Abstract
Objective. Glucocorticoid injections are used by rheumatologists to treat chronic tendinopathy. Surprisingly, the mechanisms by which corticosteroids induce pain relief in this condition have not been investigated. Previous studies have shown local substance P (SP) levels to be correlated with tendon pain and tissue pathology. The objective of this study was to determine whether SP production in human tenocytes is modulated by exposure to dexamethasone.
Methods. Human tendon fibroblasts were cultured in the presence or absence of dexamethasone (1–400 nM), an inhibitor of the glucocorticoid receptor, RU486, recombinant TGF-β (2.5 or 5.0 ng/ml) or an inhibitor of the TGF-β receptor (A83.01), recombinant human IL-1β and IL-6. Expression levels of the genes encoding for SP (TAC1) and its preferred receptor (NK1R), IL-1α, IL-1β and IL-6 were determined with quantitative PCR and protein levels of SP were examined by EIA and western blot.
Results. Exposure of human tendon cells to dexamethasone resulted in a time-dependent reduction of mRNA for SP in both hamstrings and Achilles tenocytes, whereas NK1R was unaffected. The reduction of SP mRNA was dependent on signalling through the glucocorticoid receptor. SP protein was substantially decreased by dexamethasone. Dexamethasone also prevented induction of SP by IL-1β and by cyclic mechanical loading.
Conclusion. This study demonstrates that dexamethasone treatment of human tendon fibroblasts reduces the expression of SP through a glucocorticoid receptor–dependent pathway. Drugs interfering with SP signalling could be a future target in the treatment of tendinopathy.
Keywords: corticosteroids, tendinopathy, soft tissue rheumatology, inflammation
Introduction
Glucocorticoid injections are frequently used as a treatment for chronic tendinopathy, although clinicians are now increasingly aware of caveats to this approach [1]. Several randomized trials and systematic reviews have demonstrated acute pain relief in patients with tendinopathy who receive corticosteroid injections, despite the risks of rupture or long-term recurrence of tendon pain [2]. Surprisingly, the mechanism by which dexamethasone induces acute pain relief in tendinopathy has not been investigated.
Many tendons in the body can be afflicted by chronic pain and thickening (tendinopathy), and the socio-economic impact of conditions such as rotator cuff tendinopathy is substantial. It has previously been shown that the pain of rotator cuff tendinopathy is correlated with the local tissue level of substance P (SP) and also that SP can be endogenously produced by tendon fibroblasts, particularly when these cells are subjected to mechanical loading [3, 4]. Tendinopathy is often accompanied by degeneration of the underlying tendon structure (tendinosis), and the elevation of SP in repetitively loaded tendon both precedes and can induce key features of tendinosis in an animal model [5].
In this study we examined whether SP production in tenocytes is modulated by exposure to dexamethasone. We found that dexamethasone specifically and time-responsively inhibited the production of SP mRNA (TAC1 gene) and protein by primary human tendon cells.
Methods
Cell culture
Tendon cells were obtained from the midportion of healthy Achilles tendons (Umeå University) or the semitendinosus hamstrings tendon from donors undergoing a reconstruction of the anterior cruciate ligament [ACL; University of British Columbia (UBC)]. Donors were male and female recreational athletes aged between 22 and 40 years (n = 6). The study was approved by the local ethics committee (UBC, Umeå University) and cell culture was conducted in Canada (hamstrings) or Sweden (Achilles). Written informed consent was obtained according to the Declaration of Helsinki (most recently at the General Assembly in October 2008). Tendon biopsies were washed and minced into 3- to 5-mm pieces, then enzymatically digested at 37°C under constant agitation using collagenase (clostridopeptidase A; Sigma, St Louis, MO, USA) in Dulbecco’s Modified Eagle’s Medium (D-MEM; Invitrogen, Carlsbad, CA, USA) at a concentration of 1.5 mg/ml. For the semitendinosus biopsies, this was followed by incubation in 0.25% trypsin for 3 min. The digestion product was centrifuged at 800 g for 5 min and resuspended and cultured in D-MEM supplemented with 10% fetal bovine serum (FBS; Invitrogen), 2 mM l-glutamine (Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2 in air. The medium was changed every third day. Confluent cells were harvested using 0.05% trypsin with EDTA (Invitrogen) and resuspended in medium and seeded at a 1:3 ratio. Only cells from passages 3–5 were used for experiments. The culture method and passage number resulted in consistent expression levels of tenocyte markers, including scleraxis, tenomodulin and COL1A2 (data not shown).
Experimental condition and substances for experimental design
Cells were cultured in 10% FBS. Dexamethasone (Sigma) was used at concentrations of 1–400 nM for mRNA analysis (6 and 12 h duration) and 1–100 nM for protein analysis (12 h duration). For mRNA analysis, the glucocorticoid receptor (GR) antagonist RU486 (Tocris Bioscience, Bristol, UK) was used at concentrations of 1, 10 and 100 nM for 12 h simultaneously with dexamethasone. Tendon cells were stimulated with 2.5 or 5.0 ng/ml TGF-β (R&D Systems, Minneapolis, MN, USA) with concentrations of 0, 0.1 and 1 nM A83.01 (Tocris) as an antagonist of the TGF-β pathway. RU486 and A83.01 were prepared in dimethyl sulphoxide (DMSO) and dexamethasone in absolute ethanol. Controls were incubated for the same duration as experimental conditions with the same volume of vehicle (ethanol or DMSO), which did not exceed 0.1%. The tendon cells were also stimulated with recombinant human IL-1β and IL-6 (R&D Systems) at concentrations of 0, 0.1, 1 and 10 ng/ml for 6 and 12 h. Tendon cells were seeded on a Bioflex culture plate membrane treated with collagen I (Flexcell International Corporation, Hillsborough, NC, USA) and were subjected to 10% cyclic strain with a frequency of 1 Hz for 2 h/day as previously described [4]. Before the third bout of cyclic strain, 10 nM dexamethasone was added to the culture medium of loaded cells and the same volume of vehicle (ethanol) was added to the unloaded cells and loaded cells. The tendon cells were then incubated for 6 h.
RNA isolation, reverse transcription and quantitative PCR
Primary tendon cells were seeded on six-well plates at a density of 1.2 × 105 in triplicate. At the termination of the experiments, total RNA was extracted using an RNA extraction kit (Qiagen, Venlo, The Netherlands). RNA was reverse transcribed using a high-capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA) and a thermal cycler (Eppendorf Mastercycler EP gradient S; Eppendorf North America, Hauppauge, NY, USA). Quantitative PCR (qPCR) was performed using Sybr green and TaqMan probes for TAC1 and NKR1, respectively, and data were normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S rRNA genes. Also the Sybr green probes for IL-1α, IL-1β and IL-6 were used. The primer sequences for TAC1 (forward; GATCAAGGAGGAACTGCCGGAGC; reverse: GGAATCAGCATCCCGTTTGCCCA), IL-1α (forward: CGCCAATGACTCAGAGGAAGA; reverse: AGGGCGTCATTCAGGATGAA), IL-1β (forward: AATCTGTACCTGTCCTGCGTGTT; reverse: TGGGTAATTTTTGGGATCTACACTCT), IL-6 (forward: GGTACATCCTCGACGGCATCT; reverse: GTGCCTCTTTGCTGCTTTCAC) and GAPDH (forward: TCTTTTGCGTCGCCAGCCGAG; reverse: TGACCAGGCGCCCAATACGAC) were designed and tested using Primer-BLAST (National Center for Biotechnology Information, Bethesda, MD, USA) and GeneRunner (version 3.05) and synthesized by Life Technologies (Grand Island, NY, USA). TaqMan probes for NKR1 and 18S RNA from Life Technologies (4351372 and 4352930E, respectively) were used. The cycle threshold (Ct) values were normalized to the Ct values of housekeeping genes with stable expression (GAPDH and 18S RNA) to obtain the relative gene expression; this value was expressed as the fold change compared with controls harvested at each time point.
Immunocytochemistry
Cells cultured on eight-well chamber slides (Falcon; BD, Franklin Lakes, NJ, USA) at a density of 1.5 × 104 cells/well were labelled with rabbit polyclonal antibodies against SP (Serotec, Oxford, UK) or scleraxis (Abcam, Cambridge, UK). Cells were fixed in 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 5 min, washed in phosphate buffer (PBS) 4 × 3 min and blocked in swine normal serum (1:20) for 15 min. The culture slides were incubated with primary antibody (SP, concentration 1:100; scleraxis, concentration 1:25) for 60 min at 37°C. After additional washing and blocking in normal serum the secondary antibody, TRITC/swine anti-rabbit (tetramethylrhodamine; Dako, Glostrup, Denmark) at a concentration of 1:40, was incubated for 30 min at 37°C. Finally, the cells were washed and mounted in Vectashield Hard Set Medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). The slides were examined using a Zeiss Axioskop 2 Plus microscope with epifluorescence and an Olympus DP70 digital camera.
Western blot
Cells were cultured in six-well plates at a density of 2 × 105 cells/well, in triplicate, and at the termination of the experiment the triplicates were pooled and lysed in radio-immunoprecipitation assay (RIPA) lysis buffer supplemented with a protease inhibitor cocktail (1:200; Sigma). The western blot process was performed as previously described [4]. Briefly, lysate was separated by electrophoresis at 160 V for 45 min in a 12% Tris–glycine extended (TGX) gel and subsequently transferred to a polyvinylidene fluoride transfer membrane at 100 V for 60 min. Following blocking, the membrane was incubated with the primary antibody against SP (Santa Cruz Biotechnology, Dallas, TX, USA) at a concentration of 1:200 and afterwards the secondary antibody, donkey anti-goat horseradish peroxidase (HRP; Santa Cruz) was used at a concentration of 1:1000. The bands were detected using a chemiluminescent HRP substrate (GE Healthcare, Little Chalfont, UK) and visualized on high-performance chemiluminescence film (GE Healthcare). The membranes were reprobed with an antibody against β-actin to confirm equal total protein loading.
SP enzyme immunoassay
Cells were cultured in six-well plates at a density of 2 × 105 cells/well in triplicate, then lysed in a lysis buffer (100 Mm Tris–HCI buffer, pH 7.0, containing 1 M NaCl, 2% bovine serum albumin, 4 mM EDTA, 0.2% Triton X-100 and 0.02% sodium azide) supplemented with a protease inhibitor cocktail at a concentration of 1:200. The lysates were centrifuged at 13 000 g at 4°C for 15 min and the supernatant was collected. Protein concentration was determined with Protein Assay Dye Reagent Concentrate (Bio-Rad, Hercules, CA, USA). SP concentration was quantified using 500 μg of total protein per sample, using a commercially available immunoassay kit (Phoenix Pharmaceuticals, Burlingame, CA, USA) with a linear detection range of 0.07–2.24 ng/ml. The absorbance was read at 450 nm.
Statistical analysis
All experiments were repeated using tendon cells derived from at least three separate patients. Data obtained from qPCR, SP EIA and western blot experiments were analysed with a one-way analysis of variance followed by Tukey’s multiple comparison test.
Results
Dexamethasone down-regulates SP expression
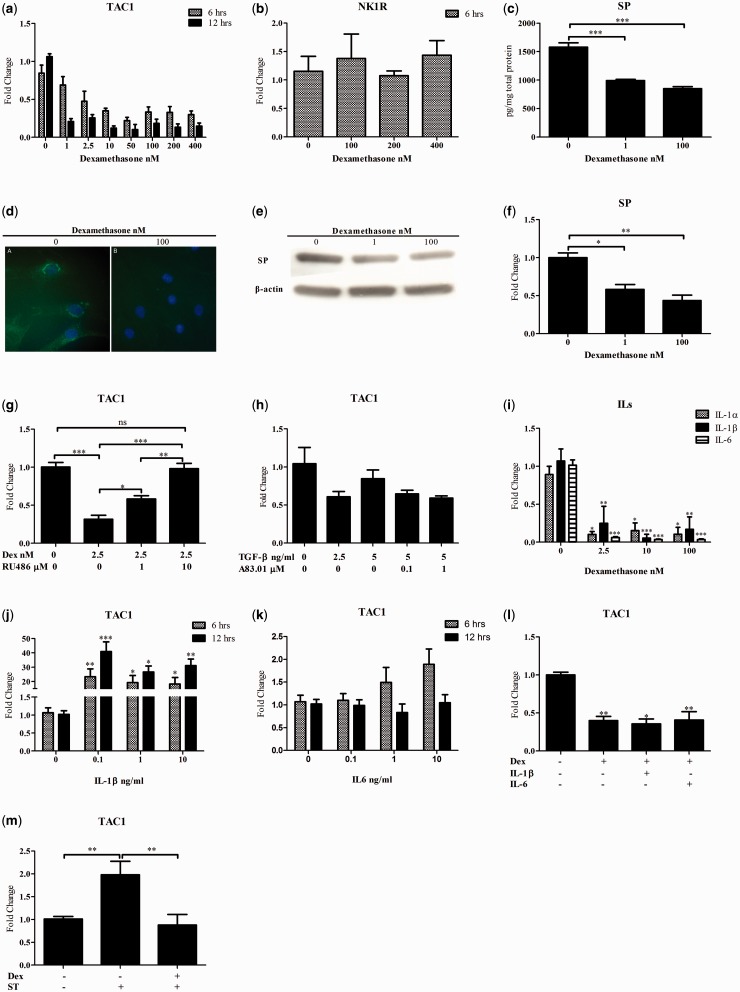
SP is encoded by the TAC1 gene. The level of TAC1 mRNA, as measured by qPCR, was significantly down-regulated after dexamethasone exposure in a time-dependent manner (Fig. 1a). This effect was specific to the examined gene, as evidenced by the fact that there was no significant effect of dexamethasone on the expression of NK1R mRNA (Fig. 1b).
Fig. 1.
Dexamethasone decreases TAC1 expression
Quantitative PCR data for (a) TAC1 and (b) NK1R for tendon cells after 6 and 12 h of incubation with increasing concentrations of dexamethasone. (c) EIA, (d) immunocytochemistry and (e and f) Western blot demonstrate reduced SP protein level in the cell lysates of tendon cells after 12 h of incubation with 1 and 100 nM dexamethasone. (g) The reduction of TAC1 expression by dexamethasone is specifically mediated through the GR, as evidenced by the lack of effect of dexamethasone when the GR antagonist RU486 at a concentration of 10 µM was simultaneously applied. The effect of RU486 was shown to be significantly dose dependent. (h) TGF-β and the inhibitor of TGF-β had no significant effect on TAC1 mRNA expression. (i) Dexamethasone after a 6 h incubation reduces the expression of IL-1α/β and IL-6 in tendon cells. (j) Recombinant human IL-1β induces the expression of TAC1 after 6 and 12 h of incubation, (k) but the induction by recombinant human IL-6 is not significant. (l) Incubation of hamstring tendon cells with dexamethasone for 12 h at a concentration of 2.5 nM (with or without 1 ng/ml of IL-1β and IL-6) inhibits TAC1 expression. (m) Six hours of incubation of hamstring tendon cells with 10 nM dexamethasone prevents induction of TAC1 in tendon cells subjected to cyclic strain (ST; 1 Hz = 10% strain, 2 h/day for 3 days; mean (s.e.); ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001). Data in panels b, g–m were generated from the hamstring tendon cells, panels c–f are from the Achilles tendon cells and panel a is from both.
Dexamethasone reduces SP production
The production of SP protein was significantly reduced after exposure to dexamethasone at concentrations of 1 and 100 nM for 12 h as compared with untreated cells (Fig. 1c). These data are consistent with the subjective immunocytochemistry evaluation (Fig. 1d) as well as western blot results showing that dexamethasone at concentrations of 1 and 100 nM reduced the expression of SP after 12 h (Fig. 1e and f).
Dexamethasone reduces SP expression through the GR
The reduced expression of TAC1 following dexamethasone exposure is significantly prevented by simultaneous exposure of the GR inhibitor RU486 after 12 h. At a concentration of 10 µM RU486, the effect of dexamethasone on TAC1 expression was negated (Fig. 1g). Stimulation of tendon cells with TGF-β with or without the presence of A83.01 as an antagonist of the TGF-β pathway had no significant effect on TAC1 mRNA expression (Fig. 1h).
Dexamethasone reduces the expression of interleukins
The tendon cells exposed to dexamethasone for 6 h showed reduced expression of IL-1α, IL-1β and IL-6 (Fig. 1i).
Dexamethasone inhibits the expression of SP induced by IL-1β
Stimulation of tendon cells with IL-1β significantly induced the expression of TAC1 (Fig. 1j). However, dexamethasone prevented the induction of TAC1 expression by IL-1β and IL-6 (Fig. 1l).
Dexamethasone inhibits the expression of SP induced by cyclic strain
Hamstring tendon cells subjected to cyclic loading showed significant up-regulation of TAC1 expression compared with non-strained cells. In contrast, adding dexamethasone at a concentration of 10 nM suppressed the induced expression of TAC1 by cyclic loading (Fig. 1m).
Discussion
In the current study we asked whether corticosteroids might influence expression levels of SP by tendon fibroblasts. Our data confirm that exposure of human cultured tendon fibroblasts to dexamethasone reduces SP mRNA and protein. Our results further show that the reduction of SP following dexamethasone exposure is mediated through the GR. The inhibition of SP mRNA by dexamethasone occurs even in the presence of the stimulatory effect of IL-1β, IL-6 and mechanical loading. The results of the study could help explain why glucocorticoid treatments lead to acute pain relief in patients suffering from tendinopathy.
The aetiology of chronic tendon pain is not completely understood, but evidence is accumulating that low-grade inflammation probably plays a role, as in OA. In the rotator cuff, pain levels are remarkably correlated with tissue levels of SP [3], and SP expression is also present in the tendons and bursae of people with greater trochanteric pain syndrome [6]. Interestingly, although peripheral SP-containing nerves are known to exist and are more extensive in chronically injured tendon, local tendon fibroblasts also appear to be a local source of SP. This finding is in keeping with recent studies demonstrating that SP is locally up-regulated by repetitive overuse in tendon fibroblasts both in vivo and in vitro [4, 7].
In cases of chronic tendinopathy, lack of inflammatory cellular infiltration in surgical biopsies has led some to challenge the involvement of inflammation in the aetiology of this disorder. However, several studies have shown inflammatory reactions in the early development of tendinopathy [8]. More recently, macrophages and T and B lymphocytes have been detected not only in the early phase, but also in chronic tendinopathy. Other studies have demonstrated increased levels of cyclooxygenase 2 and IL-6 [9]. Evidence thus points in the direction of one or more roles for inflammation during the development of tendinopathy; even if a particular inflammatory cell, activity or cytokine is not detected at a particular time point in a given study, it does not necessarily imply that the pathology is devoid of inflammation.
Dexamethasone is a commonly used glucocorticoid. Ihara and Nakanishi [10] showed that dexamethasone down-regulated the preferred SP receptor (NK1R) mRNA in a pancreatic acinar cell line. In contrast, our data did not show any significant effect of dexamethasone on NKR1 in tendon cells. Dexamethasone is an agonist of the GR. Binding of dexamethasone to the GR facilitates its translocation to the nucleus, where trans-repression of transcription factors such as nuclear factor κB and activator protein 1 by the GR negatively regulates inflammatory activity [11, 12]. King et al. [13] showed that GR transactivation by dexamethasone is involved in glucocorticoid-mediated repression of many IL-1β-induced inflammatory genes. Our results also showed that dexamethasone inhibits the expression of SP induced by IL-1β. Our study thus supports the notion of a negative regulatory effect of the GR on endogenously produced inflammatory mediators; however, further studies are needed to examine the crosstalk between GR and other transcription factors in the modulation of SP in tendon cells.
Rheumatology key messages.
Exposure of human tendon fibroblasts to dexamethasone results in reduced production of substance P.
Substance P reduction may contribute to the pain relief that many tendinopathy patients experience after anti-inflammatory treatment.
Non-corticosteroid drugs that interfere with substance P signalling could be a future target in the treatment of tendinopathy.
Acknowledgements
A.S. received a clinical scholar award from the Michael Smith foundation for health research. R.M. received a PhD scholarship from Worksafe BC. The authors acknowledge the provision of surgical biopsy material by Dr Hakan Alfredson. The authors also wish to acknowledge Attia Riaz and Dr Hayedeh Behzad for their assistance and Dr Patrik Danielson, Dr Håkan Alfredson and Dr Vincent Duronio for their guidance and logistical help.
Funding: The study was funded by a grant from the Canadian Institutes of Health Research, Institute of Musculoskeletal Arthritis (Priority Announcement 126604-1).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Metcalfe D. Glucocorticoid injections in lesions of the achilles tendon—a systematic review. Rheumatology. 2008;47(Suppl 2):II183–4. [Google Scholar]
- 2.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751–67. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 3.Gotoh M, Hamada K, Yamakawa H, Inoue A, Fukuda H. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res. 1998;16:618–21. doi: 10.1002/jor.1100160515. [DOI] [PubMed] [Google Scholar]
- 4.Backman LJ, Fong G, Andersson G, Scott A, Danielson P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PLoS One. 2011;6:e27209. doi: 10.1371/journal.pone.0027209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backman LJ, Andersson G, Wennstig G, Forsgren S, Danielson P. Endogenous substance P production in the Achilles tendon increases with loading in an in vivo model of tendinopathy-peptidergic elevation preceding tendinosis-like tissue changes. J Musculoskelet Neuronal Interact. 2011;11:133–40. [PubMed] [Google Scholar]
- 6.Fearon AM, Twin J, Dahlstrom JE, et al. Increased substance P expression in the trochanteric bursa of patients with greater trochanteric pain syndrome. Rheumatol Int. 2014 Feb 23 doi: 10.1007/s00296-014-2957-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–6. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvist MH, Lehto MU, Jozsa L, Jarvinen M, Kvist HT. Chronic achilles paratenonitis. An immunohistologic study of fibronectin and fibrinogen. Am J Sports Med. 1988;16:616–23. doi: 10.1177/036354658801600611. [DOI] [PubMed] [Google Scholar]
- 9.Legerlotz K, Jones ER, Screen HR, Riley GP. Increased expression of IL-6 family members in tendon pathology. Rheumatology. 2012;51:1161–5. doi: 10.1093/rheumatology/kes002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ihara H, Nakanishi S. Selective inhibition of expression of the substance P receptor mRNA in pancreatic acinar AR42J cells by glucocorticoids. J Biol Chem. 1990;265:22441–5. [PubMed] [Google Scholar]
- 11.Tsurufuji S, Sugio K, Takemasa F. The role of glucocorticoid receptor and gene expression in the anti-inflammatory action of dexamethasone. Nature. 1979;280:408–10. doi: 10.1038/280408a0. [DOI] [PubMed] [Google Scholar]
- 12.Vandevyver S, Dejager L, Tuckermann J, Libert C. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 2013;154:993–1007. doi: 10.1210/en.2012-2045. [DOI] [PubMed] [Google Scholar]
- 13.King EM, Chivers JE, Rider CF, et al. Glucocorticoid repression of inflammatory gene expression shows differential responsiveness by transactivation- and transrepression-dependent mechanisms. PLoS One. 2013;8:e53936. doi: 10.1371/journal.pone.0053936. [DOI] [PMC free article] [PubMed] [Google Scholar]