current threshold for genetic testing. We found that more than 50% of BRCA carriers are missed by the FH-based approach, which is consistent with some earlier reports in which 40% to 63% (1–3) of carriers in population cohorts and 50% to 75% of carriers from cancer case series unselected for FH (4–9) lacked a strong FH of cancer. New gene-sequencing technologies (10) and the falling cost of genetic testing will make it feasible to test large populations in the near future. This could lead to new approaches capable of detecting a larger proportion of carriers of high-penetrance mutations and a change from the current FH-based approach. Systematic BRCA founder mutation (FM) testing in a low-risk Ashkenazi Jewish (AJ) population is acceptable and is not associated with differences in short-term psychological or quality-of-life outcomes compared with an FH-based approach (11). The AJ population could be the first population for whom population-based testing is feasible.
A health-economic evaluation is essential for an overall assessment of the balance of costs and health benefits in the context of setting public health policy for genetic testing of high-penetrance cancer gene mutations. Decision analytical modeling compares the expected costs and consequences of decision options by synthesizing information from multiple sources and applying mathematical techniques, usually with computer software (12). The current clinical approach, which uses high-risk families for case identification, is more cost-effective than no genetic screening with a cost-effectiveness ratio (CER) of 4294 euros per life-year-gained reported (13). In addition, preventive surgery is more cost-effective than screening in known BRCA and mismatch repair (MMR) gene carriers (14–16). However, health-economic data using truly population-based ascertainment are limited. There is only one cost-utility analysis comparing population-based screening with no screening in the AJ population (17), which found that screening would prevent 2811 ovarian cancers in the United States, for a (discounted) program cost of $8300 per quality-adjusted life-year (QALY). However, to date, the cost-effectiveness of a population-based approach has not been compared with an FH-based approach. Genetic Cancer Prediction through Population Screening (GCaPPS) is a randomized trial (ISRCTN73338115) comparing outcomes of population and FH-based approaches for genetic testing in UK AJ women. In order to provide policy makers with the best available evidence, we use data from the GCaPPS trial to describe a decision analysis model comparing both population and FH-based approaches for genetic-testing in AJ women.
Methods
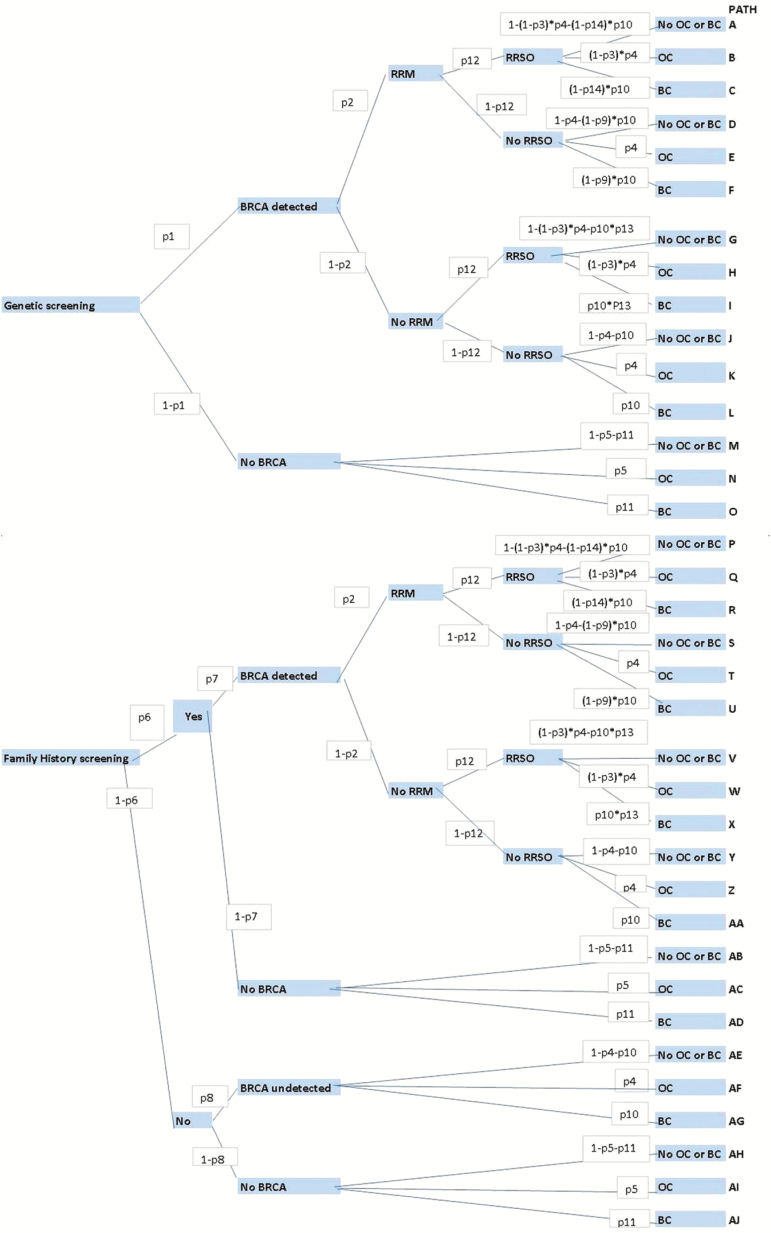
A decision analytic model (Figure 1) was developed to compare the lifetime costs and effects of genetically screening all UK AJ women age 30 years and older for BRCA FM, compared with the current practice of screening using FH-based criteria (≥10% mutation risk) (Supplementary Table 1, available online). The model assumes that all women in the population screening arm and only those with a strong FH in the FH arm are offered genetic counseling and genetic testing. A 71% uptake rate of genetic testing (estimated from the GCaPPS study) was incorporated into the model. Genetic testing involved analysis for the three BRCA FM associated with Jewish descent in a National Health Service (NHS) genetics laboratory. In line with current guidelines (18,19) women testing positive were offered risk-reducing salpingo-oophorectomy (RRSO) to reduce their ovarian cancer risk (20,21) and MRI/mammography screening or risk-reducing mastectomy (RRM) (22) to reduce their breast cancer risk. Use of a selective estrogen receptor modulator (SERM) for breast cancer chemoprevention (23) was also included in the model as part of a sensitivity analysis, but ovarian cancer screening was excluded, as its clinical value remains uncertain (24). In line with guidelines on the reference case for economic evaluation from the National Institute of Health and Clinical Excellence (NICE), all costs and outcomes were discounted at 3.5% (25).
Figure 1.
Decision model structure. The upper part of the model structure reflects a population-based approach to BRCA testing, and the lower part of the model depicts a family history (FH)–based approach. Each decision point in the model is called a “node,” and each path extending from a node is called a decision “branch.” Each branch represents a mutually exclusive course or outcome. Each decision is given a probability (probabilities p1 to p14 used in the model are explained in Table 1) highlighted in a white box along the decision branch. Values for each outcome are calculated. Cancer incidence was estimated by summing the probabilities of pathways ending in ovarian or breast cancer. Final outcomes (blue boxes on the right of the figure) of each path include development of breast cancer (BC), ovarian cancer (OC) and no breast/ovarian cancer (no OC or BC). BC = breast cancer; No OC or BC = no ovarian cancer or breast cancer developed; OC = ovarian cancer; RRSO = risk-reducing salpingo-oophorectomy; RRM = risk-reducing mastectomy.
Probabilities
All pathway probabilities for the model are presented and explained in Table 1. A one-way sensitivity analysis involved rerunning the model at both lower and upper values/limits of the 95% confidence interval or range of all probability parameters (Table 1) used in the model (Figures 2 and 3). Cancer incidence was estimated by summing the probabilities of pathways ending in ovarian or breast cancer. The current estimated UK population of AJ women is 114400 (26,27). The total population impact was estimated by multiplying the results per woman by 81224 (0.71*114400). Additionally, the effect if all 114400 women underwent testing was also calculated.
Table 1.
Probabilities of different pathways in the model*
| Probability | Value (95% CI) | Description | Source |
|---|---|---|---|
| P1 | 0.0245 (0.0131 to 0.0416) | Population prevalence of BRCA FM | GCaPPS |
| P2 | 0.52 (0.39 to 0.67) | Probability that carrier will undergo RRM | Evans (71) |
| P3 | 0.96 (0.8 to 0.96) | Reduction in risk of ovarian cancer from RRSO | Finch (20), Rebbeck (21) |
| P4 | 0.2987 (0.2485 to 0.3539) | Probability that carrier without RRSO will get ovarian cancer | Chen (61) |
| P5 | 0.0185 (0.0005 to 0.0989) | Probability that a noncarrier will get ovarian cancer | CRUK (84) |
| P6 | 0.1238 (0.1043 to 0.1454) | Probability of having a positive FH | GCaPPS |
| P7 | 0.0938 (0.0637 to 0.1763) | BRCA prevalence in FH positive individuals | GCaPPS |
| P8 | 0.0203 (0.0114 to 0.0332) | BRCA prevalence in FH negative individuals | GCaPPS |
| P9 | 0.91 (0.62 to 0.98) | Reduction in breast cancer risk from | RRM without RRSO | Rebbeck (22) |
| P10 | 0.53 (0.44 to 0.62) | Probability that carrier without RRM will get breast cancer | Chen (61) |
| P11 | 0.13 (0.11 to 0.14) | Probability that a noncarrier will get breast cancer with screening | CRUK (46), ONS (85) |
| P12 | 0.55 (0.30 to 0.75) | Probability that carrier will follow-up with RRSO | Manchanda (62) |
| P13 | 0.49 (0.37 to 0.65) | Reduction in risk of breast cancer from RRSO alone | Rebbeck (21) |
| P14 | 0.95 (0.78 to 0.99) | Reduction in risk of breast cancer from RRM with RRSO | Rebbeck (22) |
* CI = confidence interval; FH = family history; FM = founder mutations; GCaPPS = Genetic Cancer Prediction through Population Screening study; RRSO = risk-reducing salpingo-oophorectomy; RRM = risk-reducing mastectomy.
P1: The probability of carrying a BRCA FM in the AJ population (p1 = 0.0245) is taken from the GCaPPS study, as it provides UK-based data and is consistent with reports from other countries (2,86).
P2: The probability that BRCA1/2 carrier will undergo RRM is taken from an analysis of UK BRCA1/2 carriers by Evans et al. 2009. A composite uptake rate (p2 = 0.52) for BRCA1 (60% RRM rate) and BRCA2 (43% RRM rate) carriers weighted for the relative prevalence of BRCA1 and BRCA2 FM found in the London AJ population was computed (71).
P3: The reduction in ovarian cancer risk obtained from RRSO (p3 = 0.96) is taken from previous studies, which report a 4% residual risk of primary peritoneal cancer following RRSO (20).
P4: A wide range of ovarian cancer risks have been reported for BRCA carriers, with higher penetrance estimates found in carriers ascertained from high-risk families with multiple cancer cases (87). Our analysis uses ovarian cancer penetrance figures (40% for BRCA1, 18% for BRCA2) from a meta-analysis, corrected for ascertainment (61). To simplify the analysis, we have used a composite risk for BRCA1 and BRCA2 carriers (p4 = 0.2987), weighted for the relative prevalence of BRCA1 and BRCA2 FM found in the London AJ population. The BRCA1 population prevalence is 0.0132, and BRCA2 population prevalence is 0.0113 (GCaPPS study). The overall risk of ovarian cancer in BRCA carriers is calculated as ((0.0132*0.4)/2.45 + (0.0113*0.18)/2.45).
P5: The risk of ovarian cancer in a low-risk population (p5 = 0.0185) is obtained from Cancer Research UK (84).
P6: The probability of having a strong FH of cancer fulfilling the current clinical criteria (FH-positive) is obtained from the population-based GCaPPS study (p6 = 0.1238 or 128/1034).
P7, P8: The BRCA prevalence in FH-positive (p7 = 0.09375) and FH-negative (p8 = 0.0203) individuals is also obtained from the GCaPPS study in which 12/128 BRCA carriers detected were FH-positive and 15/740 were FH-negative.
P9: Reduction in breast cancer risk from RRM in BRCA carriers not undergoing RRSO is taken from the PROSE study data by Rebbeck et al., JCO 2004 (22).
P10: The breast cancer penetrance for BRCA carriers (57% for BRCA1 and 49% for BRCA2) is taken from a meta-analysis, corrected for ascertainment (61). To simplify the analysis, we have used a composite risk for BRCA1 and BRCA2 carriers (P10 = 0.53) weighted for the relative prevalence of BRCA1 and BRCA2 FM found in the London AJ population. The BRCA1 population prevalence is 0.0132, and BRCA2 population prevalence is 0.0113 (GCaPPS study). The overall risk of breast cancer in BRCA carriers is calculated as ((0.0132*0.57)/2.45 + (0.0113*0.49)/2.45).
P11: The risk of breast cancer in a low-risk population is taken from Cancer Research UK and UK Office for National Statistics data (46,85).
P12: Undergoing RRSO can be a complex decision-making process, and RRSO rates ranging from 0.3 to 0.75 have been reported in the literature (62,71,72,88). We have used the RRSO rate recently reported in high-risk women from London (p2 = 0.55), as it reflects the views of carriers from a London population and is within the range reported in the literature (62).
P13: The reduction in breast cancer risk in premenopausal women undergoing RRSO is taken from a meta-analysis by Rebbeck et al. (21).
P14: Reduction in breast cancer risk from RRM in BRCA carriers undergoing RRSO is taken from the PROSE study data by Rebbeck et al., JCO 2004 (22).
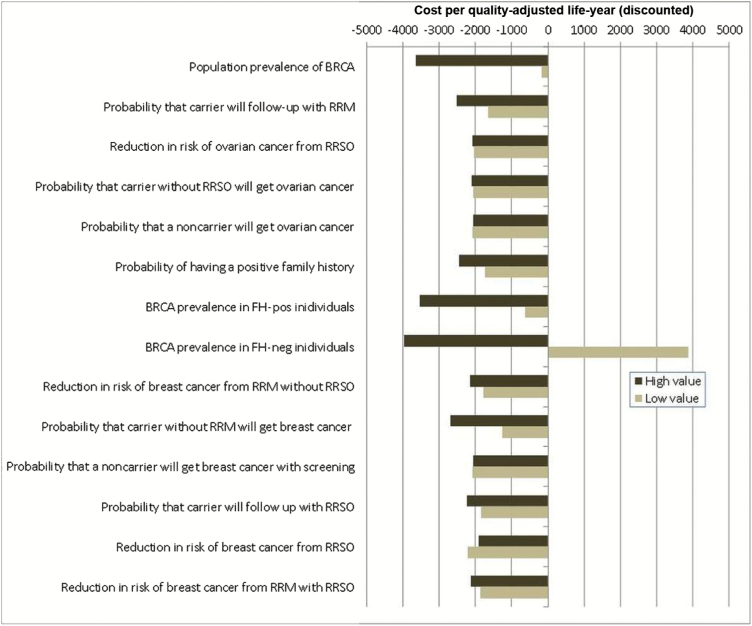
Figure 2.
Deterministic sensitivity analysis for model probabilities. One-way sensitivity analysis for all probabilities in terms of the incremental cost-effectiveness ratio (ICER) of population-based screening compared with a family history (FH)–based approach for BRCA testing. X-axis: ICER: cost (£) per quality-adjusted life-year (QALY) (discounted). Y-axis: probability parameters in the model. The model is run at both lower and upper values/limits of the 95% confidence interval or range of all probability parameters given in Table 1. “High value” represents outcomes for upper limit, and “Low value” represents outcomes for lower limit of the probability parameter. Outcomes to the left of the midline “0” value on the X-axis indicate that the model is cost saving. FH = family history; neg = negative; pos = positive; RRSO = risk-reducing salpingo-oophorectomy; RRM = risk-reducing mastectomy.
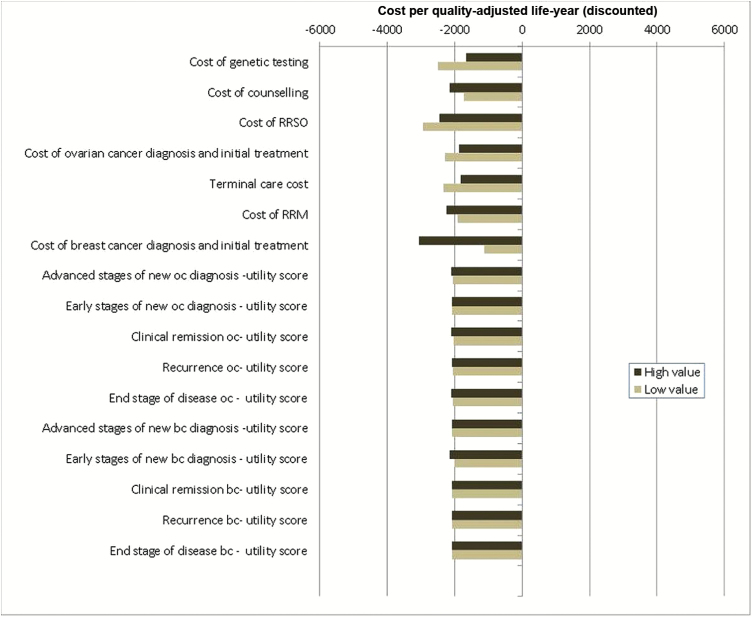
Figure 3.
Deterministic sensitivity analysis for model costs and utilities. One-way sensitivity analysis for all model costs and utility-score parameters in terms of the incremental cost-effectiveness ratio (ICER) of population-based screening compared with a family history (FH)–based approach for BRCA testing. X-axis: ICER: cost (£s) per quality-adjusted life-year (QALY) (discounted). Y-axis: cost and utility-score parameters in the model. The model is run at both lower and upper values/limits of the cost and utility-score parameters given in Table 2. “High value” represents outcomes for upper limit, and “Low value” represents outcomes for lower limit of these parameters. Outcomes to the left of the midline “0” value on the X-axis indicate that the model is cost saving. This analysis suggests that variation in costs and utility scores do not statistically significantly affect model outcomes. BC = breast cancer; FH = family history; neg = negative; OC = ovarian cancer; pos = positive; RRSO = risk-reducing salpingo-oophorectomy; RRM = risk-reducing mastectomy.
Costs
All costs are reported at 2010 prices and where required have been converted using the Hospital and Community Health Service Index (28). These are described in detail in Table 2.
Table 2.
Summary of costs used in model (2010 prices)*
| Item | Cost (£) | Source |
|---|---|---|
| Cost of genetic testing | 50 | GCaPPS |
| Cost of counseling | 33 | GCaPPS, PSSRU unit costs of health and social care (89) |
| Cost of RRSO (and HRT) | 2222 | NHS reference costs (90), BNF ( 91 ) |
| Cost of ovarian cancer diagnosis and initial treatment | 15,753 | NHS reference costs (90), NICE guideline (92) |
| Yearly cost of ovarian cancer treatment and follow-up: years 1–2 | 612 | NHS reference costs (90), NICE guideline (92) |
| Yearly cost of ovarian cancer treatment and follow-up: years 3–5 | 262 | NHS reference costs (90), NICE guideline (92) |
| Terminal care cost with ovarian cancer | 14,716 | National Audit Office (93) |
| Cost of breast cancer screening general | 330 | Robertson 2011 (94), NHS reference cost (90) |
| Cost of breast cancer screening BRCA carriers | 5983 | NHS reference costs (90), NICE guideline famial breast cancer (19) |
| Cost of RRM | 3222 | NHS reference costs (90), weighted for 21% complication rate (18,95) |
| Cost of breast cancer treatment | 15039 | NHS reference costs (90), NICE guideline advanced breast cancer (50), NICE guidelines early and locally advanced breast cancer (96) |
| Yearly cost of breast cancer follow-up and adjuvant treatment if any (eg, Tamoxifen): years 1–5 | 1914 | BNF (91), Robertson 2011 (94), NHS reference costs (90), NICE guidelines early and locally advanced breast cancer (96) NICE guideline advanced breast cancer (50) National Costing Report. Implementing NICE guidance 2009 (47) |
* All costs were varied by +/-30% in one-way sensitivity analysis. BNF = British National Formulary; GCaPPS = Genetic Cancer Prediction through Population Screening study; HRT = hormone replacement therapy; NHS = National Health Service; NICE = National Institutes for Health and Clinical Excellence; PSSRU = Personal Social Services Research Unit; RRSO = risk-reducing salpingo-oophorectomy, RRM = risk-reducing mastectomy.
† Cost of genetic counseling/testing: based on pretest counseling time (45 minutes), 71% genetic testing uptake (GCaPPS study), and national unit cost assumed for genetic counselling = £44/h of client contact from PSSRU Unit costs of Health and Social Care 2010 (89,97).
RRSO costs: based on national reference costs for an upper genital tract laparoscopic/endoscopic intermediate procedure (90). Costs of HRT (from BNF [91]) assume HRT is given from average age of RRSO to the average age of menopause (51 years).
Ovarian cancer costs: Costs for ovarian cancer diagnosis and treatment were derived from national reference costs and a recent ovarian cancer guideline from NICE (90,92). We assumed cost of diagnosis includes a pelvic examination, ultrasound scan, CA125 test, CT scan, percutaneous biopsy, and peritoneal cytology.
The cost of treatment included the reference cost for a lower and upper genital tract very complex major procedure and administration of chemotherapy based on six cycles of carboplatin and paclitaxel treatment. It was assumed that in years 1 and 2 treated survivors would have a further three consultant visits, a CT scan, and four CA125 tests each year. In years three to five years postsurgery, it was assumed that survivors would have two consultant visits and two CA125 tests. We were conservative in our cost estimates and did not include costs for additional investigations, treatment of recurrence, or management of complications in the analysis.
Costs for terminal care for ovarian cancer were derived from end-of-life costs for cancer patients based on a report from the National Audit Office, UK (93).
In line with NICE recommendations, future healthcare costs not associated with ovarian cancer were not considered (25).
Breast Cancer Costs: Breast Cancer diagnosis and treatment costs were derived predominantly from: “National costing report- Implementing NICE guidance (Feb 2009),” which provides estimates of the national cost impact arising from implementation of NICE guidelines for diagnosis and treatment of early/locally advanced breast cancer and advanced breast cancer in England, UK (47); from UK Department of Health NHS reference costs 2010–2011 (90); the BNF (91) and other relevant NICE guidelines on breast cancer care in general and high risk populations (19,50,96).
Cost of breast cancer screening: assumes for noncarriers routine mammography (eight mammograms between 50–70 years), as per UK NHS breast cancer screening program (98). Cost of breast screening for BRCA carriers is based on annual mammogram from age 40 to 69 years and annual MRI from age 30 to 49 years, as per NICE guidelines for familial breast cancer (19).
Cost of RRM: obtained from NHS reference costs (90) weighted for a 21% complication rate (18,95).
Cost of breast cancer treatment: In the general population, 10% of breast cancer is noninvasive DCIS; 90% breast cancer is invasive; 95% of invasive breast cancer is early and locally advanced (41% Stage 1, 45% stage 2, 9% stage 3 [45–48]); 5% of invasive breast cancer is advanced breast cancer (stage 4) (45–47); 35% of early and locally advanced breast cancer will progress to advanced breast cancer (NICE costing report, 2009) (47). In BRCA carriers, 20% of cancers are DCIS and 80% invasive (61% stage1) (18,49).
The cost of diagnosis includes clinical examination, mammogram, ultrasound, and biopsy.
Mean prevalence of Axillary lymph node metastasis in early invasive breast cancer is 31.4% (systematic reviews within the NICE breast cancer guideline [96] and breast cancer clinical outcome measures [BCCOM] project [99]). 30% node-positive rate is assumed for BRCA breast cancer (based on screening studies in familial breast cancer, breast cancer case series, and Early Breast Cancer Trialists’ Collaborative Group data) (49,100–103).
Cost of sentinel lymph node biopsy (SLNB): from NICE national costing report (47). SLNB for staging axilla for early invasive breast cancer and no evidence of lymph node involvement on Ultrasound (US)/negative US–guided biopsy (73% of invasive cancers).
Cost of axillary lymph node dissection (ALND): assumed to be 25% of cost of breast surgery, as per NICE guideline development group recommendation (47), undertaken for lymph node–positive cancers (31% early and locally advanced invasive cancers) (47,96).
Breast surgery costs: This includes costs of breast conserving surgery (assumed for all noninvasive cancers, and 75% of early/locally advanced [stage 1–3] invasive cancers); and costs of mastectomy with reconstruction (for 25% early/locally advanced cancers). Costs are obtained from the national NHS reference costs (90).
Radiotherapy and chemotherapy: Invasive breast cancers that are not low risk (99,104,105) receive adjuvant treatment in line with NICE guidelines. Costs include radiotherapy costs for 60% of early invasive/locally advanced, radiotherapy and chemotherapy costs for 40% early invasive/locally advanced, and chemotherapy costs for all advanced cancers. Radiotherapy costs include planning and 40Gy, 15 times over three weeks (NICE guidelines [96]) or palliative treatment, taken form national NHS reference costs (90). Chemotherapy costs (based on polychemotherapy) (100) include administration costs, costs of 1st and 2nd line therapy, and toxicity from NICE guidelines (47,50).
All costs are adjusted for BRCA breast cancers for difference in stage at presentation and 20% cancers being noninvasive.
70% general population invasive breast cancers are ER-positive; 15% early invasive breast cancers and 25% advanced breast cancers are HER2-positive (50,96). 27% BRCA1 and 67% BRCA2 breast cancers are ER-positive; 5% BRCA1 and 14% BRCA2 breast cancers are HER2-positive (101–103,106–108). ER and HER2 testing costs are obtained from a local NHS trust and included for all breast cancers.
Endocrine therapy costs: As per NICE guidelines (47,96), ER-positive invasive breast cancers receive Tamoxifen 20mg/day (premenopausal)/ Anastrazole 1mg/day (postmenopausal) for five years: costs from the BNF (91). Rates are adjusted for BRCA carriers, ER positivity, and menopause status.
Biphosphonate costs: 74% patients with advanced breast cancer will develop bone metastases, and 65% patients with bone metastases are offered bisphosphonates (47,109,110). As per NICE guidelines, costs (from BNF [91]) assume that 50% patients receive oral clodronate and ibandronic acid, and 50% receive intravenous zoledronic acid or pamidronate (47).
Cost of Trastuzumab: for HER2-positive patients, given at three-week intervals for one year or until disease recurrence as per NICE guidelines. Costs obtained from NICE costing report (47).
35% of early/locally advanced breast cancer progress to advanced breast cancer (NICE guidelines) (47). Recurrence rates for early/locally advanced breast cancer (from the US National Surgical Adjuvant Breast and Bowel Project [NSABP]): 15.9% for node-positive (111) and 11% for node-negative (112) breast cancer: composite recurrence rate = 12.6% (weighted for 31% node-positive and 69% node-negative disease). Recurrence rate for advanced/metastatic breast cancer is 66% (34% relapse free five-year survival) (52).
Follow-up costs: includes annual mammograms and six monthly consultations. MRI scan for all stage 4 cancers. Costs include a progression rate of 35% from early and locally advanced to advanced disease (47), and 66% relapse rate for advanced disease (52).
Costs for terminal care for breast cancer were derived from end-of-life costs for cancer patients based on a report from the National Audit Office, UK (93). In line with NICE recommendations, future healthcare costs not associated with breast cancer were not considered (25).
Chemoprevention (sensitivity analysis): Tamoxifen/Raloxifene for five years (19,23), from BNF (91).
†For more detailed explanation, see Supplementary Table 2 (available online).
Life-years
Life expectancy for women not developing cancer (age 53 years) was based on life-tables from the Office of National Statistics (29). The mean age for breast/ovarian cancer onset was age 41.7/51.5 years for BRCA1 and 45.6/58.9 years for BRCA2 AJ women, respectively (30). To simplify the analysis, we used average ages for breast (43.5 years) and ovarian cancer (54.9 years) onset for BRCA1/BRCA2 carriers, obtained by assigning weights to the individual ages of onset for the relative population prevalence of BRCA1 and BRCA2 FM ((1.32*AgeBRCA1)/2.45 + (1.13*AgeBRCA2)/2.45)). The mean ages for sporadic breast and ovarian cancer in AJ-women were 57 years and 63 years, respectively (31–33). In the absence of AJ-specific survival data, five-year survival rates were assumed to be the same as the general UK population (34). This model incorporates potential survival differences between BRCA1, BRCA2, and sporadic ovarian cancers (35,36). No statistically significant survival difference between BRCA and sporadic breast cancers has been reported (37,38). For ovarian cancer, the five-year survival for BRCA1 is 44% (95% confidence interval [CI] = 40% to 48%) and for BRCA2 52% (95% CI = 46% to 58%) (35), giving a composite five-year survival for BRCA1+BRCA2 (weighted by BRCA1 and BRCA2 prevalence) of 0.477 ((0.44*1.32/2.45)+(0.52*1.13/2.45)). After five years’ survival, the probability of death was assumed to be the same as the general population.
Quality-Adjusted Life-years (QALYs)
QALY is a measurement that expresses changes in length of life, while at the same time incorporating reductions in quality-of-life. Calculation of QALYs requires knowledge of quality-of-life adjustment or utility weights for each health state in the model. Utility weights are an indication of an individual’s preference for specific health states where 1 equals perfect health and 0 equals death. QALY equals survival in life-years multiplied by utility weight. No studies that measured utility scores directly for women undergoing RRSO were identified. A Dutch study found no measurable impact on generic quality-of-life in high-risk women undergoing RRSO (39). Therefore, we did not include a utility decrement for RRSO in our analysis. Havrilesky (40) reported detailed utility estimates related to various health states following ovarian cancer treatment using visual analogue scale and time-trade-off (TTO) methods. As visual scales for comparing health state preferences are subject to inherent biases and are generally less accurate (41), we utilized the TTO scores. We assumed 70% of women present with ovarian cancer at advanced stages (42,43), with a lower utility score for a new diagnosis at 0.55 (SD = 0.29), while the remainder presenting at early stages have a higher utility score of 0.81 (SD = 0.26). The end-stage-of-life utility score, where ovarian cancer patients did not survive the next year, was 0.16 (SD = 0.25). Of those that survived initial chemotherapy, the chance of recurrence with early disease was 10.5% annually (44), and with advanced disease this would be 20.6% (42). For women with recurrent disease, the mean utility value was 0.5 (range = 0.4–0.61), and for women in remission the utility value was 0.83 (SD = 0.25) (40).
Of general population breast cancer, 10% is noninvasive/DCIS, 90% is invasive, 95% of invasive cancer is early and locally advanced (41% = stage 1, 45% = stage 2, 9% = stage 3) (45–48), and 5% is advanced (stage 4) (45–47). In BRCA carriers, 20% of cancers were DCIS and 80% invasive (61% = stage 1) (18,49). Utility weights for breast cancer were assumed as follows: advanced breast cancer was 0.65, early or locally advanced breast cancer was 0.71, remission was 0.81, recurrence was 0.45; these were obtained from NICE guidelines (50,51). For those who survived initial chemotherapy, the chance of breast cancer recurrence/progression with early or locally advanced disease was 35% (47), and for recurrence with advanced disease it was 66% (52).
Statistical Analysis
For each branch of the decision model, the probability of being in each branch was calculated by multiplying together the path probabilities. The total costs and effects in terms of life-years and QALYs were then estimated by weighting the values for each branch by the probability of being in each branch. The incremental cost-effectiveness ratio (ICER) was estimated by dividing the difference in cost by the difference in effect. ICER = (Cost A–Cost B)/(Effect A–Effect B). By comparing this ICER with the cost-effectiveness threshold used by NICE (£20000-£30000/QALY) (53), it was possible to determine whether or not population screening for all women was cost-effective compared with FH-based testing. To explore uncertainty in the results and robustness of the model, a one-way sensitivity analysis was undertaken by varying each parameter in the model and then rerunning the model to assess the impact on overall results. Probabilities and utility scores were varied according to their 95% confidence intervals/range, where available, or by +/-10%, and costs were varied by +/-30%. In addition to the one-way sensitivity results, a probabilistic sensitivity analysis (PSA) was undertaken as recommended by NICE methods guidance (25,54). Any variation in model parameters/variables is likely to occur in parallel rather than independently of each other. In the PSA all variables are varied simultaneously across their distributions to further explore model uncertainty. We assigned costs a gamma distribution, probabilities a beta distribution, and utilities a log-normal distribution as suggested in the literature (55). The results of 1000 simulations were plotted on a cost-effectiveness acceptability curve showing the proportion of simulations that indicated that the intervention was cost-effective at different willingness-to-pay thresholds. Other scenarios also explored included: 1) breast cancer prophylaxis with SERMs (tamoxifen/raloxifene) in BRCA carriers (19,23) and 2) women opting for genetic testing at age 50 years (average age of menopause) with a median age for RRSO and RRM at 54 years (just below the weighted average age of ovarian cancer onset in BRCA1/BRCA2 carriers).
Results
The discounted and undiscounted lifetime costs, life-years, and QALYs for each branch in the decision model are given in Table 3. Overall, a population-screening approach saved more life-years (0.090) and QALYs (0.101) than an FH-based approach. This difference equated to 33 days’ gain in life expectancy for AJ women using a population-screening strategy compared with an FH-based one. Discounted results show a smaller overall gain in life-years and QALYs and overall cost difference, as discounting adjusts costs and outcomes that occur in the future and the cost savings generated through prevention of future ovarian cancer cases is valued less. The baseline discounted ICER was -£2079 per QALY, indicating that population-based screening not only saves more QALYs but is also cost saving and is highly cost-effective in AJ women. This is well below the NICE threshold of £20000-£30000/QALY. Population-based screening also lowered ovarian cancer incidence by 0.34% (from 2.49% to 2.15%) and lowered breast cancer incidence by 0.62% (from 13.31% to 12.69%). Assuming that 71% of the estimated 114000 AJ women in the UK (26,27) undergo testing, the overall impact of a population-based strategy is a reduction in ovarian cancer and breast cancer by 276 and 508 cases, respectively, at a discounted cost savings of £3.7 million. Should the entire population undergo testing, the number of cancers potentially prevented would increase to 388 for ovarian and 715 for breast cancers at a discounted cost savings of £5.2 million.
Table 3.
Model outcomes for costs, life-years, and quality-adjusted life-years (QALYs), undiscounted and discounted
| Population screening | Undiscounted | Discounted | |||||
|---|---|---|---|---|---|---|---|
| Probability | Cost, £ | Life-years | QALYs | Cost, £ | Life-years | QALYs | |
| A. PS, carrier, RRM, RRSO, no OC/BC | 0.0068 | 5197 | 53.00 | 53.00 | 3819 | 23.40 | 23.40 |
| B. PS, carrier, RRM, RRSO, OC | 0.0001 | 30 343 | 38.20 | 37.22 | 13550 | 19.75 | 19.39 |
| C. PS, carrier, RRM, RRSO, BC | 0.0002 | 27 936 | 46.92 | 45.63 | 28013 | 21.48 | 20.74 |
| D. PS, carrier, RRM, no RRSO, no OC/BC | 0.0038 | 2976 | 53.00 | 53.00 | 2766 | 23.40 | 23.40 |
| E. PS, carrier, RRM, no RRSO, OC | 0.0017 | 28 121 | 38.20 | 37.22 | 12497 | 19.75 | 19.39 |
| F. PS, carrier, RRM, no RRSO, BC | 0.0003 | 25 715 | 46.92 | 45.63 | 26960 | 21.48 | 20.74 |
| G. PS, carrier, no RRM, RRSO, no OC/BC | 0.0047 | 8297 | 53.00 | 53.00 | 5025 | 23.40 | 23.40 |
| H. PS, carrier, no RRM, RRSO, OC | 0.0001 | 33 442 | 38.20 | 37.22 | 16256 | 19.75 | 19.39 |
| I. PS, carrier, no RRM, RRSO, BC | 0.0017 | 31 036 | 46.92 | 45.63 | 29219 | 21.48 | 20.74 |
| J. PS, carrier, no RRM, no RRSO, no OC/BC | 0.0009 | 6075 | 53.00 | 53.00 | 3972 | 23.40 | 23.40 |
| K. PS, carrier, no RRM, no RRSO, OC | 0.0016 | 31 221 | 38.20 | 37.22 | 13703 | 19.75 | 19.39 |
| L. PS, carrier, no RRM, no RRSO, BC | 0.0028 | 28 814 | 46.92 | 45.63 | 28166 | 21.48 | 20.74 |
| M. PS, noncarrier, no OC/BC | 0.8355 | 423 | 53.00 | 53.00 | 213 | 23.40 | 23.40 |
| N. PS, noncarrier, OC | 0.0181 | 25 568 | 38.63 | 37.83 | 9274 | 19.99 | 19.72 |
| O. PS, noncarrier, BC | 0.1219 | 24 616 | 49.16 | 47.87 | 8868 | 22.50 | 22.05 |
| Family history screening | 1.0000 | ||||||
| P. FH pos, carrier, RRM, RRSO, no OC/BC | 0.0032 | 5197 | 53.0000 | 53.0000 | 3819 | 23.3988 | 23.3988 |
| Q. FH pos, carrier, RRM, RRSO, OC | 0.00004 | 30 343 | 38.2031 | 37.2150 | 13550 | 19.7536 | 19.3902 |
| R. FH pos, carrier, RRM, RRSO, BC | 0.0001 | 27 936 | 46.9236 | 45.6273 | 28013 | 21.4791 | 20.7432 |
| S. FH pos, carrier, RRM, no RRSO, no OC/BC | 0.0018 | 2976 | 53.0000 | 53.0000 | 2766 | 23.3988 | 23.3988 |
| T. FH pos, carrier, RRM, no RRSO, OC | 0.0008 | 28 121 | 38.2031 | 37.2150 | 12497 | 19.7536 | 19.3902 |
| U. FH pos, carrier, RRM, no RRSO, BC | 0.0001 | 25 715 | 46.9236 | 45.6273 | 26960 | 21.4791 | 20.7432 |
| V. FH pos, carrier, no RRM, RRSO, no OC/BC | 0.0022 | 8297 | 53.0000 | 53.0000 | 5025 | 23.3988 | 23.3988 |
| W. FH pos, carrier, no RRM, RRSO, OC | 0.0000 | 33 442 | 38.2031 | 37.2150 | 14756 | 19.7536 | 19.3902 |
| X. FH pos, carrier, no RRM, RRSO, BC | 0.0008 | 31 036 | 46.9236 | 45.6273 | 29219 | 21.4791 | 20.7432 |
| Y. FH pos, carrier, no RRM, no RRSO, no OC/BC | 0.0004 | 6075 | 53.0000 | 53.0000 | 3972 | 23.3988 | 23.3988 |
| Z. FH pos, carrier, no RRM, no RRSO, OC | 0.0007 | 31 221 | 38.2031 | 37.2150 | 13703 | 19.7536 | 19.3902 |
| AA. FH pos, carrier, no RRM, no RRSO, BC | 0.0013 | 28 814 | 46.9236 | 45.6273 | 28166 | 21.4791 | 20.7432 |
| AB. FH pos, noncarrier, no OC/BC | 0.0961 | 423 | 53.0000 | 53.0000 | 213 | 23.3988 | 23.3988 |
| AC. FH pos, noncarrier, OC | 0.0021 | 25 568 | 38.6324 | 37.8321 | 9274 | 19.9915 | 19.7169 |
| AD. FH pos, noncarrier, BC | 0.0140 | 24 616 | 49.1623 | 47.8660 | 8868 | 22.4951 | 22.0482 |
| AE. FH neg, carrier, no OC/BC | 0.0030 | 330 | 53.0000 | 53.0000 | 120 | 23.3988 | 23.3988 |
| AF. FH neg, carrier, OC | 0.0053 | 25 475 | 38.2031 | 37.2150 | 9851 | 19.7536 | 19.3902 |
| AG. FH neg, carrier, BC | 0.0095 | 23 069 | 46.9236 | 45.6273 | 24314 | 21.4791 | 20.7432 |
| AH. FH neg, noncarrier, no OC/BC | 0.7352 | 330 | 53.0000 | 53.0000 | 120 | 23.3988 | 23.3988 |
| AI. FH neg, noncarrier, OC | 0.0159 | 25 475 | 38.6324 | 37.8321 | 9182 | 19.9915 | 19.7169 |
| AJ. FH neg, noncarrier, BC | 0.1073 | 24 524 | 49.1623 | 47.8660 | 8775 | 22.4951 | 22.0482 |
| Average population screening | 0.1484* | 4156 | 52.1912 | 52.0088 | 1677 | 23.2049 | 23.1406 |
| Average family history screening | 0.1587* | 4233 | 52.1016 | 51.9078 | 1741 | 23.1799 | 23.1096 |
| Incremental (difference) | -0.0096 | -77 | 0.090 | 0.101 | -64 | 0.025 | 0.031 |
| Cost per quality-adjusted life-year | -767 | -2079 | |||||
| Total population screening effect | 837† | -4467529 | 5166 | 5827 | -3718526 | 1442 | 1789 |
| Jewish population screened | 81 224 | ||||||
* Cancer incidence. BC = breast cancer; FH = family history; neg = negative; OC = ovarian cancer; pos = positive; PS = population screening; QALY = quality-adjusted life-year; RRSO = risk-reducing salpingo-oophorectomy, RRM = risk-reducing mastectomy.
† Reduction in total number of cancer cases.
The results of the one-way sensitivity analysis (Figures 2,3) indicate that the upper/lower utility values, costs, penetrance estimates, and rate of uptake of preventive/risk-reducing surgery have little influence on the overall results, and the model is cost saving at both upper and lower limits of these variables. However, the model is highly sensitive to the overall BRCA prevalence and BRCA prevalence in FH-negative women (Figure 2). At the lower limits of overall BRCA prevalence and BRCA prevalence in FH-positive individuals, the intervention was still just cost saving at -£183/QALY and -£631/QALY, respectively. At the lowest value for BRCA prevalence in FH-negative women, the ICER equals £3877 per QALY, well under the NICE threshold of £20000 to £30000 per QALY, indicating that population screening was still cost-effective, but no longer cost saving. At the highest BRCA prevalence rates in FH-positive and FH-negative women, the intervention was both more effective and cost saving.
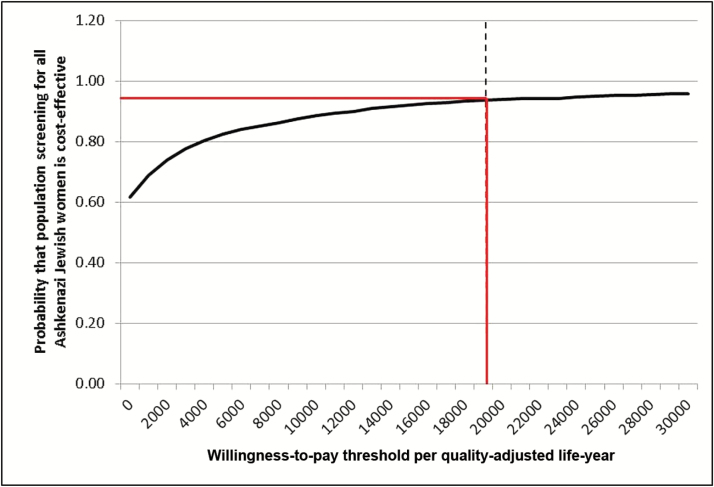
The probabilistic sensitivity analysis results (Figure 4) shows that at a threshold of £20000 94% of simulations indicate that population screening in AJ women is a cost-effective intervention compared with current NHS policy. A scenario incorporating breast cancer prophylaxis with tamoxifen (premenopausal)/raloxifene (postmenopausal) in BRCA carriers, as recently recommended by NICE (19), achieved a greater cost savings per QALY of -£2472 for population-based screening compared with FH-based testing. A further scenario where unaffected women age 50 years were screened for BRCA mutations (with a median age for RRSO and RRM = 54 years), also achieved a higher cost savings per QALY of -£2946.
Figure 4.
Cost-effectiveness acceptability curve. Probabilistic sensitivity analysis in which all model parameters/variables are varied simultaneously across their distributions to further explore model uncertainty. X-axis: Incremental cost-effectiveness ratio in terms of cost (£s)/quality-adjusted life-year. Y-axis: proportion of simulations. The results of 1000 simulations were plotted on a cost-effectiveness acceptability curve showing the proportion of simulations (Y-axis), which indicated that the intervention was cost-effective at different willingness-to-pay thresholds (X-axis). The solid red line marks the proportion of simulations found to be cost-effective at the £20 000 threshold used by NICE. 94% simulations are cost-effective in this analysis.
Discussion
According to NICE, a cost-effectiveness analysis comparing the relative health outcomes and costs of interventions is the preferred form of economic evaluation, and QALY is the most suitable determinant of health benefit, reflecting both mortality and health-related quality-of-life effects (25). Value-based judgments are used to assign an appropriate price for health outcomes. This analysis focused on whether the cost differential between different interventions/strategies is worthwhile in terms of changes in health outcomes. Our decision analysis model addressed the topical issue of cost-effectiveness of a new population-based BRCA testing strategy in AJ women, made possible by identification of AJ founder mutations and advances in the efficiency/cost of technology for mutation detection. Our finding that a population screening program implemented in UK AJ women older than age 30 years will be cost saving compared with the current FH-based one has important implications for clinical care, population/public health, and for health care providers and commissioners. There is potential within the UK for reduction of the number of ovarian cancers by 276 and breast cancers by 508, combined with overall cost savings of £3.7 million. Should the entire relevant UK population undergo testing, the number of cancers potentially prevented would increase to 388 for ovarian and 715 for breast cancer at a discounted cost savings of £5.2 million. This compares favorably with a number of interventions routinely used in clinical practice, which, while saving lives, cost more. For instance, the ICER for breast screening in BRCA1/BRCA2 carriers age 35 to 49 years recommended by NICE (56) is £11700(MRI alone) or £15300 (MRI+mammography) per case of cancer detected (57). The ICER for paclitaxel and cisplatin for primary treatment of ovarian cancer ranges between $6600 and $22000 per life-year gained (58,59).
This is the first report comparing cost-effectiveness of population screening for founder mutations of BRCA1/2 with the current standard of care. Our analysis incorporates impact on both breast and ovarian cancer risk and fulfills various requirements suggested by NICE for health-economic decision-making. The “time horizon” in our analysis is long enough to reflect important differences in costs and outcomes (25). We use current best practice as a comparator, QALYs to measure health outcomes, a 3.5% discount rate on costs and health outcomes, and, as far as possible, directly obtained population-based data for parameters in the analysis (25). This is the first model to incorporate BRCA prevalence in FH-negative individuals, and probabilities used in the model are consistent with other reports in the literature (2,60). A comparison of population-screening for BRCA carriers vs no screening reported an ICER of $8300 per QALY (17). This higher value compared with the cost savings in our study could be attributable to our lower costs of testing, higher health care costs in the United States compared with the UK, and the beneficial impact of breast cancer prevention found in our study but not evaluated by the earlier study (17). Another advantage of our model is that it also includes genetic counseling costs, which would be a key component of any population-based testing strategy. This approach facilitates reflection of the positive and negative consequences of genetic testing and permits participants to make informed decisions based on their own values and opinions. In order to minimize overestimating benefits of population-based screening, we have been conservative in our use of ovarian and breast cancer penetrance estimates (corrected for ascertainment) (61), as well as our costs for ovarian and breast cancer diagnosis and treatment, by including a minimal subset of baseline costs. We did not include all costs for additional investigations, treatment of recurrence, or management of complications.
The deterministic sensitivity analysis permitted scrutiny of model outcomes and identification of variables that exert the most influence (Figures 2 and 3). The 95% confidence limits for probabilities explored in our sensitivity analysis were quite wide, adding to the strength of the results. The lack of statistically significant effect on outcome despite 30% variation in costs indicates that costs of counseling, treatment, and prevention are less important in influencing the overall results. BRCA prevalence rates (P1, P8) emerged as the key variables of influence, given the higher model sensitivity to uncertainties around these values (Figure 2). That the model remains largely cost saving despite probabilities varying widely is reassuring. Even at low prevalence rates in FH-positive or FH-negative individuals, population testing remained cost-effective compared with the NICE threshold of £20000 to £30000 per QALY (53). Though we used an RRSO risk-reduction estimate (0.96) based on the residual risk of PPC (20), a lower rate of 0.8 (21) assessed in the sensitivity analysis showed it was still cost saving. RRSO rates vary with time, menopausal status, age, and parity (62). It is encouraging that even at low (0.3) RRSO rates, the model remains cost saving (Figure 2). The PSA undertaken adds to the robustness of our results and has been recommended by decision-making bodies (25). It permits simultaneous variation in probabilities of all parameters to fully characterize uncertainties in the model and its effect on overall results. That 94% of simulations on PSA were cost-effective reconfirms the health-economic benefit of a population-based approach to genetic testing.
Our model is limited by a number of assumptions. It does not incorporate any potential reduction in QALY following RRSO. Although RRSO is associated with worse menopausal symptoms and sexual function compared with women undergoing screening, no difference in generic quality-of-life has been reported (39,63,64) RRSO has also been linked with a higher risk of cardiovascular disease (65,66) and osteoporosis. We felt that these downsides in premenopausal women may be balanced by the decrease in vasomotor symptoms, cardiovascular sequelae, and improved sexual function and osteo-protection seen with hormone replacement therapy (HRT) (63,65,67–69), as well as the reduction in cancer worry, perceived risk, and high overall satisfaction observed following RRSO (39,63). RRSO also halves the risk of subsequent breast cancer in premenopausal women (21), and available evidence suggests that HRT does not alter the statistically significant benefit of reduction in breast cancer risk obtained from bilateral oophorectomy (70).
Of BRCA carriers, 21% to 67% undergo risk-reducing mastectomy (71–73). Addition of risk-reducing mastectomy or screening to RRSO further increases life expectancy in BRCA carriers (74). Our model incorporates the impact of breast screening already prevalent in the UK and includes the benefits of reduction in breast cancer risk obtained from RRSO (21) and risk-reducing mastectomy (RRM) (22). Our analysis does not incorporate any potential reduction in QALY following RRM. Although a negative impact on sexual pleasure and body image has been reported, no difference over time in sexual habit, discomfort, or activity was documented (75). Lack of adverse effects on anxiety, depression, and health-related quality-of-life over time has also been reported (18,75,76). Additionally, any negative effects of RRM could be balanced by findings of significant decreases in anxiety scores, improved social activity (75), reports of a majority of women finding RRM results to be consistent with their expectations, and high satisfaction with overall cosmetic results (76–79).
Although it can be hypothesized that FH-positive women may be more likely to adapt to their increased risk while FH-negative women have less of an opportunity to consider/adapt to these issues before being tested, we did not find FH to statistically significantly affect quality-of-life outcomes in our GCaPPS trial. Not all of those undergoing genetic counseling will opt for genetic testing, and a reduced genetic testing uptake rate is built into the model costs. A total of 1034 (71%) of 1450 people who made a genetic counseling appointment in the GCaPPS study underwent BRCA testing. This equals 1034 (89%) of the 1168 who attended pretest counseling. FH did not statistically significantly influence uptake of testing in our population-based trial; hence, uptake using either FH or population approaches in our decision model is assumed to be similar (71%). This estimate lies well within the range of genetic testing rates (varying from 66%–90%) (80–83), reported by previous studies of varying ascertainment and sizes.
The intermarriage rate in the Jewish community is likely to lead to a fall in BRCA founder mutation prevalence. Twenty-five percent of Jewish marriages in the UK and 44% in the United States are to non-Jews, although only 50% of the population is married. Hence, overall, the impact of this is unlikely to affect more than 25% of the population. Assuming a BRCA prevalence of 2.5% for 75%, and 1% for 25% of the population, the overall population BRCA prevalence will be about 2.1%. Even if BRCA prevalence were 1% for 50% of the population, the overall population prevalence would be about 1.75%. These extremes are well within the 95% confidence intervals accounted for in our sensitivity analysis, which shows the model to be cost-effective.
Implementation of any national screening program has many challenges and raises important issues of logistics and quality control. It also requires raising public (and health professional) awareness/education, community engagement, and information dissemination via media campaigns, which have an added cost. In the UK, screening programs are centrally coordinated by the national screening committee with fail-safe procedures and involve close coordination with GP/primary care and public health physicians. In addition, there is need for clearly defined downstream secondary and tertiary care pathways, developed in close coordination with clinical genetics teams, breast surgeons, gynecologists, and others responsible for the management of women found to be at high risk. While these pathways exist for high-risk women, they would need to be expanded prior to program implementation. It would not be sensible or feasible for all women to undergo pretest counseling in tertiary high-risk cancer genetics clinics within a hospital setting, so a community-based approach would need to be explored. We have demonstrated that successful recruitment to such a population-based program for pretest counseling and BRCA testing outside a hospital setting is possible using a community- or high street–based model. More efficient, acceptable, and cost-effective ways of delivering information on genetic risk will be needed for any population-based testing program to become a reality, and this area should be the focus of further research.
Health-economic assessments are critical for determining the appropriateness of resource allocation for cost-intensive population-based interventions. Rising health care costs and the ever-increasing price of new ovarian/breast cancer treatments and drug therapies in a challenging economic environment further magnify the importance of newer cost-effective preventive strategies. An important advantage of population screening is the ability to identify BRCA carriers without a strong FH of cancer undetectable by an FH-based approach. This translates to 2322 additional UK AJ women (population prevalence =2.03%, 95% CI = 1.14% to 3.32%) who may benefit from access to screening/preventive options. The lack of statistically significant difference in short-term outcomes of anxiety, depression, quality-of-life, health anxiety, and overall impact of genetic testing between FH and population-based approaches reconfirms that population-based genetic testing in the majority of Jewish people does not harm quality-of-life or psychological well-being, or lead to excessive health concerns (11).
The high cost-effectiveness of population-based testing in AJ women demonstrated in this analysis combined with the above findings should justify a change in the current paradigm, which is limited to an FH-based approach to BRCA testing in the AJ population. We conclude that introduction of systematic population testing for AJ BRCA1/2 founder mutations could save both lives and financial resources. Finally, we emphasise that the results from our analysis are related to population-based BRCA testing in AJ women and cannot be directly extrapolated to non-AJ populations with lower prevalence rates for BRCA1/2 mutations. Nevertheless, as the cost of testing falls and the acceptance/understanding of this type of health intervention evolves in our societies, it is likely to become an increasingly important area for research and evaluation.
Funding
The trial is funded by the cancer charity The Eve Appeal.
Supplementary Material
Ethics approval and trial registration: The Genetic Cancer Prediction through Population Screening (GCaPPs) study received full ethics approval from the Institute of Child Health/Great Ormond Street Hospital Research Ethics Committee on June 8, 2008 (Research Ethics Committee [REC] Reference number 08/ H0713/44). The study was registered with the International Standard Randomized Controlled Trial Number Register - ISRCTN 73338115 (http://www.controlled-trials.com/ISRCTN73338115). Contribution to authorship: IJ conceived the GCaPPs study. IJ and RM developed concept and design of the GCaPPS study. RM, RL, IJ, and UM were responsible for literature search and design of the HE study. RM and RL developed the model. RM, RL, AM, MR, and MB were involved in the health-economic and statistical analysis. RM, KL, and MB were involved in data collection and analysis. RM and RL prepared the tables and figures. RM, IJ, UM, KL, JW, SG, LS, NB, RD, AK, HD, YW, CC, IT, AMG, and UB were involved in running the GCaPPS study. YW did the genetic testing. RT and CJ were collaborators and helped with data collection from genetic laboratories. RM and RL prepared the first draft of the manuscript. All authors critically contributed to and revised the manuscript and approved the .nal version.
Disclaimers/conflict of interest statement: IJ and UM have a financial interest in Abcodia, Ltd., a company formed to develop academic and commercial development of biomarkers for screening and risk prediction. IJ is a member of the board of Abcodia Ltd. and a Director of Women’s Health Specialists Ltd. IJ holds an NIHR Senior Investigator Award. The other authors declare no conflict of interest.
Role of Funding Source: The study was funded by The Eve Appeal charity. The funding body (The Eve Appeal charity) had no role in the study design, data collection, analysis, or interpretation, the writing of the report, nor decision to submit for publication. The research team was independent of funders. The study was supported by researchers at the National Institute for Health Research, University College London Hospitals Biomedical Research Centre.
We are particularly grateful to the women and men who participated in the GCaPPS trial. We are grateful to the entire medical, nursing, and administrative staff who work on the GCaPPS trial and to the independent members of the trial steering committee and data monitoring committee. We are grateful to the numerous supporting Jewish charities, community and religious organizations, as well as numerous members of the Jewish community for their time, advice, and support. We are grateful to Robert Liston, Vijay Devineni, and Andy Ryan for their help with designing the trial management system and for IT support. We are grateful to the various regional genetic units in London (Great Ormond Street Hospital, Kennedy Galton Centre Northwick Park Hospital, Guys Hospital, and Royal Marsden Hospital) and the West Midlands Regional Genetics Service for their support of the study. We are grateful to the teams at Boots Pharmacy, Norwood, Jewish Care, Ovacome, Agudas Israel Housing Association, Academic Study group on Israel and the Middle East, Liberal Judaism, Movement for Reform Judaism, Indian Jewish Association, Stamford Hill Group Practice, and Lane End Medical Centre for their support. IJ holds a National Institute for Health Research Senior Investigator award.
References
- 1. Levy-Lahad E, Gabai-Kapara E, Kaufman B, et al. Identification of BRCA1/BRCA2 carriers by screening in the healthy population and its implications. In: American Society of Clinical Oncology, Annual meeting. J Clin Oncol. 2011;29(Suppl);abstr 1513. [Google Scholar]
- 2. Hartge P, Struewing JP, Wacholder S, et al. The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet. 1999;64(4):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metcalfe KA, Poll A, Royer R, et al. Screening for founder mutations in BRCA1 and BRCA2 in unselected Jewish women. J Clin Oncol. 2010;28(3):387–391. [DOI] [PubMed] [Google Scholar]
- 4. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. [DOI] [PubMed] [Google Scholar]
- 5. Hopper JL, Southey MC, Dite GS, et al. Population-based estimate of the average age-specific cumulative risk of breast cancer for a defined set of protein-truncating mutations in BRCA1 and BRCA2. Australian Breast Cancer Family Study. Cancer Epidemiol Biomarkers Prev. 1999;8(9):741–747. [PubMed] [Google Scholar]
- 6. Peto J, Collins N, Barfoot R, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999;91(11):943–949. [DOI] [PubMed] [Google Scholar]
- 7. Hirsh-Yechezkel G, Chetrit A, Lubin F, et al. Population attributes affecting the prevalence of BRCA mutation carriers in epithelial ovarian cancer cases in israel. Gynecol Oncol. 2003;89(3):494–498. [DOI] [PubMed] [Google Scholar]
- 8. Moller P, Hagen AI, Apold J, et al. Genetic epidemiology of BRCA mutations--family history detects less than 50% of the mutation carriers. Eur J Cancer. 2007;43(11):1713–1717. [DOI] [PubMed] [Google Scholar]
- 9. de Sanjose S, Leone M, Berez V, et al. Prevalence of BRCA1 and BRCA2 germline mutations in young breast cancer patients: a population-based study. Int J Cancer. 2003;106(4):588–593. [DOI] [PubMed] [Google Scholar]
- 10. Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26(10):1135–1145. [DOI] [PubMed] [Google Scholar]
- 11. Manchanda R, Loggenberg K, Burnell M, et al. Population-based testing for brca1/2 mutations does not cause short term psychological harm: results from a randomized trial (GCaPPS). Int J Gynecol Cancer. 2012;22(8, Suppl 3):E153. Oral Presentation at IGCS conference, Vancouver, October 2012. [Google Scholar]
- 12. Petrou S, Gray A. Economic evaluation using decision analytical modelling: design, conduct, analysis, and reporting. BMJ. 2011;342:d1766. [DOI] [PubMed] [Google Scholar]
- 13. Balmana J, Sanz J, Bonfill X, et al. Genetic counseling program in familial breast cancer: analysis of its effectiveness, cost and cost-effectiveness ratio. Int J Cancer. 2004;112(4):647–652. [DOI] [PubMed] [Google Scholar]
- 14. Anderson K, Jacobson JS, Heitjan DF, et al. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann Intern Med. 2006;144(6):397–406. [DOI] [PubMed] [Google Scholar]
- 15. Yang KY, Caughey AB, Little SE, et al. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) Families. Familial Cancer. 2011;10(3):535–543. [DOI] [PubMed] [Google Scholar]
- 16. Kwon JS, Sun CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer. 2008;113(2):326–335. [DOI] [PubMed] [Google Scholar]
- 17. Rubinstein WS, Jiang H, Dellefave L, et al. Cost-effectiveness of population-based BRCA1/2 testing and ovarian cancer prevention for Ashkenazi Jews: a call for dialogue. Genet Med. 2009;11(9):629–639. [DOI] [PubMed] [Google Scholar]
- 18. Nelson HD, Fu R, Goddard K, et al. In. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer: Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Rockville, MD; 2013. [PubMed] [Google Scholar]
- 19. NICE. Familial breast cancer: Classification and care of people at risk of familial breast cancer and management of breast cancer and related risks in people with a family history of breast cancer. In. NICE clinical guideline CG164 ed. London, UK: National Institute for Health and Care Excellence; 2013. [Google Scholar]
- 20. Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296(2):185–192. [DOI] [PubMed] [Google Scholar]
- 21. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22(6):1055–1062. [DOI] [PubMed] [Google Scholar]
- 23. Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacobs I. Screening for familial ovarian cancer: the need for well-designed prospective studies. J Clin Oncol. 2005;23(24):5443–5445. [DOI] [PubMed] [Google Scholar]
- 25. NICE. Guide to the methods of technology appraisal. In. N1618 ed. London, UK: National Institute for Health and Clinical Excellence (NICE); 2008. [PubMed] [Google Scholar]
- 26. Graham D, Schmool M, Waterman S. Jews in Britain: a snapshot from the 2001 Census. In: London, UK: Institute for Jewish Policy Research; 2007. [Google Scholar]
- 27. Office of National Statistics. Census 2001: National report for England and Wales, Part1, Section-2. Table S149: Sex and age by religion. In: London, UK: Office of National Statistics; 2003, 182–183. [Google Scholar]
- 28. Curtis L. Unit Costs of Health and Social Care 2011. In: Canterbury, Kent: Personal Social Services Research Unit (PSSRU); 2011. [Google Scholar]
- 29. Office of National Statistics. Lifetable for females in the UK. Available at: http://www.ons.gov.uk/ons/taxonomy/index.html?nscl=Interim+Life+Tables. Accessed June 2012. [Google Scholar]
- 30. Kadouri L, Hubert A, Rotenberg Y, et al. Cancer risks in carriers of the BRCA1/2 Ashkenazi founder mutations. J Med Genet. 2007;44(7):467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283(17):2260–2265. [DOI] [PubMed] [Google Scholar]
- 32. SEER. Surveillance Epidemiology and End Results. SEER Stat Fact Sheets: Ovary 2005–2009. Available at: http://seer.cancer.gov/statfacts/html/ovary.html. Accessed June 2012. [Google Scholar]
- 33. ONS. Cancer Registrations in England. In: London, UK: Office of National Statistics; 2010. [Google Scholar]
- 34. Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer. 2009;101(Suppl 2):S115–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolton KL, Chenevix-Trench G, Goh C, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307(4):382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walters S, Nur U, Rachet B, et al. (2010). Cancer survival in England: one-year and five-year survival for 21 common cancers, by sex and age. Office for National Statistics, Statistical Bulletin. [Google Scholar]
- 37. Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010;119(1):13–24. [DOI] [PubMed] [Google Scholar]
- 38. Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357(2):115–123. [DOI] [PubMed] [Google Scholar]
- 39. Madalinska JB, Hollenstein J, Bleiker E, et al. Quality-of-life effects of prophylactic salpingo-oophorectomy versus gynecologic screening among women at increased risk of hereditary ovarian cancer. J Clin Oncol. 2005;23(28):6890–6898. [DOI] [PubMed] [Google Scholar]
- 40. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drummond M, Sculpher M, Torrance G, et al. Methods for the economic evaluation of health care programs. Third Edition ed. Oxford Oxford University Press; 2005. [Google Scholar]
- 42. Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7(Suppl 5):20–28. [DOI] [PubMed] [Google Scholar]
- 43. Yancik R. Ovarian cancer. Age contrasts in incidence, histology, disease stage at diagnosis, and mortality. Cancer. 1993;71(2 Suppl):517–523. [DOI] [PubMed] [Google Scholar]
- 44. Swart A. Long-term follow-up of women enrolled in a randomized trial of adjuvant chemotherapy for early stage ovarian cancer. In: ASCO Annual Meeting Proceedings (Part I). 2007: Abstract 25, p. 18S (June 20 Supplement): 5509. J Clin Oncol. [Google Scholar]
- 45. Lyratzopoulos G, Abel GA, Barbiere JM, et al. Variation in advanced stage at diagnosis of lung and female breast cancer in an English region 2006–2009. Br J Cancer. 2012;106(6):1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. CRUK. (2012). Breast Cancer Incidence Statistics UK 2009–2011. Breast Cancer (C50), Average Number of New Cases per Year and Age-Specific Incidence Rates, Females, UK, 2009–2011. [Google Scholar]
- 47. NICE. National costing report: Early and locally advanced breast cancer/Advanced breast cancer. In: London, UK: National Institute for Health and Clinical Excellence; 2009. [Google Scholar]
- 48. ONS. (2008). Registrations of cancer diagnosed in 2006, England. Cancer Statistics Registrations, The Office for National Statistics. [Google Scholar]
- 49. Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening-MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468. [DOI] [PubMed] [Google Scholar]
- 50. NICE. Clinical Guideline (CG81) – Advanced breast cancer: diagnosis and treatment. In: Cardiff, Wales, UK: National Collaborating Centre for Cancer, National Institute for Health and Clinical Excellence; 2009. [Google Scholar]
- 51. Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert review of pharmacoeconomics & outcomes research. 2010;10(5):553–566. [DOI] [PubMed] [Google Scholar]
- 52. Gennari A, Conte P, Rosso R, et al. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;104(8):1742–1750. [DOI] [PubMed] [Google Scholar]
- 53. NICE. Social value judgements: principles for the development of NICE guidance. In: (NICE) NIfHaCE, (ed). 2nd ed: National Institute for Health and Clinical Excellence (NICE); 2008. [PubMed] [Google Scholar]
- 54. Andronis L, Barton P, Bryan S. Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making. Health technology assessment. 2009;13(29):iii, ix-xi, 1–61. [DOI] [PubMed] [Google Scholar]
- 55. Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8(1):1–2. [DOI] [PubMed] [Google Scholar]
- 56. NICE. Familial breast cancer: The classification and careof women at risk of familial breast cancer in primary, secondary and tertiary care. In. London: National Institute for Health and Clinical Excellence; 2006. [Google Scholar]
- 57. Griebsch I, Brown J, Boggis C, et al. Cost-effectiveness of screening with contrast enhanced magnetic resonance imaging vs X-ray mammography of women at a high familial risk of breast cancer. Br J Cancer. 2006;95(7):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dedes KJ, Bramkamp M, Szucs TD. Paclitaxel: cost-effectiveness in ovarian cancer. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):235–243. [DOI] [PubMed] [Google Scholar]
- 59. NICE. (2003). Guidance on the use of paclitaxel in the treatment of ovarian cancer.
- 60. Roa BB, Boyd AA, Volcik K, et al. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet 1996;14(2):185–187. [DOI] [PubMed] [Google Scholar]
- 61. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Manchanda R, Burnell M, Abdelraheim A, et al. Factors influencing uptake and timing of risk-reducing salpingo-oophorectomy in women at risk of familial ovarian cancer: a competing risk time to event analysis. BJOG. 2012;119(5):527–536. [DOI] [PubMed] [Google Scholar]
- 63. Finch A, Metcalfe KA, Chiang JK, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011;121(1):163–168. [DOI] [PubMed] [Google Scholar]
- 64. Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89(2):281–287. [DOI] [PubMed] [Google Scholar]
- 65. Lokkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of Hormone Therapy. Maturitas. 2006;53(2):226–233. [DOI] [PubMed] [Google Scholar]
- 66. Michelsen TM, Dorum A, Dahl AA. A controlled study of mental distress and somatic complaints after risk-reducing salpingo-oophorectomy in women at risk for hereditary breast ovarian cancer. Gynecol Oncol. 2009;113(1):128–133. [DOI] [PubMed] [Google Scholar]
- 67. Madalinska JB, van Beurden M, Bleiker EM, et al. The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorectomy. J Clin Oncol. 2006;24(22):3576–3582. [DOI] [PubMed] [Google Scholar]
- 68. MacLennan AH. Hormone replacement therapy: a 2008 perspective. Obstet Gynecol Reprod Med. 2008;19(1):13–18. [Google Scholar]
- 69. Sturdee DW, Pines A, Archer DF, et al. Updated IMS recommendations on postmenopausal hormone therapy and preventive strategies for midlife health. Climacteric. 2011;14(3):302–320. [DOI] [PubMed] [Google Scholar]
- 70. Rebbeck TR, Friebel T, Wagner T, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23(31):7804–7810. [DOI] [PubMed] [Google Scholar]
- 71. Evans DG, Lalloo F, Ashcroft L, et al. Uptake of risk-reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age, and time dependent. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2318–2324. [DOI] [PubMed] [Google Scholar]
- 72. Skytte AB, Gerdes AM, Andersen MK, et al. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: uptake and timing. Clin Genet. 2010;77(4):342–349. [DOI] [PubMed] [Google Scholar]
- 73. Friebel TM, Domchek SM, Neuhausen SL, et al. Bilateral prophylactic oophorectomy and bilateral prophylactic mastectomy in a prospective cohort of unaffected BRCA1 and BRCA2 mutation carriers. Clin Breast Cancer. 2007;7(11):875–882. [DOI] [PubMed] [Google Scholar]
- 74. Sigal BM, Munoz DF, Kurian AW, et al. A Simulation Model to Predict the Impact of Prophylactic Surgery and Screening on the Life Expectancy of BRCA1 and BRCA2 Mutation Carriers. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol. 2008;26(24):3943–3949. [DOI] [PubMed] [Google Scholar]
- 76. Isern AE, Tengrup I, Loman N, et al. Aesthetic outcome, patient satisfaction, and health-related quality of life in women at high risk undergoing prophylactic mastectomy and immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2008;61(10):1177–1187. [DOI] [PubMed] [Google Scholar]
- 77. Brandberg Y, Arver B, Johansson H, et al. Less correspondence between expectations before and cosmetic results after risk-reducing mastectomy in women who are mutation carriers: a prospective study. Eur J Surg Oncol. 2012;38(1):38–43. [DOI] [PubMed] [Google Scholar]
- 78. Metcalfe KA, Esplen MJ, Goel V, et al. Psychosocial functioning in women who have undergone bilateral prophylactic mastectomy. Psychooncology. 2004;13(1):14–25. [DOI] [PubMed] [Google Scholar]
- 79. Wasteson E, Sandelin K, Brandberg Y, et al. High satisfaction rate ten years after bilateral prophylactic mastectomy - a longitudinal study. Eur J Cancer Care (Engl). 2011;20(4):508–513. [DOI] [PubMed] [Google Scholar]
- 80. Chaliki H, Loader S, Levenkron JC, et al. Women’s receptivity to testing for a genetic susceptibility to breast cancer. Am J Public Health. 1995;85(8 Pt 1):1133–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tambor ES, Rimer BK, Strigo TS. Genetic testing for breast cancer susceptibility: awareness and interest among women in the general population. Am J Med Genet. 1997;68(1):43–49. [PubMed] [Google Scholar]
- 82. Press NA, Yasui Y, Reynolds S, et al. Women’s interest in genetic testing for breast cancer susceptibility may be based on unrealistic expectations. Am J Med Genet. 2001;99(2):99–110. [DOI] [PubMed] [Google Scholar]
- 83. Phillips KA, Warner E, Meschino WS, et al. Perceptions of Ashkenazi Jewish breast cancer patients on genetic testing for mutations in BRCA1 and BRCA2. Clin Genet. 2000;57(5):376–383. [DOI] [PubMed] [Google Scholar]
- 84. CRUK. Ovarian Cancer - UK Incidence Statistics. Available at: http://info.cancerresearchuk.org/cancerstats/types/ovary/incidence/. Accessed March 2013. [Google Scholar]
- 85. ONS. (2013). Cancer Registration Statistics, England, 2011. Office for National Statistics. [Google Scholar]
- 86. Bahar AY, Taylor PJ, Andrews L, et al. The frequency of founder mutations in the BRCA1, BRCA2, and APC genes in Australian Ashkenazi Jews: implications for the generality of U.S. population data. Cancer. 2001;92(2):440–445. [DOI] [PubMed] [Google Scholar]
- 87. Evans DG, Shenton A, Woodward E, et al. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bradbury AR, Ibe CN, Dignam JJ, et al. Uptake and timing of bilateral prophylactic salpingo-oophorectomy among BRCA1 and BRCA2 mutation carriers. Genet Med. 2008;10(3):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Curtis L. Unit Costs of Health and Social Care 2010. In: Personal Social Services Research Unit (PSSRU); 2010. [Google Scholar]
- 90. Department of Health PbR Team. NHS 2010–11 reference costs publication. In: Department of Health; 2011. [Google Scholar]
- 91. BNF. British National Formulary 67. London, UK: BMJ Group, and the Pharmaceutical Press (Royal Pharmaceutical Society of Great Britain); 2014. [Google Scholar]
- 92. NICE. Ovarian cancer: the recognition and initial management of ovarian cancer. In: London: National Institute for Health and Clinical Excellence (NICE); 2011. [Google Scholar]
- 93. NAO. End of life care. In: Burr TCaAG, ed. London: National Audit Office (NAO), House of Commons; 2008. [Google Scholar]
- 94. Robertson C, Arcot Ragupathy SK, Boachie C, et al. The clinical effectiveness and cost-effectiveness of different surveillance mammography regimens after the treatment for primary breast cancer: systematic reviews registry database analyses and economic evaluation. Health Technol Assess. 2011;15(34):v-vi, 1–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Contant CM, Menke-Pluijmers MB, Seynaeve C, et al. Clinical experience of prophylactic mastectomy followed by immediate breast reconstruction in women at hereditary risk of breast cancer (HB(O)C) or a proven BRCA1 and BRCA2 germ-line mutation. Eur J Surg Oncol. 2002;28(6):627–632. [DOI] [PubMed] [Google Scholar]
- 96. NICE. Early and locally advanced breast cancer: diagnosis and treatment. In. NICE Clinical Guideline, CG80. Cardiff, Wales, UK: National Collaborating Centre for Cancer, National Institute for Health and Clinical Excellence; 2009. [Google Scholar]
- 97. Manchanda R, Loggenberg K, Burnell M, et al. Dvd-based genetic counseling is as effective and more cost-efficient than standard-counseling for BRCA testing: results from a randomized trial. Int J Gynecol Cancer. 2012;22(8, Suppl 3):E413. Presented at IGCS, conference Vancouver, October 2012. [Google Scholar]
- 98. Waldron J. Breast Screening Program, England 2008–09. In: UK: The NHS Information Centre, for Health and Social Care, Government Statistical Service; 2010. [Google Scholar]
- 99. Bates T, Kearins O, Monypenny I, et al. Clinical outcome data for symptomatic breast cancer: the Breast Cancer Clinical Outcome Measures (BCCOM) Project. Br J Cancer. 2009;101(3):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 101. Cortesi L, Turchetti D, Marchi I, et al. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer. 2006;6:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365(9473):1769–1778. [DOI] [PubMed] [Google Scholar]
- 103. Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6(1):R8–R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Blamey RW, Ellis IO, Pinder SE, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–1999. Eur J Cancer. 2007;43(10):1548–1555. [DOI] [PubMed] [Google Scholar]
- 105. Gribbin J, Dewis R. (2009). Adjuvant! Online: review of evidence concerning its validity, and other considerations relating to its use in the NHS. NICE Clinical Guidelines, No. 80: Early and Locally Advanced Breast Cancer: Diagnosis and Treatment. [Google Scholar]
- 106. Comen E, Davids M, Kirchhoff T, et al. Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi Women. Breast Cancer Res Treat. 2011;129(1):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tung N, Garber JE, Lincoln A, et al. Frequency of triple-negative breast cancer in BRCA1 mutation carriers: comparison between common Ashkenazi Jewish and other mutations. J Clin Oncol. 2012;30(35):4447–4448. [DOI] [PubMed] [Google Scholar]
- 108. Chappuis PO, Nethercot V, Foulkes WD. Clinico-pathological characteristics of BRCA1- and BRCA2-related breast cancer. Semin Surg Oncol. 2000;18(4):287–295. [DOI] [PubMed] [Google Scholar]
- 109. Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10(2):169–180. [DOI] [PubMed] [Google Scholar]
- 110. Lipton A, Hershey S. (2006). Bone Metastases in Breast Cancer. Business Briefing: US Oncology Review. [Google Scholar]
- 111. Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24(13):2028–2037. [DOI] [PubMed] [Google Scholar]
- 112. Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27(15):2466–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.