Abstract
Objective. To estimate the prevalence of inadequate pain relief (IPR) among patients with symptomatic knee OA prescribed analgesic therapy and to characterize patients with IPR.
Methods. Patients ≥50 years old with physician-diagnosed knee OA who had taken topical or oral pain medication for at least 14 days were recruited for this prospective non-interventional study in six European countries. Pain and function were assessed using the Brief Pain Inventory (BPI) and the WOMAC; quality of life (QoL) was assessed using the 12-item short form. IPR was defined as an average pain score of >4 out of 10 on BPI question 5.
Results. Of 1187 patients enrolled, 68% were female and the mean age was 68 years (s.d. 9); 639 (54%) met the definition of IPR. Patient responses for the BPI average pain question were well correlated with responses on the WOMAC pain subscale (Spearman r = 0.64, P < 0.001). In multivariate logistic regression, patients with IPR had greater odds of being female [adjusted odds ratio (adjOR) 1.90 (95% CI 1.46, 2.48)] and having OA in both knees [adjOR 1.48 (95% CI 1.15, 1.90)], higher BMI, longer OA duration, depression or diabetes. Patients with IPR (vs non-IPR) were more likely to have worse QoL, greater function loss and greater pain interference.
Conclusion. IPR is common among patients with knee OA requiring analgesics and is associated with large functional loss and impaired QoL. Patients at particular risk of IPR, as characterized in this study, may require greater attention towards their analgesic treatment options.
Trial registration: https://clinicaltrials.gov/ (NCT01294696).
Keywords: analgesic therapy, inadequate pain relief, knee, osteoarthritis
Introduction
Since 1990 the prevalence of musculoskeletal disorders has increased 45% and these disorders represent the second largest cause of disability worldwide, accounting for 21% of global years lived in disability [1]. Of the musculoskeletal disorders, OA represents the most common form of joint disease [2, 3]. Risk factors including older age, female gender, obesity and a history of manual labour result in susceptibility of the joints to damage, repair failure and OA [3–5].
OA of the knees accounts for 83% of the total OA burden and ranks among the top 10 non-communicable diseases for global disability-adjusted life years (DALYs; i.e. years of life lost and years lived with disability) [1, 3]. Clinical outcomes for people with knee OA typically involve pain, limitations of daily living activities, physical disability and overall reduction of quality of life (QoL).
As there is no cure for OA, the goals of OA therapies are to reduce the levels of pain and inflammation, slow cartilage degradation, improve function and reduce disability. Evidence-based guidelines recommend a broad range of pharmacological and non-pharmacological therapies for OA pain management [6–13]. Commonly used therapies and treatment modalities include paracetamol, NSAIDs, opioids, topical analgesics and IA injection of corticosteroid [5–12]. It is not possible to predict which therapy will work for any individual patient, and response is not uniform across populations [9, 14].
In both OA clinical trials and clinical practice, outcome assessments have traditionally been limited to evaluation of pain intensity [15]. The focus of clinical trials for OA analgesic therapies has been on treatment response, while the consequences of inadequate response are less well investigated [16]. Moreover, the outcomes of clinical trials do not directly translate to the real world, where patients may experience greater pain, have multiple co-morbidities and use their pain medications intermittently.
Thus an important issue facing people with OA and their health care providers is inadequate pain relief (IPR). As a concept, IPR has not been widely explored in OA, and there is limited understanding of the relationship between IPR and patient factors, treatment patterns and QoL. A better understanding of IPR—and of the characteristics of patients likely to experience IPR—may help clinicians to identify patients who require close monitoring and careful selection of therapy for OA.
The objectives of this multinational prospective observational study, the Study of Osteoarthritis Real World Therapies (SORT), were (i) to estimate the prevalence of IPR among patients with symptomatic knee OA prescribed analgesic therapy, (ii) to assess the characteristics of patients with IPR and (iii) to examine the relationship between IPR and health outcomes, comparing patients with IPR versus non-IPR in terms of history and clinical care at baseline.
Methods
Study design and patients
This prospective longitudinal cohort study was conducted at 53 centres in six European countries (the UK, France, Germany, Portugal, The Netherlands and Italy). Centres included both general and specialist practices, varying by country (see supplementary Table S1, available at Rheumatology Online). Patients were recruited from November 2011 to January 2012. This analysis presents baseline pain data, demographic data and medication use, as well as the associations with other health outcomes at baseline. Clinical and demographic data for patients who declined to participate were not collected.
Patients eligible for study enrolment were ≥50 years old with a physician’s diagnosis of OA of one or both knees [17] and were prescribed, at the direction of a physician, topical or oral pain medications for at least 14 days before entering the study. Patients were excluded if they had any arthritis other than primary OA, partial or total joint replacement in the affected knee(s) or treatment with DMARDs.
As this was a real-world observational study, no medications or interventions were provided under the study protocol. Participation in the study did not influence the choice of treatment; patients’ treatments were determined by their attending physicians. Patients could elect to withdraw at any time without impact on their medical care. The study protocol was approved by the local ethics committee at each site and each patient gave written informed consent according to the Declaration of Helsinki. The study was registered with ClinicalTrials.gov (NCT01294696).
Data collection
At study entry, demographic information, medical history and medication use were collected. Patients completed questionnaires regarding their knee OA, including assessments of pain level, joint stiffness, physical functioning, satisfaction with and response to treatment and QoL. All questionnaires were provided to patients in their local language.
Patients were instructed to assess pain caused by OA in the affected knee(s) using the Brief Pain Inventory (BPI). The BPI is a comprehensive instrument validated in several languages for assessing pain severity and pain interference with function [18–21]. Pain relief was derived using question 5 of the BPI, which assesses average pain: Please rate your pain by circling the one number that best describes your pain on the average. The numerical rating scale ranges from 0 (no pain) to 10 (pain as bad as you can imagine). This single question is similar to how physicians would evaluate their patients’ pain in most clinical encounters and is of clear clinical relevance.
Other patient self-assessments were collected, including pain, stiffness and physical function in the knee as measured by the WOMAC questionnaire (0–100 mm visual analogue scale) [22] and QoL as assessed by the Medical Outcomes Trust 12-item Short Form Health Survey (SF-12) [23].
Patients also reported their satisfaction with symptomatic treatment for knee OA, as measured by two questions regarding pain relief and tolerability of medication over the previous 7 days. These questions were assessed on a five-item categorical scale ranging from very dissatisfied to very satisfied. Patients and investigators reported the response to therapy on a five-item scale from no response to excellent response using the Patient Global Assessment of Response to Therapy Questionnaire (PGART) and the Investigator Global Assessment of Response to Therapy Questionnaire (IGART) [24], respectively.
Study endpoint
The primary endpoint was the patient’s assessment of pain relief at study entry. In clinical trials, assessments of pain caused by OA are based on cut-off points for patient-reported pain, function and global assessments [25]. Different pain cut-off points have been used previously to categorize pain severity [26] and manageable pain [27]. In a recent review of pain measures and associated cut-off points, it was reported that patients reported that ≤3 on a 10 point scale is thought to be no worse than mild pain [28]
Question 5 of the BPI, which assesses average pain, defines mild pain as a pain score of 0–4, moderate pain as 5–6 and severe pain as 7–10 [29]. In this study, IPR was defined as a score >4 (moderate or severe pain) on BPI question 5; scores of 0–4 were classified as non-IPR.
Statistical analyses
The primary analysis was a cross-sectional comparison of IPR vs non-IPR patient cohorts at baseline. Study endpoints, patient characteristics and treatments for knee OA were summarized using descriptive statistics. Categorical variables were compared using the χ2 test or Fisher’s exact test, while continuous variables were compared using the Mann–Whitney Wilcoxon test.
We examined the robustness of the cut-off point of 4 on the BPI in identifying the prevalence of IPR by comparing adjacent cut-off points found in the literature (pain ≤3 as no worse than mild pain and pain >5 [29]) and observed the distribution of patients classified as IPR. In addition, we reviewed the distribution of the BPI average score and its association with the WOMAC pain subscale using Spearman’s rank-order correlation coefficient. We also compared the different IPR cut-off points using the WOMAC pain subscale, WOMAC physical function subscale and SF-12 Physical Component Summary scores. To accomplish this, following Tan et al. [30], we employed an analysis of variance (ANOVA) using IPR categories derived from the BPI average score as the independent variable and the other measures as dependent variables.
To identify the characteristics of patients who were more likely to have IPR, a multivariate logistic regression model was employed, adjusting for country effects. The following variables were assessed: age, female sex, BMI, years since OA diagnosis, clinical diagnosis of both knees, co-morbidities (cardiovascular disease, renal impairment/failure, diabetes, depression, hyperlipidaemia, hypertension) and a number of different classes of medication.
All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA). Statistical significance was set at P < 0.05.
Results
A total of 1187 consenting patients were enrolled in the study at 53 centres in six countries. Table 1 summarizes patient characteristics at baseline and Supplementary Table S2, available at Rheumatology Online, presents patient enrolment numbers by country. The mean patient age was 68 years, 68% of patients were women and only 19% of patients were in paid employment at the time of study enrolment. The most common physician-reported co-morbidity was hypertension, affecting 58% of patients. Just over half of the patients (52%) had OA affecting both knees, while nearly one-quarter each had an affected left or right knee (Table 1).
Table 1.
Baseline demographic characteristics of all patients and by knee pain relief at baseline
| All patients (n = 1187) | IPR (n = 639) | non-IPR (n = 548) | P-value | |
|---|---|---|---|---|
| Age, mean (s.d.), years | 67.8 (9.4) | 68.0 (9.8) | 67.6 (9.0) | 0.484 |
| Female, n (%) | 808 (68.1) | 479 (75.0) | 329 (60.0) | <0.001 |
| BMI, mean (s.d.), kg/m2 | 29.4 (5.5) | 30.0 (5.7) | 28.7 (5.1) | <0.001 |
| Number of classes of medication, mean (s.d.)a | 1.5 (0.7) | 1.5 (0.7) | 1.4 (0.7) | 0.008 |
| Currently in paid employment, n (%)b | 229 (19.4) | 103 (16.3) | 126 (23.1) | 0.003 |
| Current smoker, n (%) | 95 (8.0) | 49 (7.7) | 46 (8.4) | 0.431 |
| Co-morbidity, n (%) | ||||
| Cardiovascular diseasec | 265 (22.3) | 154 (24.1) | 111 (20.3) | 0.124 |
| Hypertension | 692 (58.3) | 396 (62.0) | 296 (54.0) | 0.007 |
| Hyperlipidaemia | 533 (44.9) | 297 (46.5) | 236 (43.1) | 0.242 |
| Depressiond | 298 (25.1) | 197 (30.8) | 101 (18.4) | <0.001 |
| Diabetes | 213 (17.9) | 145 (22.7) | 68 (12.4) | <0.001 |
| GI conditione | 157 (13.2) | 92 (14.4) | 65 (11.9) | 0.229 |
| Renal impairment/failured | 80 (6.7) | 53 (8.3) | 27 (4.9) | 0.027 |
| Years since OA diagnosis, mean (s.d.) | 6.2 (6.2) | 6.9 (6.6) | 5.4 (5.6) | <0.001 |
| Median (IQR) | 4.5 (1.3, 9.6) | 5.5 (1.5, 10.6) | 3.7 (0.9, 8.4) | <0.001 |
| Clinical diagnosis of OA, n (%) | ||||
| Left knee only | 286 (24.1) | 146 (22.9) | 140 (25.6) | 0.307 |
| Right knee only | 282 (23.8) | 120 (18.8) | 162 (29.6) | <0.001 |
| Both knees | 619 (52.2) | 373 (58.4) | 246 (44.9) | <0.001 |
| Hip | 290 (24.4) | 168 (26.3) | 122 (22.3) | 0.119 |
| Spine | 522 (44.0) | 289 (45.2) | 233 (42.5) | 0.379 |
| Physician-reported disability—partial or total, n (%) | 154 (13.0) | 98 (15.3) | 56 (10.2) | 0.009 |
| Hip replacement—partial or total, n (%) | 70 (5.9) | 45 (7.0) | 25 (4.6) | 0.083 |
| Analgesic medication for OA, n (%) | ||||
| NSAID | 773 (65.1) | 389 (60.9) | 384 (70.1) | <0.001 |
| Paracetamol | 470 (39.6) | 282 (44.1) | 188 (34.3) | <0.001 |
| Alternative therapyf | 211 (17.8) | 104 (16.3) | 107 (19.5) | 0.149 |
| Opioid-containing medication | 254 (21.4) | 169 (26.5) | 85 (15.5) | <0.001 |
| Other | 50 (4.2) | 32 (5.0) | 18 (3.3) | 0.150 |
IPR was defined as an average Brief Pain Inventory pain score of moderate or greater pain (score >4) and non-IPR was defined as a score ≤4. aThe classes of medication are NSAID, paracetamol, alternative therapy, opioid-containing medication and other. bEmployment data were missing for eight patients—five in the IPR cohort and three in the non-IPR cohort. cCardiovascular disease included congestive heart failure or established ischaemic heart disease, peripheral arterial disease or cerebrovascular disease. dCurrent or prior diagnosis. eGI conditions included IBD, active peptic ulceration or GI bleeding, perforation related to previous NSAID use, recurrent peptic ulcer or GI haemorrhage. fAlternative therapies included glucosamine, chondroitin and hyaluronate. GI: gastrointestinal; IQR: interquartile range; IPR: inadequate pain relief.
Prevalence of IPR
At the baseline assessment, 639/1187 patients [54% (95% CI 51%, 57%)] reported IPR as measured on the BPI average pain score. The prevalence of IPR was quite robust to the definition using different cut-off points. With a cut-off point of ≤3 defining patients with no worse than mild pain, the inverse number of patients was 846/1187 [71% (95% CI 69%, 74%)] meeting the definition for IPR (i.e. a BPI score > 3). A cut-off point > 5 classified 414 [35% (95% CI 32%, 38%)] patients as having IPR.
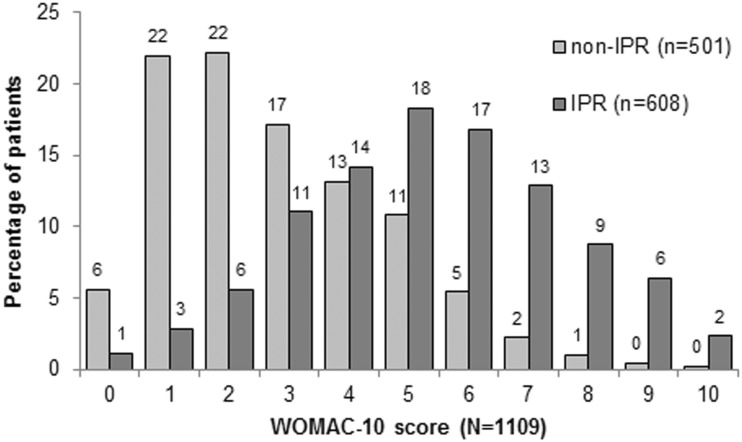
Patient responses for the BPI average pain question were well correlated with responses on the WOMAC pain subscale (Spearman’s r = 0.64, P < 0.001, n = 1109), further demonstrating that the concept of IPR is not an artefact of how IPR is defined and assessed. Fig. 1 depicts the distribution of patients in the IPR and non-IPR cohorts according to WOMAC pain subscale scores, which were converted to a 0–10 point scale to facilitate comparison.
Fig. 1.
Score distribution for the WOMAC pain subscale score (converted to a 0–10 scale) by score on BPI question 5 as IPR (score >4) and non-IPR (score ≤4)
Percentages are patients in each cohort (IPR and non-IPR); 78 patients were missing WOMAC pain subscale data. IPR: inadequate pain relief; BPI: Brief Pain Inventory.
The cut-off point of 4 was optimal in our sample, with the highest F values in the ANOVAs when BPI pain severity, BPI pain interference and SF-12 Physical Component Summary scores were the dependent variables and near optimal when the WOMAC pain subscale and WOMAC physical function subscale were the dependent variables (see Supplementary Table S3, available at Rheumatology Online).
Characteristics of patients with IPR
While patients in both the IPR and non-IPR cohorts were of similar mean age (68 years) and included similar percentages of current smokers (8%), the two groups were significantly different for multiple characteristics, as outlined in Table 1. Patients reporting IPR were more likely to be female and less likely to be employed; they had a higher mean BMI and a longer mean duration of OA; and they had a greater likelihood of co-morbid hypertension, current or prior depression, diabetes and current or prior renal impairment or failure (P ≤ 0.027 for all comparisons). In addition, a significantly greater percentage of patients with IPR had OA affecting both knees (P < 0.001) and physician-reported partial or total disability (P = 0.009; Table 1). While NSAIDs were the most commonly prescribed medication in both cohorts (65% overall), patients in the IPR cohort were significantly more likely (P < 0.001) to be prescribed paracetamol (44% vs 34% in non-IPR cohort) or opioid-containing medication (26% vs 16%).
After adjusting for country fixed effects, there were several significant predictors of IPR (Table 2). Specifically, patients who reported IPR had greater odds of being female and having higher BMI, more years with OA diagnosis, a clinical diagnosis of OA in both knees, current or prior depression and a diagnosis of diabetes.
Table 2.
Multivariable analyses examining the odds of IPR, adjusted for country fixed effects (n = 1187)
| Adjusted OR | 95% CI | |
|---|---|---|
| Female | 1.90 | 1.46, 2.48 |
| BMI | 1.03 | 1.01, 1.06 |
| Years since OA diagnosis | 1.04 | 1.02, 1.06 |
| Clinical OA diagnosis of both knees | 1.48 | 1.15, 1.90 |
| Cardiovascular disease | 1.18 | 0.86, 1.62 |
| Renal impairment/failure | 1.29 | 0.77, 2.20 |
| Baseline diabetes diagnosis | 2.09 | 1.47, 3.01 |
| Baseline depression | 1.89 | 1.41, 2.54 |
| Baseline hyperlipidaemia | 0.88 | 0.68, 1.14 |
| Baseline hypertension | 1.15 | 0.88, 1.51 |
| Number of different classes of medication | 1.04 | 0.86, 1.26 |
Overall significance of the model: likelihood ratio χ2 = 143.69, df = 17, P < 0.001. OR: odds ratio.
IPR and health outcomes
As shown in Table 3, patients in the IPR cohort had significantly greater overall pain severity and much worse pain interference on the BPI compared with patients in the non-IPR cohort (P < 0.001 for all comparisons). Similarly, the WOMAC subscale scores (pain, physical function and stiffness) were significantly worse (P < 0.001) for patients in the IPR cohort.
Table 3.
IPR and health outcomes by pain relief status
| IPR (n) | IPR (n = 639) | Non-IPR (n) | Non-IPR (n = 548) | P-value | |
|---|---|---|---|---|---|
| BPI, severity,a mean (s.d.) | 637 | 5.8 (1.4) | 547 | 2.7 (1.3) | <0.001 |
| BPI, interference,a mean (s.d.) | 636 | 5.3 (2.0) | 548 | 2.5 (1.8) | <0.001 |
| WOMAC pain subscale, mean (s.d.) | 608 | 53.6 (21.5) | 501 | 28.5 (18.4) | <0.001 |
| Median (IQR) | 608 | 53.2 (39.1–68.8) | 501 | 25 (13.6–40) | |
| WOMAC stiffness subscale, mean (s.d.) | 610 | 56.8 (26.3) | 502 | 33.7 (25.3) | <0.001 |
| Median (IQR) | 610 | 59.8 (41.5–77.5) | 502 | 30 (12.5–50.5) | |
| WOMAC physical function subscale, mean (s.d.) | 601 | 55.0 (21.1) | 498 | 29.9 (19.4) | <0.001 |
| Median (IQR) | 601 | 56 (41–70.9) | 498 | 26.9 (14.3–42.0) | |
| PGART | |||||
| Excellent | 629 | 24 (3.8) | 540 | 53 (9.8) | <0.001 |
| Good | 629 | 164 (26.1) | 540 | 247 (45.7) | <0.001 |
| Fair | 629 | 259 (41.2) | 540 | 177 (32.8) | 0.004 |
| Poor | 629 | 137 (21.8) | 540 | 41 (7.6) | <0.001 |
| No response | 629 | 45 (7.2) | 540 | 22 (4.1) | 0.031 |
| IGART | |||||
| Excellent | 637 | 13 (2.0) | 546 | 44 (8.1) | <0.001 |
| Good | 637 | 155 (24.3) | 546 | 262 (48.0) | <0.001 |
| Fair | 637 | 282 (44.3) | 546 | 165 (30.2) | <0.001 |
| Poor | 637 | 156 (24.5) | 546 | 67 (12.3) | <0.001 |
| No response | 637 | 31 (4.9) | 546 | 8 (1.5) | 0.001 |
Scoring on the BPI ranges from 0 (best) to 10 (worst); scoring on the WOMAC ranges from 0 (best) to 100 (worst). aThe BPI severity score is the mean of responses to questions 3–6; the BPI interference score is the mean of responses to questions 9A–G. IPR: inadequate pain relief; BPI: Brief Pain Inventory; PGART: Patient Global Assessment of Response to Therapy; IGART: Investigator Global Assessment of Response to Therapy; IQR: interquartile range.
The Patient and Investigator Global Assessments of Response to Therapy showed significantly worse responses (P ≤ 0.004) to immediate prior therapy for patients in the IPR cohort versus the non-IPR cohort (Table 3). In addition, responses for all QoL domains on the SF-12 were significantly worse for those with IPR (Table 4). Overall, 51% of patients in the IPR cohort reported their general health status to be fair or poor as compared with 31% of the non-IPR cohort (P < 0.001). At baseline, 48% of patients with IPR reported dissatisfaction with response to treatment and 38% reported dissatisfaction with tolerability of medication.
Table 4.
Questionnaire results for QoL on the SF-12
| IPR (n) | IPR (n = 639) | Non-IPR (n) | Non-IPR (n = 548) | P-value | |
|---|---|---|---|---|---|
| General health status, n (%) | |||||
| Excellent/very good/ good | 636 | 309 (48.6) | 545 | 378 (69.4) | <0.001 |
| Poor/fair | 636 | 327 (51.4) | 545 | 167 (30.6) | <0.001 |
| Physical functioning scale | 633 | 27.9 (26.9) | 546 | 49.8 (28.1) | <0.001 |
| Role physical scale | 638 | 39.2 (25.1) | 545 | 59.3 (23.8) | <0.001 |
| Bodily pain scale | 639 | 37.4 (24.7) | 548 | 59.7 (23.3) | <0.001 |
| General health scale | 636 | 41.1 (25.2) | 545 | 52.1 (22.5) | <0.001 |
| Vitality scale | 633 | 40.4 (25.5) | 540 | 53.2 (23.5) | <0.001 |
| Social functioning scale | 631 | 57.4 (29.3) | 544 | 78.4 (23.8) | <0.001 |
| Role emotional scale | 636 | 57.2 (28.8) | 548 | 74.5 (23.0) | <0.001 |
| Mental health scale | 633 | 56.0 (23.4) | 541 | 70.3 (19.7) | <0.001 |
| Physical Component Summary | 624 | 35.0 (8.0) | 533 | 41.2 (8.2) | <0.001 |
| Mental Component Summary | 624 | 45.6 (11.3) | 533 | 51.8 (9.0) | <0.001 |
QoL: quality of life; SF-12: 12-item Short Form Health Survey. Data are presented as mean (s.d.) unless otherwise specified. Scoring on the SF-12 subscales ranged from 0 (worst) to 100 (best). IPR: inadequate pain relief.
Discussion
The results of this observational study indicate that IPR is a highly prevalent problem among patients with OA. After ≥2 weeks of physician-prescribed treatment, more than half of patients with knee OA reported IPR, as defined by moderate to severe pain (score >4) using the BPI average pain question. The prevalence of IPR could be as high as 70% if a pain score ≤3 (no worse than mild pain [29]) was used as the appropriate treatment target. As demonstrated in this study, patient responses on the BPI were well correlated with responses on the WOMAC pain subscale. Moreover, patients in the IPR cohort reported significantly worse scores on pain severity, pain interference, WOMAC function subscale and QoL (SF-12 Physical Component Summary) than patients in the non-IPR cohort.
Patients with IPR were a largely identifiable population who were more likely to be female and have longer disease duration, bilateral knee OA, greater opioid use and a higher prevalence of certain co-morbidities (hypertension, depression and diabetes). Clinicians should recognize this constellation of characteristics as constituting special risk, since patients with IPR were significantly more likely to be unemployed and to be considered partially or totally disabled by their physician (15% vs 10% of those in the non-IPR cohort).
The fact that 54% of patients in this real-world setting had persistent moderate to severe pain suggests that currently prescribed pain treatments for knee OA are not meeting the needs of the majority of patients. The most commonly prescribed analgesic medications reported by patients were NSAIDs, followed by paracetamol and opioid-containing medications. These findings are consistent with those of prior observational studies indicating that management of OA in primary care may not conform to the recommendations in clinical practice guidelines or to the intensity of patients’ pain [31–36].
For patients with knee OA, the importance of pain relief and functional improvement was evident in the results of a recently reported discrete choice experiment that assessed patient preferences with regard to NSAID-related benefits [36]. The patients with self-reported OA in that study ranked reduction in ambulatory pain and reduced difficulties associated with daily activities as the most important benefits.
The clinical characteristics of our patient population reflect those reported for the general population with OA with regard to age, sex and co-morbidities [4], suggesting that study patients are representative of patients with knee OA and that our findings are generalizable. Patient and investigator assessments of response to therapy were generally in agreement. Study results across the multiple and varied assessments of pain and functional outcomes were consistent, reinforcing the robustness of the findings as well as illustrating the marked differences between patients with IPR and non-IPR. It is noteworthy that patient assessments of pain relief were associated with other patient-reported assessments of function and QoL. This finding suggests that assessing pain relief in the clinic is an efficient way of assessing the patient’s overall health status.
A possible limitation of the study is that of selection bias, as not all invited patients consented to participate in the study, and we are not certain that non-participants were similar to patients. Evidence of selective non-participation (by patients ≥80 years, of lower socio-economic group and currently employed) was seen in an earlier prospective study of knee pain and knee OA in the general population, although the authors found no effect on the distribution of WOMAC scores [37]. Our analysis is cross-sectional, therefore the causal link between IPR and functional status cannot be fully characterized. The 12-month follow-up period of this study will enable us to document the clinical course of pain and pain management in patients with OA. From the longitudinal analysis we can demonstrate an association between pain intensity and variability to a range of other health benefits (e.g. QoL, functional status and productivity).
Conclusion
IPR is common among patients with knee OA requiring analgesics, affecting 54% of patients in this study, and is associated with large functional loss and impaired QoL. There appears to be a patient profile at particular risk of IPR. Practitioners should actively evaluate pain management and the effectiveness of treatment response. In discussions with patients, practitioners should ensure that patients are communicating their concerns about pain medication and have a thorough understanding of their analgesic treatment options.
Rheumatology key messages.
Inadequate pain relief is common among patients with knee OA requiring analgesics.
Patients with inadequate pain relief have functional loss and reduced quality of life.
Inadequate pain relief predictors include female sex, higher BMI, longer OA duration, bilateral knee OA, depression and diabetes.
Supplementary Material
Acknowledgements
Medical writing and editorial assistance was provided by Elizabeth V. Hillyer, DVM. This assistance was funded by Merck Sharp & Dohme, a subsidiary of Merck & Co., Whitehouse Station, NJ, USA.
Funding: This work was supported by Merck Sharp & Dohme, a subsidiary of Merck & Co., Whitehouse Station, NJ, USA. The sponsor was involved with the writing, study design, collection, analysis and interpretation of data.
Disclosure statement: P.M.P. was a full-time employee of Merck at the time this study was designed and conducted. C.J.P. has received honoraria from Merck. C.M.B. is an employee of Merck. F.R. has received honoraria from MSD for the SORT study. S.R. has worked as a consultant on this project and others at MSD. SDT, SVE & PM are employees of MSD and is a shareholder of Merck & Co., Inc. M.A.F.vdL. has provided consultancy services to Pfizer, Novartis and MSD and has received research grants from AbbVie, Pfizer, MSD, BMS and Roche. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Mody GM, Brooks PM. Improving musculoskeletal health: global issues. Best Pract Res Clin Rheumatol. 2012;26:237–49. doi: 10.1016/j.berh.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Conaghan PG, Dickson J, Grant RL. Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336:502–3. doi: 10.1136/bmj.39490.608009.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–99. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 11.Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–55. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roddy E, Zhang W, Doherty M, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee—the MOVE consensus. Rheumatology. 2005;44:67–73. doi: 10.1093/rheumatology/keh399. [DOI] [PubMed] [Google Scholar]
- 13.Porcheret M, Jordan K, Croft P. Treatment of knee pain in older adults in primary care: development of an evidence-based model of care. Rheumatology. 2007;46:638–48. doi: 10.1093/rheumatology/kel340. [DOI] [PubMed] [Google Scholar]
- 14.Bennell KL, Hunter DJ, Hinman RS. Management of osteoarthritis of the knee. BMJ. 2012;345:e4934. doi: 10.1136/bmj.e4934. [DOI] [PubMed] [Google Scholar]
- 15. Osteoarthritis Research Society International (OARSI). OARSI Primer. http://primer.oarsi.org/ (20 December 2013, date last accessed)
- 16.Taylor SD, Everett SV, Taylor TN, Watson DJ, Taylor-Stokes G. A measure of treatment response: patient and physician satisfaction with traditional NSAIDs for osteoarthritis control. Open Access Rheum Res Rev. 2013;5:69–76. doi: 10.2147/OARRR.S41940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman RD. Criteria for classification of clinical osteoarthritis. J Rheumatol Suppl. 1991;27:10–2. [PubMed] [Google Scholar]
- 18.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in Pain Research and Therapy. Vol. 12. Issues in pain measurement. New York, NY, USA: Raven Press, 1989:391–403. [Google Scholar]
- 19.Caraceni A, Mendoza TR, Mencaglia E, et al. A validation study of an Italian version of the Brief Pain Inventory (Breve Questionario per la Valutazione del Dolore) Pain. 1996;65:87–92. doi: 10.1016/0304-3959(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 20.Radbruch L, Loick G, Kiencke P, et al. Validation of the German version of the Brief Pain Inventory. J Pain Symptom Manage. 1999;18:180–7. doi: 10.1016/s0885-3924(99)00064-0. [DOI] [PubMed] [Google Scholar]
- 21.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 23.Stewart AL, Hays RD, Ware JE., Jr The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–35. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Navarra S, Rubin BR, Yu Q, Smugar SS, Tershakovec AM. Association of baseline disease and patient characteristics with response to etoricoxib and indomethacin for acute gout. Curr Med Res Opin. 2007;23:1685–91. doi: 10.1185/030079907x210750. [DOI] [PubMed] [Google Scholar]
- 25.Sadosky AB, Bushmakin AG, Cappelleri JC, Lionberger DR. Relationship between patient-reported disease severity in osteoarthritis and self-reported pain, function and work productivity. Arthritis Res Ther. 2010;12:R162. doi: 10.1186/ar3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapstad H, Hanestad BR, Langeland N, Rustoen T, Stavem K. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Musculoskelet Disord. 2008;9:55. doi: 10.1186/1471-2474-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelman DC, Hoffman DL, Seifeldin R, Dukes EM. Development of a metric for a day of manageable pain control: derivation of pain severity cut-points for low back pain and osteoarthritis. Pain. 2003;106:35–42. doi: 10.1016/s0304-3959(03)00274-4. [DOI] [PubMed] [Google Scholar]
- 28.Moore RA, Straube S, Aldington D. Pain measures and cut-offs—‘no worse than mild pain’ as a simple, universal outcome. Anaesthesia. 2013;68:400–12. doi: 10.1111/anae.12148. [DOI] [PubMed] [Google Scholar]
- 29. Pain Research Group. Brief Pain Inventory. http://www3.mdanderson.org/depts/prg/bpi.htm#currnt_validation (20 December 2013, date last accessed)
- 30.Tan G, Thornby JI, Jensen MP, Rintala DDH. Categorizing pain in patients seen in a veterans health administration hospital: pain as the fifth vital sign. Psychol Serv. 2008;5:239–50. [Google Scholar]
- 31.Snijders GF, den Broeder AA, van Riel PL, et al. Evidence-based tailored conservative treatment of knee and hip osteoarthritis: between knowing and doing. Scand J Rheumatol. 2011;40:225–31. doi: 10.3109/03009742.2010.530611. [DOI] [PubMed] [Google Scholar]
- 32.Richette P, Hilliquin P, Bertin P, et al. Comparison of general practitioners and rheumatologists’ prescription patterns for patients with knee osteoarthritis. BMC Musculoskelet Disord. 2011;12:72. doi: 10.1186/1471-2474-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porcheret M, Jordan K, Jinks C, Croft P. Primary care treatment of knee pain—a survey in older adults. Rheumatology. 2007;46:1694–700. doi: 10.1093/rheumatology/kem232. [DOI] [PubMed] [Google Scholar]
- 34.Hunter DJ, Neogi T, Hochberg MC. Quality of osteoarthritis management and the need for reform in the US. Arthritis Care Res. 2011;63:31–8. doi: 10.1002/acr.20278. [DOI] [PubMed] [Google Scholar]
- 35.Muller S, Bedson J, Mallen CD. The association between pain intensity and the prescription of analgesics and non-steroidal anti-inflammatory drugs. Eur J Pain. 2012;16:1014–20. doi: 10.1002/j.1532-2149.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauber AB, Arden NK, Mohamed AF, et al. A discrete-choice experiment of United Kingdom patients’ willingness to risk adverse events for improved function and pain control in osteoarthritis. Osteoarthritis Cartilage. 2013;21:289–97. doi: 10.1016/j.joca.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Peat G, Thomas E, Handy J, et al. The Knee Clinical Assessment Study – CAS(K). A prospective study of knee pain and knee osteoarthritis in the general population: baseline recruitment and retention at 18 months. BMC Musculoskelet Disord. 2006;7:30. doi: 10.1186/1471-2474-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.