Abstract
The output of alternative splicing depends on the cooperative or antagonistic activities of several RNA-binding proteins (RBPs), like Ptbp1 and Esrp1 in Xenopus. Fine-tuning of the RBP abundance is therefore of prime importance to achieve tissue- or cell-specific splicing patterns. Here, we addressed the mechanisms leading to the high expression of the ptbp1 gene, which encodes Ptbp1, in Xenopus epidermis. Two splice isoforms of ptbp1 mRNA differ by the presence of an alternative exon 11, and only the isoform including exon 11 can be translated to a full-length protein. In vivo minigene assays revealed that the nonproductive isoform was predominantly produced. Knockdown experiments demonstrated that Esrp1, which is specific to the epidermis, strongly stimulated the expression of ptbp1 by favoring the productive isoform. Consequently, knocking down esrp1 phenocopied ptbp1 inactivation. Conversely, Ptbp1 repressed the expression of its own gene by favoring the nonproductive isoform. Hence, a complex posttranscriptional mechanism controls Ptbp1 abundance in Xenopus epidermis: skipping of exon 11 is the default splicing pattern, but Esrp1 stimulates ptbp1 expression by favoring the inclusion of exon 11 up to a level that is limited by Ptbp1 itself. These results decipher a posttranscriptional mechanism that achieves various abundances of the ubiquitous RBP Ptbp1 in different tissues.
INTRODUCTION
Alternative splicing relies on the selection of different splice sites within a pre-mRNA and allows different mRNA isoforms to be produced from a given gene. Deep sequencing of mRNA across several human tissues has revealed that up to 94% of human gene products are subject to alternative splicing, indicating that it is a widespread means of regulating gene expression. The selection of the splice isoforms of an mRNA is specific to cell types or developmental stages. Hence, alternative splicing promotes specific proteomes that in turn specify the cellular identity (1, 2).
cis-Acting regulatory elements within a pre-mRNA control alternative splicing. A single alternative splice event is generally under the control of a combination of positive and negative controls. Several RNA-binding proteins (RBPs), including those of the hnRNP and SR subfamilies, directly interact with these cis-acting regulatory sequences and either cooperate or antagonize to dictate the final outcome of the splicing reaction (3). The tissue specificity of alternative splicing can rely on the tissue-specific expression of RBPs that regulates pre-mRNA splicing, as shown for NOVA-1 in neurons (4). It can also be achieved through small changes in the relative levels or activities of ubiquitous factors (5). For example, the RBPs TIA1 and PTBP1 play antagonistic roles in the control of FAS alternative splicing (6), and it can be anticipated that subtle tissue-specific changes in the TIA1-to-PTBP1 ratio may lead to significant changes in the splicing pattern of FAS. Phosphorylations and, potentially, cell-type-specific phosphorylations have also been shown to affect the local concentration of splicing factors around pre-mRNA by altering their intracellular localization (7), protein-protein interactions (8), or RNA-protein interactions (9). Therefore, the relative abundances of RBPs involved in alternative splicing regulation have to be fine-tuned to support cell-type-specific splicing patterns.
Several RBPs negatively and directly self-regulate. Most of them control their abundance by promoting unstable isoforms of their mRNAs. For example, the RBPs CELF2 (CUGBP2), TIA1, PTBP1, HNRNPL, SRSF3 (Srp20), TRA2B, and SRSF2 commit the splicing of their own pre-mRNA toward isoforms that contain a premature termination codon, which targets them to rapid degradation by the nonsense-mediated decay (NMD) pathway (10–15). ELAVL1 (HuR) favors a distal site of cleavage/polyadenylation, resulting in a long 3′ untranslated region of ELAVL1 mRNA that contains several AU-rich elements, common triggers of mRNA degradation (16). TARDBP (TDP-43) directly promotes the decay of its mRNA (17). PABPC3 [cytoplasmic poly(A) binding protein] and SRSF1 (ASF/SF2) repress the translation of their own mRNA (18, 19).
Self-regulatory mechanisms tend to minimize variations of RBP amounts. However, the amounts of RBPs may significantly differ from one tissue to another. This is the case for PTBP1. In HeLa cells, PTBP1 favors a splicing isoform of PTBP1 mRNA that contains a premature termination codon and is targeted for rapid degradation (12). This mechanism is expected to ensure a constant level of PTBP1 in mammalian cells. However, the PTBP1 gene is expressed in several tissues at different levels, and this differential expression is important. In neuronal progenitors, for example, PTBP1 represses neuronal mRNAs, such as the mRNA encoding PSD-95. Upon neuronal differentiation, PTBP1 is repressed, leading to the expression of neuronal genes (20, 21). The repression of PTBP1 is even sufficient to induce a transdifferentiation of fibroblasts to neurons (22). The control of the amount of PTBP1 is therefore a key regulator of neuronal differentiation. Similarly, the downregulation of the murine PTBP1 homologue (Ptbp1) may be necessary during mouse spermatogenesis to achieve meiosis-specific splicing patterns (23).
Here, we used the Xenopus model to address how differential levels of expression of the PTBP1 homologue, ptbp1, are achieved in two different tissues: the epidermis, where ptbp1 mRNA is abundant, and the somites, where it is barely present (24). We hypothesized that the RBP Esrp1 (also known as Rbm35a) could contribute to the high level of ptbp1 expression. Esrp1 is the amphibian homologue of human ESRP1, which has initially been identified by screening for factors that favor an epithelial isoform of FGFR2 mRNA (25). ESRP1 and its paralog, ESRP2, are specifically expressed in epithelial cells and are key determinants of epithelial identity (26, 27). We demonstrate that esrp1 and ptbp1 are coexpressed in Xenopus epidermis, and we identify a mechanism by which the Esrp1 protein modulates ptbp1 pre-mRNA splicing and the Ptbp1 protein level.
MATERIALS AND METHODS
Antibodies, plasmids, and in vitro transcription.
Anti-ESRP1 antibodies were kindly provided by Russ Carstens (25). Anti-Ptbp1 antibodies have been described previously (28). The anti-PCNA, anti-V5, and secondary antibodies were from Sigma (catalog number P8825), Invitrogen (catalog number R960), and Jackson, respectively.
The WT-esrp1-V5 plasmid was made by amplification of the esrp1 open reading frame (ORF) from Image clone 5571123 (Imagenes) using the following primers: forward primer AGATCTTTCACCATGACTGCTGTTTCTCCGGAT (the bold ATG is the translation initiation codon) and reverse primer AGCGGCCGCAATACAAACCCATTCTTTGG. The resulting product was cloned between the BglII and NotI sites of the pT7TS-V5 vector (28). The same procedure was used to construct the esrp1-V5 plasmid, using the following forward primer: GGATCCGCCACCATGACCGCCGTCTCGCCTGACTACCTGGTGGTT (restricted by BamHI). The RNA transcribed from this plasmid contains several point mutations in the 5′ region of the ORF (underlined nucleotides in the primer sequence), making it immune to the morpholino (MO) antisense oligonucleotide against esrp1. The WT-ptbp1-V5 and ptbp1-V5 plasmids have been described previously (28, 29). We constructed the keratin-ptbp1 minigene by amplifying the region of the Xenopus ptbp1 gene between exons 10 and 12 from the Xenopus genome with the following primers: forward primer tgagctcactagtcccGACTTGGCATCCCTGGAAAC and reverse primer ccatggccgcgggcccCAAGTTGAGCTTGGTTCCCAT (the plasmid sequences used for cloning are in lowercase). The first 81 nucleotides of exon 10 were omitted to remove two potential AUG start codons. The resulting PCR product was cloned into the SmaI-linearized pBS-keratin plasmid (30) by Gibson assembly (New England BioLabs).
The matrices for in vitro transcription were prepared by PCR amplification using combinations of the following primers: a forward primer in intron 10 (aaattaatacgactcactatagGGAGACAACCTATCCTTCAAAAATATTAAC; the sequence for T7 transcription is in lowercase), a forward primer in exon 11 (aaattaatacgactcactatagGGAGAGTTACACCCCAATGCCTCTTTATTC; the sequence for T7 transcription is in lowercase), a reverse primer in exon 11 (CAATGTGGGGTTACGGAGAA), a reverse primer in proximal intron 11 (GAAAAAGTAATAAGTGTGAAAAACATACC), and a reverse primer in distal intron 11 (GCCATAGAAAGTTATATTAACAGTAAGTTG). Radiolabeled RNAs were obtained from these matrices by in vitro transcription (MEGAscriptT7; catalog number AM1334; Ambion) in the presence of [α-32P]UTP (catalog number BLU507X; Perkin-Elmer).
Xenopus embryo manipulations.
Xenopus embryos at the two-cell stage were injected in both blastomeres with one or several of the following: a control morpholino antisense oligonucleotide (CCTCTTACCTCAGTTACAATTTATA; 20 ng, unless otherwise stated; GeneTools), morpholino antisense oligonucleotide against ptbp1 (AACAATTCCTTCCATGGCACACTAA; 20 ng), a morpholino antisense oligonucleotide against esrp1 (AATCCGGAGAAACAGCAGTCATGGT; 20 ng, unless otherwise stated), the keratin-ptbp1 minigene plasmid (200 pg), and in vitro transcripts (transcribed from their respective plasmids using an mMessage mMachine T7 kit [200 ng; Ambion]). The embryos were generally cultured until stages 28 to 30 (tail bud) (31). Alternatively, embryos injected with an esrp1 mRNA were arrested at stage 15 (neurula) due to massive death in later stages, and embryos to be photographed were arrested at stage 35 (tadpole). When required, the embryos were manually dissected with fine forceps in 5% Ficoll.
Western blotting and RNA isolation.
Embryo extracts were prepared by snap-freezing pools of embryos in liquid nitrogen and then crushing them in 10 μl per embryo of homogenization buffer (20 mM Tris-HCl, pH 7.4, 4 mM EDTA). Protein extracts for Western blot analyses were prepared by adding 1 volume of 220 mM KCl, 10 mM MgCl2, 20 mM Tris-HCl, pH 7.4, 40 mM NaF, 40 mM glycerophosphate, 10 mM dithiothreitol, 4 mM EDTA, 8% glycerol, 2% IGEPAL CA-630, 2% deoxycholate, protease inhibitor cocktail (catalog number P8340; Sigma). After centrifugation (12,000 × g, 15 min, 4°C), the soluble material was resolved by denaturing gel electrophoresis and analyzed by Western blotting. Enhanced chemifluorescence (Amersham Bioscience) and a Storm molecular imager (Molecular Dynamics) were used for visualization and quantification. Total RNAs were extracted from the embryo extracts using the Tri Reagent (Sigma) and treated with Turbo DNase (Ambion). Then, cDNAs were synthesized using random primers and SuperScript II reverse transcriptase (RT; Invitrogen).
Protein-RNA-binding assays.
All protein-RNA-binding experiments were carried out with protein extracts prepared as described above from embryos injected with esrp1-V5 mRNA. For unlabeled immunoprecipitations, embryo extracts were diluted in 10 volumes of PXL buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1% IGEPAL CA-630, 0.1% SDS, 0.5% deoxycholate). This diluted extract was incubated with 40 μl of protein G-Dynabeads (40 μl beads/ml diluted extract; Invitrogen) previously incubated with control mouse immunoglobulin or antibodies against the V5 epitope (6 μg/40 μl beads; catalog number R-960-25; Invitrogen). After four washes in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 1 M NaCl, 0.5 mM EDTA, 0.5% IGEPAL CA-630, 0.1% SDS, 1% deoxycholate), the beads were eluted in SDS buffer for Western blot analysis or RNA extraction with the Tri Reagent. For cross-linking experiments, 20 pmol of 32P-labeled in vitro transcripts was incubated with 50 μl of embryo extract for 30 min on ice before UV irradiation (4,000 μJ/cm2; Stratalinker). The extracts were treated with 2 μl RNase A (catalog number A7973; Promega) before immunoprecipitation as described above.
Real-time and radioactive PCR.
Real-time PCR was performed by the use of Sybr Green chemistry (Power Sybr green PCR master mix; Applied Biosystems) in an ABI Prism 7900 system (Applied Byosystems). The primer sequences (5′ to 3′) are given below: for eef1a1, GAGAGGGAAGCTGCTGAGATGG and CCACAGGGAGATGTCAATGGTA; for ptbp1 (exon 3 [e3] to e4), AAGGCAAAAACCAGGCCTT and GCAGTGCTGCTTGAGCTCTA; for ptbp1 (intron 7 [i7] to e8), TGTCCTGACATGGGATCTCTTA and GAAATCCAGCACCAGCATAAG; for ptbp1 (e8 to e11), TTATGCTGGTGCTGGATTTC and AAGAGGCATTGGGGTGTAAC; for ptbp1 (e11 to e12), GTTACACCCCAATGCCTCTTTA and CAAGTTGAGCTTGGTTCCCA; for ptbp1 (e11 to i11), GTTACACCCCAATGCCTCTTTA and AGCAACTTCTGTTATGCACCA; for ptbp1 (e13 to e14), GCCCCTCCGTACAACTGTAT and CAGATGGCGGAATGTTTGAG; and for ptbp2, CAGCTTGCAATGAGTCATCTAA and ACATTGCCTCCAGTGTTTGTAA.
The radioactive PCRs were performed with 32P-end-labeled forward primers specific for each minigene. The number of PCR cycles was optimized for each pair of primers. The amplimers were resolved on an 8% native polyacrylamide gel and quantified by PhosphorImager analysis (Amersham Bioscience). The primer sequences (5′-3′) are given below: for eef1a1, the same primers described above; for the minigene, GTCCAATTATGCACACCACAGAAGT and TGAGCTCACTAGTCCCGACTTG; for ptbp1, GATTGAATTGCACAACCATGATA and CCCTTGGGAATGGTTTCTGATA; for ptbp1 (e1-e4), ATGGAAGGCATTGTTCAAG and CTGGAAGCTTACGAAGATGG; for ptbp1 (e10-e13), GGTCGACTTGGTATTCATGG and GATACAGTTGTACGGAGGGG; for tpm1a-α7, GAGTTTGCAGAGAGGACAGTA and TGGAAAGGGTACGGAGGTAAGC; and for tpm1a-O5, GAGTTTGCAGAGAGGACAGTA and CGGAATTCCTGGCACTCAAGAGCAAG.
RESULTS
ptbp1 and esrp1 are coexpressed in Xenopus embryos.
Two ESRP paralogs, ESRP1 and ESRP2, exist in mammals (26, 27). However, we identified by genomic and EST databases searches (32) only one esrp gene in Xenopus tropicalis (GenBank accession number NM_001005057.1) and Xenopus laevis (GenBank accession number NM_001086055). This gene is more closely related to human or murine ESRP1 than to ESRP2 (Fig. 1A), and here we name it Xenopus esrp1.
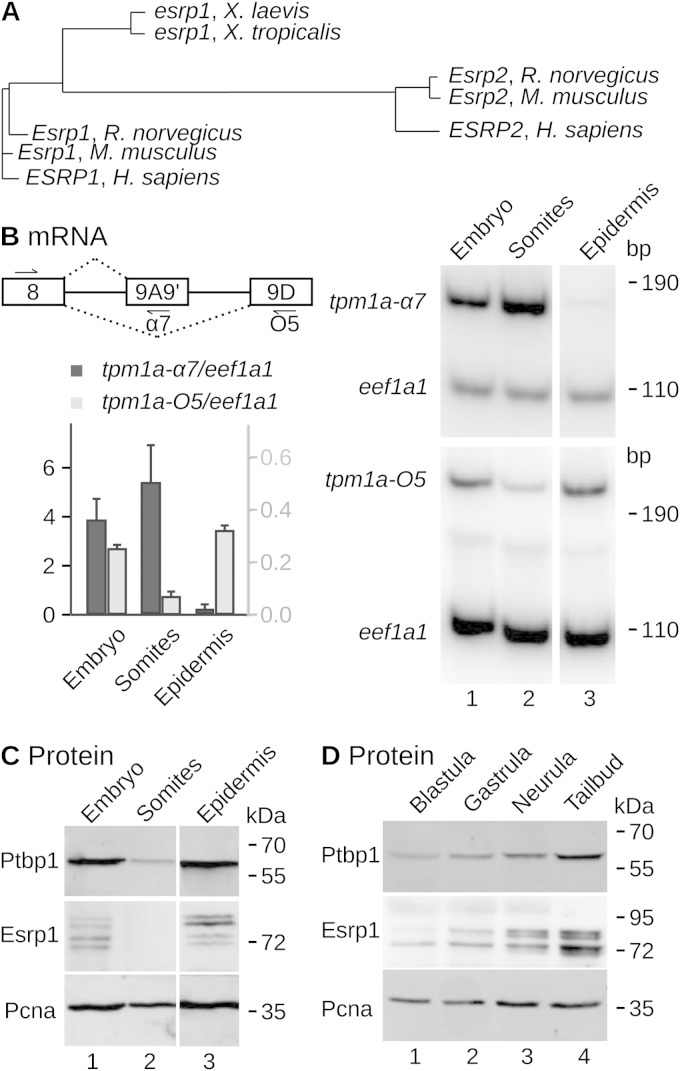
FIG 1.
Coexpression of ptbp1 and esrp1 in Xenopus embryos. (A) Phylogenetic tree showing the distances between Xenopus esrp1 (Xenopus laevis and Xenopus tropicalis), human (Homo sapiens) ESRP1 and ESRP2, and rodent (Rattus norvegicus and Mus musculus) Esrp1 and Esrp2. (B) (Top left) Schematic drawing of tpm1a pre-mRNA. Constitutive exon 8 is spliced either to terminal exon 9A9′ to produce the α7 isoform or to terminal exon 9D to produce the O5 isoform. An additional exon, 9B, was omitted for clarity. The arrows reveal the positions of the PCR primers. (Right) The somites and the epidermis were dissected from stage 30 Xenopus embryos. The relative abundances of the indicated isoforms of the tpm1a mRNAs and of the eef1a1 mRNA (EF1α, as a loading control) were estimated in the two fractions and total nondissected sibling embryos by radioactive semiquantitative RT-PCR. (Lower left) Quantification from 3 similar experiments (mean ± SD). (C and D) The relative abundances of Ptbp1, Esrp1, and Pcna (as a loading control) in dissected embryos (C) or in total embryos arrested at different stages of development (D) were measured by Western blotting. The blastula is stages 6 to 8, the gastrula is stages 9 to 11, the neurula is stage 15, and the tail bud is stages 28 to 30 (31). In all panels, the positions of the molecular mass markers are indicated on the right of the gels.
To test the coexpression of ptbp1 and esrp1 in Xenopus, tail bud embryos (stages 28 to 30) were dissected into two fractions: the epidermis of ectodermal origin and the somites, which are of mesodermal origin and are the prospective muscles and bones. Analysis of the levels of tpm1a-α7 and -O5 mRNAs was used to assess the purity of the fractions. These two isoforms differ by the terminal exon (Fig. 1B) and are specific to myotomal and nonmuscle cells, respectively (33, 34). As expected, the α7 isoform was essentially restricted to the somitic fraction, while a high level of the O5 isoform was present in the epidermal fraction (Fig. 1B). The Ptbp1 protein showed different abundances in the two dissected fractions (Fig. 1C). Normalization to the amount of Pcna revealed that Ptbp1 is ∼8 times more abundant in the epidermis than in the somites. Using a monoclonal antibody directed against mammalian ESRP1 (25), we detected Xenopus Esrp1 as four bands of unequal intensity (Fig. 1C). The same observation was previously made in human cells and was attributed to posttranslational modifications (25). Importantly, the global signal for Esrp1 was strong in the epidermis and undetectable in the somites. Hence, both ptbp1 and esrp1 genes are more strongly expressed in the epidermis than in the somites. Furthermore, during Xenopus development, the amount of Esrp1 protein continuously increased at least up to the tail bud stage, very much like the amount of the Ptbp1 protein (Fig. 1D). Overall, our data show that both esrp1 and ptbp1 are strongly expressed in the epidermis and that their levels increase in concert during development.
ptbp1 requires Esrp1 for high expression in epidermis.
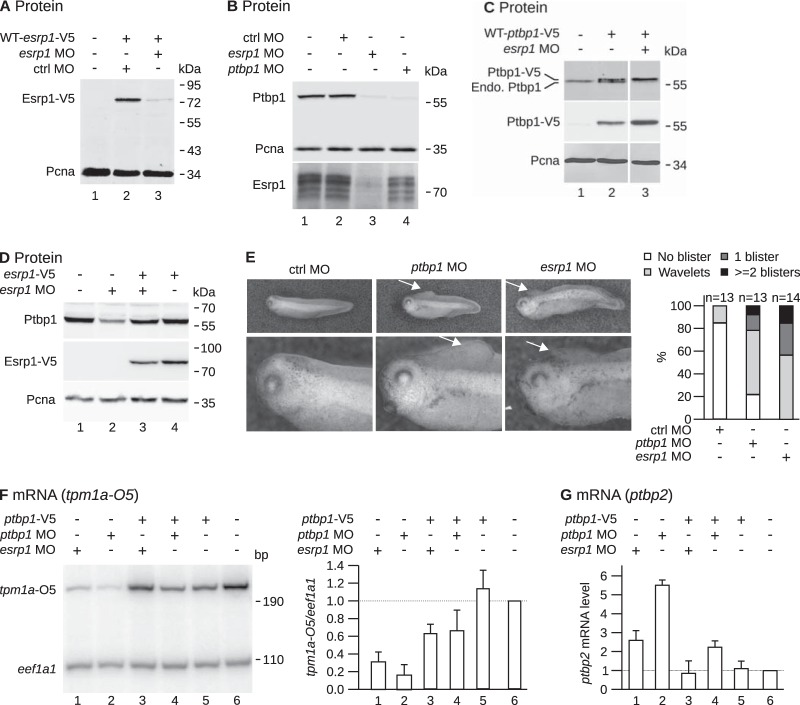
The expressions of ptbp1 and esrp1 correlate with each other. We then tested whether the selective downregulation of either of them could impact the expression of the other. Morpholino antisense oligonucleotides targeting ptbp1 mRNA (ptbp1 MO) have been described (29). We designed a morpholino antisense oligonucleotide targeting esrp1 mRNA (esrp1 MO) that efficiently reduces the translation of an esrp1 reporter mRNA (Fig. 2A). We injected these two morpholinos or a control morpholino into both blastomeres of a two-cell embryo, and we measured the levels of endogenous Esrp1 and Ptbp1 proteins after development (Fig. 2B). As expected, ptbp1 MO and esrp1 MO prevented the accumulation of the Ptbp1 and Esrp1 proteins, respectively. Importantly, the esrp1 MO strongly reduced the Ptbp1 protein level (Fig. 2B, lane 3). We checked to see if the esrp1 MO was acting by directly impairing ptbp1 translation. To do so, we injected an mRNA encoding a V5-tagged version of Ptbp1 alone or in combination with the esrp1 MO. The morpholino reduced the abundance of endogenous Ptbp1, as was already observed, but not that of the V5-tagged protein (Fig. 2C). Hence, the esrp1 MO cannot directly target ptbp1 mRNA. More generally, in BLAST analyses we found a potential target of the esrp1 MO in the Xenopus genome only in esrp1 mRNA, suggesting that the MO is specific. We carried out a rescue experiment to confirm these findings. While the esrp1 MO reduced the amount of the Ptbp1 protein, as previously observed (Fig. 2D, compare lanes 1 and 2), the coinjection of an esrp1 mRNA immune to the MO restored a normal level of Ptbp1 (lane 3). Altogether, these data show that Esrp1 is required to achieve high levels of endogenous Ptbp1.
FIG 2.
The knockdown of esrp1 reduces the abundance of the Ptbp1 protein. (A) An mRNA encoding a V5-tagged version of Esrp1 with the wild-type (WT) 5′ region (WT-esrp1-V5) was injected with either a control (ctrl) or the esrp1 MO in two-cell embryos. The embryos were allowed to develop until stage 15 (neurula) before protein extraction and Western blot analysis using anti-V5 and anti-Pcna (loading control) antibodies. (B) The indicated morpholinos were injected in both blastomeres of two-cell embryos. The embryos were allowed to develop until stages 28 to 30 (tail bud) before protein extraction and Western blot analysis. (C) An mRNA encoding a V5-tagged version of Ptbp1 with the wild-type 5′ region (WT-ptbp1-V5) was injected alone or in combination with the esrp1 MO in both blastomeres of two-cell embryos. The embryos were allowed to develop until stages 28 to 30 before protein extraction for Western blot analysis using anti-Ptbp1, anti-V5, and anti-Pcna (loading control) antibodies. Endo., endogenous. (D) esrp1 MO and/or an mRNA encoding a V5-tagged version of Esrp1 immune to the esrp1 MO (esrp1-V5 mRNA) was injected in both blastomeres of two-cell embryos. The embryos were allowed to develop until stage 15 before protein extraction and Western blot analysis. (E) The indicated morpholinos were injected in both blastomeres of two-cell embryos. The embryos were allowed to develop until stage 35 (tadpole), and the presence of blisters was recorded. (Left) Representative embryos (arrows point to the blisters); (right) quantification of the blister phenotype. (F) An mRNA encoding a V5-tagged version of Ptbp1 (which is immune to the ptbp1 MO) and/or the indicated morpholinos were injected in both blastomeres of two-cell embryos. The embryos were allowed to develop until stages 28 to 30. The total RNAs were extracted and quantified by semiquantitative RT-PCR with a forward primer hybridizing to the constitutive exon 8 and a reverse primer hybridizing to the nonmuscular exon 9D of tpm1a mRNA, to amplify the nonmuscular (O5) isoform (Fig. 1B). Primers specific for eef1a1 mRNAs were also used. (Left) Results of a representative experiment; (right) quantification from 3 independent experiments (mean ± SD). The tpm1a-O5/eef1a1 ratios were normalized by the ratio in the noninjected embryos in each experiment to correct for the different specific activities of the radiolabeled primers. (G) Quantification by real-time PCR of ptbp2 mRNA abundance relative to eef1a1 mRNA abundance in embryos injected with the same molecules used for the assay whose results are presented in panel F (mean ± SD from 3 independent experiments).
The amount of Ptpb1 is likely to influence its activity as a posttranscriptional regulator. The strong downregulation of ptbp1 in esrp1 morphants may therefore have consequences similar to those of the knockdown of ptbp1. We tested this prediction by comparing the phenotypes of esrp1 and ptbp1 morphants and by comparing the fates of selected RNAs in esrp1 and ptbp1 morphants. As previously observed (29), ptbp1 morphants display blister-like structures in the dorsal fin (Fig. 2E). About 80% of the embryos injected with the ptbp1 MO displayed this phenotype, demonstrating a high penetrance (Fig. 2E). The embryos injected with the esrp1 MO massively died at the tail bud stages, which was not the case for embryos injected with the ptbp1 MO. However, all the embryos that survived up to stage 35 displayed blister-like structures with variable severities (Fig. 2E). This suggests that unknown RNAs whose misregulation in ptbp1 morphants causes the appearance of blisters are similarly misregulated in esrp1 morphants. To confirm that the set of genes differentially expressed in ptbp1 morphants overlaps the set of genes differentially expressed in esrp1 morphants, we examined selected RNAs in esrp1 morphants. tpm1a pre-mRNA can mature to a muscle-specific isoform (α7) or a nonmuscle isoform (O5) (Fig. 1B), and Ptbp1 favors the nonmuscle isoform (29, 35). Accordingly, ptbp1 knockdown reduced the abundance of the O5 isoform down to 17% of the initial value (95% confidence interval, 6 to 27%) (compare lanes 2 and 6 in Fig. 2F), and a high level of O5 was restored by the coinjection of a ptbp1 mRNA (compare lanes 2 and 4 in Fig. 2F) (P = 0.04, Student's t test). Importantly, esrp1 knockdown also reduced the amount of the O5 isoform down to 31% of the initial value (95% confidence interval, 22 to 40%) (compare lanes 1 and 6 in Fig. 2F), and the level of the O5 isoform rose when ptbp1 mRNA was coinjected (compare lanes 1 and 3 in Fig. 2F) (P = 0.007, Student's t test). This shows that the decrease of O5 in esrp1 morphants is due to the downregulation of ptbp1. In human, the PTBP1 and PTBP2 paralogue genes encode two related RNA-binding proteins, and they negatively cross-regulate. Hence, the downregulation of PTBP1 upregulates PTBP2 (36). It is also the case in Xenopus, since ptbp2 mRNA was upregulated 5.5-fold in ptbp1 morphants (95% confidence interval, 4.6- to 6.4-fold) (Fig. 2G, lanes 2 and 6). A low ptbp2 mRNA level was restored by the coinjection of a ptbp1 mRNA (compare lanes 2 and 4 in Fig. 2G) (P = 0.006, Student's t test). ptbp2 was upregulated 2.6-fold in esrp1 morphants (95% confidence interval, 1.7- to 3.4-fold) (Fig. 2G, lane 1). esrp1 MO was less efficient than ptbp1 MO at stimulating ptbp2 expression (compare lanes 1 and 2 in Fig. 2G), probably because the esrp1 MO does not act in organs like brain, where ptbp1 and ptbp2 (24), but not esrp1, are expressed. Nevertheless, the abundance of ptbp2 mRNA was reduced in esrp1 morphants by the coinjection of ptbp1 mRNA (compare lanes 1 and 3 in Fig. 2G) (P = 0.01, Student's t test). Hence, inhibition of esrp1 has the same effects as inhibition of ptbp1 on the splicing pattern of tpm1a and on the abundance of ptbp2 mRNA, and these effects are due to ptbp1 repression. Together, these data suggest that the depletion of Esrp1 profoundly affects the genetic program controlled by Ptbp1 in epidermal cells.
Esrp1 upregulates the expression of ptbp1 posttranscriptionally.
Esrp1 is responsible for the high abundance of the Ptbp1 protein. RT-quantitative PCR (qPCR) experiments run with several pairs of primers (Fig. 3A) demonstrated that the abundance of ptbp1 mRNA was significantly lower in esrp1 morphants (Fig. 3B). Hence, the reduced amount of Ptbp1 protein in esrp1 morphants relies on a decreased level of ptbp1 mRNA. We determined the abundance of ptbp1 pre-mRNA by RT-qPCR to test if this downregulation was associated with changes in transcription. This experiment, which we carried out with two different pairs of primers measuring exon-intron junctions, failed to consistently reveal changes in ptbp1 pre-mRNA abundance (Fig. 3C). Accordingly, the amounts of ptbp1 pre-mRNA were very similar in esrp1 morphants and in control embryos. We conclude that knockdown of Esrp1 does not affect ptpb1 transcription. Thus, the Esrp1 protein upregulates the expression of ptbp1 posttranscriptionally.
FIG 3.
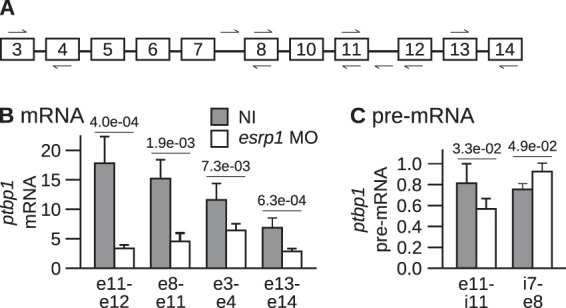
The knockdown of esrp1 reduces the abundance of ptbp1 mRNA but not that of ptbp1 pre-mRNA. (A) Positions of the primers in ptbp1 RNA used for real-time PCR. (B) Both blastomeres of two-cell embryos were injected with the esrp1 MO or left noninjected (NI). The development of the embryos was arrested at stages 28 to 30 for RNA extraction. The abundance of ptbp1 mRNA relative to that of eef1a1 mRNA was determined by real-time RT-PCR (mean ± SD from 5 independent experiments). Primers hybridizing to the indicated exons (identified by the prefix e on the x axis) were used. The P values of Student's t tests are given above the bars. (C) Same as panel B, except that pairs of primers directed against the indicated intron (i)-exon (e) junctions were used to amplify ptbp1 pre-mRNA. No amplification occurred in control experiments performed without reverse transcriptase.
The self-regulatory feedback loop of ptbp1 expression is active in epidermis despite Esrp1.
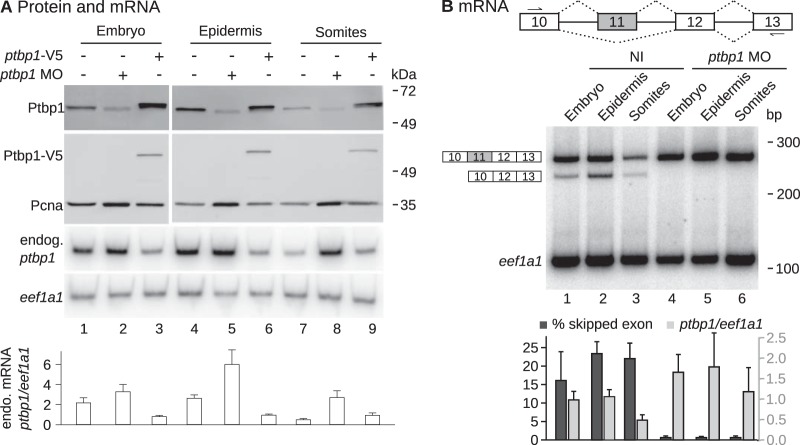
In human cells, a negative-feedback loop of PTBP1 expression operates posttranscriptionally (12). In Xenopus embryos, the Esrp1 protein upregulates the expression of ptbp1 posttranscriptionally (Fig. 3). Therefore, we hypothesized that Esrp1 could stimulate the expression of ptbp1 in Xenopus epidermis by inhibiting the negative-feedback loop. A testable prediction of this hypothesis is that Ptbp1 self-regulates in Xenopus embryos, except in epidermis. We tested this prediction by manipulating Ptbp1 levels. As already observed (Fig. 2B), the ptbp1 MO drastically reduced the abundance of the Ptbp1 protein in total embryos (Fig. 4A, top, lanes 1 and 2). This was also the case in dissected epidermis (lanes 4 and 5) as well as in somites (lanes 7 and 8). In all of these samples, the ptbp1 MO yielded a high level of ptbp1 mRNA (Fig. 4A, bottom), suggesting that reducing the level of the Ptbp1 protein by impairing the translation of its mRNA raises the amount of ptbp1 mRNA. Conversely, injection of a recombinant ptbp1-V5 mRNA resulted in a high level of Ptbp1 protein in total embryos (Fig. 4A, top, lane 3), in dissected epidermis (lane 6), and in somites (lane 9). This significantly reduced the amount of endogenous ptbp1 mRNA in total embryos and in dissected epidermis (Fig. 4A, bottom). These results show that in Xenopus embryos as well as in dissected epidermis and somites, any variation in the level of the Ptbp1 protein leads to an opposite variation in the level of ptbp1 mRNA. Hence, the ptbp1 self-regulatory loop is active in all these embryonic regions, and Esrp1 upregulates the expression of ptbp1 in epidermis, in spite of the self-regulatory loop.
FIG 4.
Self-regulatory feedback loop of ptbp1 expression in Xenopus epidermis. The ptbp1 MO or an mRNA encoding a V5-tagged version of Ptbp1 protein (ptbp1-V5 mRNA) was injected in both blastomeres of two-cell embryos. The embryos were allowed to develop until stages 28 to 30. The epidermis and the somites were dissected, or the embryos were left intact before protein and RNA extractions. (A) From top to bottom, Western blots using antibodies against Ptbp1, the V5 tag, and Pcna (loading control); results of one representative semiquantitative RT-PCR experiment using primers targeting eef1a1 and ptbp1 mRNAs, where the reverse primer targets the 3′ untranslated region of endogenous (endog.) ptbp1 mRNA (exon 15) and does not amplify the injected recombinant mRNA; and quantification of the ptbp1/eef1a1 mRNA ratios (mean ± SD from 3 independent experiments). (B) (Top) Schematic drawing of ptbp1 pre-mRNA. Exon 11 is either spliced or skipped. The arrows reveal the positions of the PCR primers (forward primer in exon 10 and reverse primer in exon 13). (Middle) Results of a representative semiquantitative RT-PCR. (Bottom) The percentage of ptbp1 mRNA excluding exon 11 (dark gray, ptbp1 mRNA without exon 11/total ptbp1 mRNA) and the total ptbp1/eef1a1 mRNA ratios (light gray) from 3 independent experiments (mean ± SD).
In human cells, PTBP1 self-regulates by activating the skipping of exon 11 of its own pre-mRNA. This yields an isoform that contains a premature termination codon, which is subsequently targeted to rapid degradation by the nonsense-mediated decay (NMD) process and is not translated to the PTBP1 protein (12). We asked whether Ptbp1 similarly modulated the inclusion of ptbp1 exon 11 in Xenopus epidermis. We carried out RT-PCR assays using primers flanking exon 11 to simultaneously amplify cDNA species containing and devoid of exon 11 (Fig. 4B). The identities of the PCR products indicated on the left of the gel in Fig. 4B were confirmed by cloning and sequencing. We calculated the percentage of skipping of exon 11 as the ratio of the amount of PCR product excluding the exon to the sum of the amounts of PCR products. A small amount of ptbp1 mRNA lacking exon 11 could be detected in control embryos (16%) (Fig. 4B, middle, lane 1). In ptbp1 morphants, the skipping of exon 11 was reduced to an almost undetectable level (lane 4). Furthermore, the total amount of ptbp1 mRNA was high. A likely explanation for this observation is that the isoform devoid of exon 11 is subject to NMD and is less stable than the isoform containing it. We could not measure the inclusion of exon 11 in embryos overexpressing ptbp1 because the amplimer obtained from the injected mRNA was indistinguishable from that obtained from the endogenous ptbp1 mRNA. Nevertheless, these data show that Ptbp1 favors the skipping of exon 11 of its own pre-mRNA in Xenopus embryos. Importantly, the same was true in somites and epidermis dissected from ptbp1 morphants (Fig. 4B, middle, lanes 5 and 6). Hence, in Xenopus, the self-regulatory feedback loop of ptbp1 is exerted by stimulating the skipping of exon 11 from its own pre-mRNA. This feedback loop occurs in epidermis, like in somites or whole embryos, despite Esrp1.
Esrp1 directly stimulates the inclusion of exon 11 in ptbp1 mRNA.
Esrp1 upregulates the expression of ptbp1 posttranscriptionally (Fig. 3). Moreover, the inclusion of exon 11 during PTBP1 pre-mRNA maturation controls the level of mature PTBP1 mRNA in human cells (12), like in Xenopus epidermis (Fig. 4). Therefore, we hypothesized that Esrp1 could modulate the inclusion of ptbp1 exon 11 in Xenopus epidermis. Compared with control embryos, the esrp1 MO dramatically increased the percentage of skipped exon 11 and decreased the amount of total ptbp1 mRNA (Fig. 5A; compare lanes 1 and 4) (P = 0.007 for the percentage of skipped exon and P = 0.02 for the RNA amount, Student's t tests). This suggests that Esrsp1 favors the accumulation of the presumably stable ptbp1 mRNA containing exon 11.
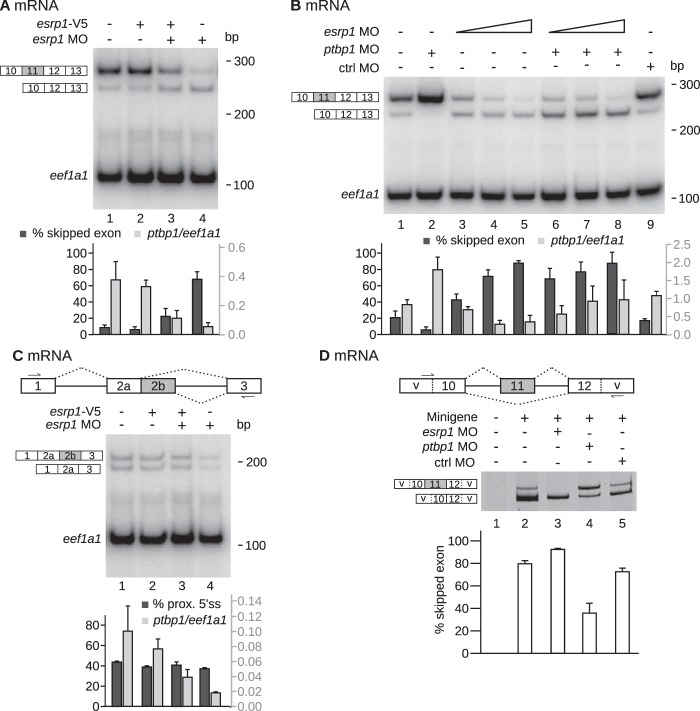
FIG 5.
Control of ptbp1 mRNA splicing pattern by Esrp1. (A and B) The indicated molecules were injected in both blastomeres of two-cell embryos. The embryos were allowed to develop until stage 15 (A) or stages 28 to 30 (B) before RNA extraction. The splicing pattern of ptbp1 mRNA was analyzed as described in the legend to Fig. 4B. (Top) Results of one representative experiment; (bottom) quantifications (mean ± SD from 3 independent experiments). (A) Injection of esrp1 MO and/or a recombinant esrp1-V5 RNA immune to the esrp1 MO. (B) Injection of morpholinos (60 ng control MO, 20 ng ptbp1 MO, 10, 20, or 40 ng esrp1 MO). (C) (Top) Schematic drawing of ptbp1 pre-mRNA. Usage of the proximal (prox.) 5′ splice site (ss) in intron 2 produces an mRNA with a short exon 2 (exon 2a). The arrows in exons 1 and 3 reveal the positions of the PCR primers. (Middle and bottom) Results of one representative experiment (middle) and quantifications (bottom; mean ± SD from 3 independent experiments), as in panel A. (D) (Top) Structure of the minigene, which encompasses the keratin promoter and the region of the ptbp1 gene between exons 10 and 12. The splicing pattern of the RNA transcribed from the minigene is assayed using primers flanking exon 11 (arrows). This assay is insensitive to the endogenous ptbp1 mRNA because both primers target vector (v) sequences and are therefore specific to the minigene. (Bottom) The minigene was injected with the indicated MO in one blastomere of two-cell embryos. The embryos were arrested at stages 28 to 30, before RNA extraction and analyses of splicing pattern. (Bottom) Quantification from 3 independent experiments (mean ± SD).
Several lines of argument indicate that this effect is specific. First, the coinjection of an immune esrp1 mRNA (esrp1-V5) together with the esrp1 MO restored a high level of ptbp1 mRNA including exon 11 (Fig. 5A, lane 3). Second, this effect was dose dependent, since increasing amounts of esrp1 MO resulted in growing percentages of ptbp1 mRNA devoid of exon 11 (Fig. 5B, lanes 1, 3 to 5, and 9). Third, Ptbp1 stimulates exon 11 skipping (Fig. 4 and 5B, lane 2), but the ptbp1 MO should have no effect in embryos injected with the esrp1 MO, which are already depleted in Ptbp1 (Fig. 2). Accordingly, the esrp1 MO promoted the accumulation of the ptbp1 isoform devoid of exon 11 similarly in the presence or the absence of the ptbp1 MO (Fig. 5B, compare lanes 6 to 8 and 3 to 5). The only difference is that the ptbp1 mRNA levels were increased by the ptbp1 MO in esrp1 morphants, possibly because the translational inhibition conferred by the ptbp1 MO allowed the isoform devoid of exon 11 to partly escape from NMD. Fourth, ptbp1 intron 2 contains two alternative 5′ splice sites whose usage results in a mature mRNA with either a short (2a) or a long (2a and 2b) exon 2. RT-PCR assays carried out with primers flanking exon 2 revealed that esrp1 MO had no effect on the ratio between these two isoforms (Fig. 5C). Hence, Esrp1 specifically stimulates the presence of exon 11 in ptbp1 mRNA.
We used a minigene approach to test the idea that Esrp1 acts during ptbp1 pre-mRNA maturation to stimulate exon 11 inclusion. The minigene consists of the keratin promoter, which is specifically active in the epidermis (28), and the region of the Xenopus ptbp1 gene between exons 10 and 12. Unlike the endogenous mRNA, the RNA obtained from the reporter minigene is NMD insensitive because it contains no open reading frame. Consequently, the relative proportions of the isoforms with and without exon 11 directly reflect the inclusion of that exon during pre-mRNA maturation. In embryos injected with the minigene only, the most abundant isoform was that devoid of exon 11 (Fig. 5D, lane 2). The same was true for embryos coinjected with a control MO (Fig. 5D, lane 5). We could not readily compare the amounts of RNA obtained from the minigene in the different situations because, generally, the transcriptional efficiency of injected minigenes is highly variable between embryos due to the random rate of mosaicism. Nevertheless, these data suggest that in the epidermis, exon 11 is a weak exon that is predominantly skipped during ptbp1 pre-mRNA maturation. As was observed for the endogenous RNA, Ptbp1 contributes to exon 11 skipping (Fig. 5D, lane 4). However, in embryos injected with the esrp1 MO and depleted in Esrp1 and, consequently, in Ptbp1 (Fig. 2B), exon 11 was almost completely skipped (Fig. 5D, lane 3). This shows that Esrp1 is required to splice in exon 11 in epidermis. Together, these data reveal a three-pronged mechanism to control exon 11 inclusion in epidermis: this exon is intrinsically very weak, but Esrp1 stimulates its inclusion, while Ptbp1 counteracts Esrp1 to a certain extent.
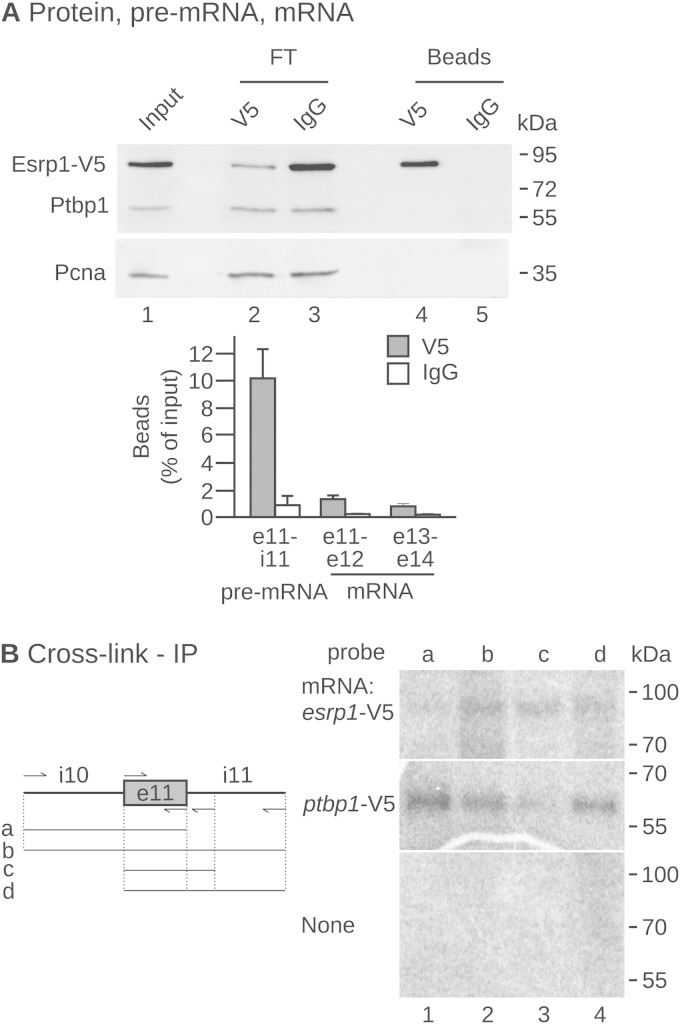
Esrp1 stimulates the inclusion of ptbp1 mRNA exon 11. This effect can be direct, if Esrp1 interacts with ptbp1 pre-mRNA. We tested this hypothesis by immunoprecipitation experiments in embryos injected with an mRNA expressing a V5-tagged version of the Esrp1 protein (Fig. 6A). The Esrp1-V5 protein was efficiently immunoprecipitated with anti-V5 but not with control antibodies (Fig. 6A, top, lanes 4 and 5). Interestingly, we failed to detect Ptbp1 in the immunoprecipitated fraction (lane 4), suggesting that the Ptbp1 and Esrp1 proteins do not interact. We next quantified ptbp1 pre-mRNAs in the Esrp1-V5 immunoprecipitate (Fig. 6A, bottom). A significant proportion of the ptbp1 pre-mRNA was detected in the sample immunoprecipitated with anti-V5 antibodies but not in the sample immunoprecipitated with control immunoglobulins. A much smaller amount of ptbp1 mRNA coimmunoprecipitated with Esrp1-V5. Hence, Esrp1 is able to bind to ptbp1 pre-mRNA. We carried out UV cross-linking experiments to identify the region of ptbp1 pre-mRNA that interacts with Esrp1. We used probes containing different regions flanking exon 11 (Fig. 6B, left) and protein extracts from embryos injected with an mRNA expressing a V5-tagged version of the Esrp1 protein. After immunoprecipitation of the cross-linked product with a V5 antibody, a signal migrating at the expected mobility for Esrp1-V5 was detected using extracts from injected embryos (Fig. 6B, top right) but not using extracts from control embryos (Fig. 6B, bottom right). The signal obtained with the full-length probe (Fig. 6B, right, lane 2) was lost when intron 11 was deleted from the probe (lane 1) but not when other regions were deleted (lanes 3 and 4). This identifies ptbp1 intron 11 to be the region binding to Esrp1. This is consistent with a previous demonstration that human ESRP proteins bound downstream of an alternative exon generally stimulate its inclusion (37). For comparison, we also carried out the same experiment with protein extracts from embryos injected with ptbp1-V5 mRNA (Fig. 6B, middle right). A signal was detectable with the full-length probe (Fig. 6B, lane 2), when intron 10 was deleted (lane 4), and when intron 11 was deleted (lane 1) but not when both introns were missing (lane 3). This indicates that Ptbp1 interacts with both ptbp1 intron 10 and ptbp1 intron 11.
FIG 6.
Esrp1 binds to ptbp1 pre-mRNA. (A) An mRNA encoding a V5-tagged Esrp1 protein was injected in both blastomeres of two-cell embryos that were allowed to develop until stage 15. The V5-tagged protein was immunoprecipitated using anti-V5 antibodies or IgGs for mock immunoprecipitations. (Top) The input, the flowthrough (FT), and the immunoprecipitated fractions analyzed by Western blotting using antibodies against V5, Ptbp1, and Pcna. Equivalent amounts of the input, flowthrough, and eluates were loaded. (Bottom) Measurement of the amounts of ptbp1 pre-mRNA and mRNA in the immunoprecipitated fractions relative to the input amount using primers for the indicated intron (i) and exon (e) pairs. These immunoprecipitated fraction-to-input ratios (mean ± SD) result from 3 independent anti-V5 and mock immunoprecipitations. (B) (Left) Matrices were obtained by PCR amplification using combinations of forward and reverse primers, indicated by arrows. They were used for in vitro transcription to obtain 4 radiolabeled RNAs encompassing different fragments of intron 10, exon 11, and intron 11 of ptbp1 pre-mRNA. IP, immunoprecipitation. (Right) The in vitro transcripts were incubated in protein extracts made from embryos previously injected with esrp1-V5 mRNA (top) or ptbp1-V5 mRNA (middle) or left uninjected (bottom). After UV cross-linking and RNase treatment, the V5-tagged proteins were immunoprecipitated, electrophoresed, and revealed by autoradiography.
DISCUSSION
Previous results in human cultured cells demonstrated that PTBP1 is able to control the maturation of its own pre-mRNA. Notably, PTBP1 promotes exon 11 skipping. The resulting mRNA contains a premature termination codon and is targeted to rapid degradation by NMD (12). This negative-feedback loop of PTBP1 expression is believed to ensure a constant level of PTBP1 in diverse cell types. Our results show that this mechanism is conserved in Xenopus. Furthermore, we demonstrated that it occurs in whole embryos and in dissected embryonic epidermis and somites. We found that Esrp1 stimulates ptbp1 expression by promoting exon 11 inclusion, and, using a minigene strategy, we observed that exon 11 skipping is the predominant splicing pattern in the epidermis (Fig. 7). These findings raise two related questions: why is exon 11 intrinsically so weak, and how do Esrp1 and Ptbp1 modulate its inclusion in two opposite directions?
FIG 7.
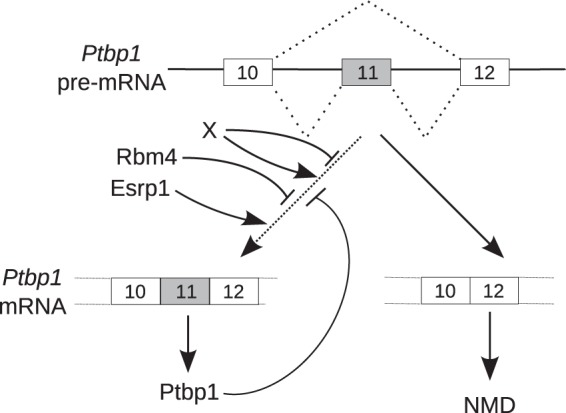
Model for the control of Ptbp1 abundance. The ptbp1 pre-mRNA matures into two isoforms differing by the presence of exon 11. The isoform devoid of exon 11 (right) contains a premature stop codon and is targeted to rapid degradation by NMD. The isoform containing exon 11 (left) is translated into the Ptbp1 protein. Exon 11 is spliced with a low efficiency, and Esrp1 is required to achieve exon 11 inclusion and, thereby, Ptbp1 accumulation in the epidermis. Ptbp1 counteracts Esrp1 to favor exon 11 skipping, which provides the basis for the ptbp1 self-regulatory feedback loop. Rbm4 also reduces exon 11 inclusion in muscle, heart, and, potentially, other tissues. Additional unknown trans-acting factors X may stimulate exon 11 splicing or skipping.
First, other trans-acting factors, in addition to Ptbp1, might reduce the inclusion of exon 11. In mice, RBM4 activates the skipping of Ptbp1 exon 11 during the differentiation of C2C12 myoblasts and, hence, downregulates Ptbp1 (38). RBM4 is highly abundant in muscle and heart but is ubiquitously expressed (39). Rbm4 is therefore a candidate protein to repress exon 11 in Xenopus somites and epidermis, but other unknown factors X might also account for its weak inclusion (Fig. 7). In addition, the predicted branch point in human PTBP1 is located 351 nucleotides upstream of exon 11. This unusually long distance may allow multiple PTBP1 molecules to bind between the branch point and the 3′ splice site to repress exon 11 (12). This region is highly conserved in Xenopus. The poor splicing efficiency of exon 11 in Xenopus may therefore arise from a distant branch point, with binding sites for negative factors being inserted between the branch point and the 3′ splice site.
A simple hypothesis to account for the antagonism between Ptbp1 and Esrp1 is that these proteins compete for binding to ptbp1 pre-mRNA. We have observed that Xenopus Ptbp1 binds to both introns 10 and 11. An RNA splice map of PTBP1 in human cells revealed that the binding of this protein on both sides of an alternative exon generally inhibits the inclusion of the exon (40). This is consistent with previous suggestions that PTBP1 could sequester alternative exons from the spliceosome by RNA looping (41, 42). It is therefore highly probable that Xenopus Ptbp1 inhibits exon 11 inclusion through a cross talk between the two flanking introns. We have also observed that the Xenopus Esrp1 protein binds to intron 11 of ptbp1 pre-mRNA. Consequently, Esrp1 might compete with Ptbp1 for intron 11 binding, hence disrupting the sequestering loop formed through exon 11.
At least three RNA-binding proteins control ptbp1 pre-mRNA maturation and, therefore, the abundance of the Ptbp1 protein in different tissues (Fig. 7). In addition, during neuronal differentiation, PTBP1 is downregulated at least in part by microRNA miR-124 (43). At least two reasons may explain this apparent requirement for such a tight control of PTBP1 protein levels. On the one hand, overexpressed PTBP1 globally reduces gene expression by modulating the efficiency of 3′ end processing (44). On the other hand, several reports point to a role of PTBP1 levels in the acquisition and maintenance of cellular identity. In HeLa cells, the exons found to be activated after knockdown of PTBP1 are enriched in brain-specific exons (45), suggesting that PTBP1 repression could be an important determinant of the splicing switch that operates during neuronal differentiation. Indeed, the repression of PTBP1 is sufficient to induce the transdifferentiation of fibroblasts to neurons (22). Similarly, caspases downregulate PTBP1 during heart differentiation, and this regulation is correlated with a modification of splicing patterns (38, 46). Hence, the PTBP1 protein is found in virtually all cell types, but a diversity of posttranscriptional controls exquisitely regulates its abundance to avoid detrimental effects.
The ESRP proteins are major splicing factors. The knockdown of ESRP1 or ESRP2 combined with splicing microarray hybridization or deep RNA sequencing revealed that hundreds of epithelium-specific splicing patterns depend on ESRP proteins (37, 47). The epithelium-specific splicing patterns are lost during the epithelial-mesenchymal transition (EMT), when ESRP genes are repressed (37, 48, 49). ESRP proteins play a role in modulating the EMT, with significant consequences for cancer progression (50–52). ESRP1 directly controls the epithelium-specific splicing pattern of EXOC7 (EXO70) mRNA (50). In addition, we observed that ptbp1 and esrp1 morphants display similar phenotypes with blister-like structures, suggesting overlapping sets of deregulated genes and that Esrp1 controls tpm1a and ptbp2 mRNAs through Ptbp1. This reveals that PTBP1 may mediate part of the posttranscriptional regulations exerted by ESRP1 in epithelia. Conversely, the regulations mediated by ESRP1 and PTBP1 may have antagonistic consequences, as shown for FGFR2 mRNA. Exon IIIb is repressed in mesenchymes, owing to PTBP1 binding to flanking intronic splice silencers (53, 54), but is activated in epithelia by ESRP (25). Together, these data show that ESRP proteins control epithelium-specific splicing through several different routes: directly by pre-mRNA binding, which in some instances antagonizes PTBP1, or indirectly by raising the PTBP1 amount.
ACKNOWLEDGMENTS
We are grateful to Russ Carstens (University of Pennsylvania School of Medicine) for the kind gift of the anti-ESRP1 antibodies and to Lawrence Chasin (Columbia University) for critical reading of the manuscript and helpful suggestions.
V.L. is a staff member of the Institut National de la Santé et de la Recherche Médicale.
REFERENCES
- 1.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. 2008. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 2.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. 2008. Alternative isoform regulation in human tissue transcriptomes. Nature 456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manley JL, Tacke R. 1996. SR proteins and splicing control. Genes Dev 10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 4.Jensen KB, Dredge BK, Stefani G, Zhong R, Buckanovich RJ, Okano HJ, Yang YY, Darnell RB. 2000. Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron 25:359–371. doi: 10.1016/S0896-6273(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Valcarcel J. 2005. Building specificity with nonspecific RNA-binding proteins. Nat Struct Mol Biol 12:645–653. doi: 10.1038/nsmb961. [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo JM, Majos N, Bonnal S, Martinez C, Castelo R, Guigo R, Bilbao D, Valcarcel J. 2005. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell 19:475–484. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Yario TA, Steitz JA. 2004. A molecular link between SR protein dephosphorylation and mRNA export. Proc Natl Acad Sci U S A 101:9666–9670. doi: 10.1073/pnas.0403533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CJ, Tang Z, Lin RJ, Tucker PW. 2007. Phosphorylation by SR kinases regulates the binding of PTB-associated splicing factor (PSF) to the pre-mRNA polypyrimidine tract. FEBS Lett 581:223–232. doi: 10.1016/j.febslet.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacke R, Chen Y, Manley JL. 1997. Sequence-specific RNA binding by an SR protein requires RS domain phosphorylation: creation of an SRp40-specific splicing enhancer. Proc Natl Acad Sci U S A 94:1148–1153. doi: 10.1073/pnas.94.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dembowski JA, Grabowski PJ. 2009. The CUGBP2 splicing factor regulates an ensemble of branchpoints from perimeter binding sites with implications for autoregulation. PLoS Genet 5:e1000595. doi: 10.1371/journal.pgen.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Guiner C, Lejeune F, Galiana D, Kister L, Breathnach R, Stevenin J, Del Gatto-Konczak F. 2001. TIA-1 and TIAR activate splicing of alternative exons with weak 5′ splice sites followed by a U-rich stretch on their own pre-mRNAs. J Biol Chem 276:40638–40646. doi: 10.1074/jbc.M105642200. [DOI] [PubMed] [Google Scholar]
- 12.Wollerton MC, Gooding C, Wagner EJ, Garcia-Blanco MA, Smith CW. 2004. Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol Cell 13:91–100. doi: 10.1016/S1097-2765(03)00502-1. [DOI] [PubMed] [Google Scholar]
- 13.Rossbach O, Hung LH, Schreiner S, Grishina I, Heiner M, Hui J, Bindereif A. 2009. Auto- and cross-regulation of the hnRNP L proteins by alternative splicing. Mol Cell Biol 29:1442–1451. doi: 10.1128/MCB.01689-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jumaa H, Nielsen PJ. 1997. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J 16:5077–5085. doi: 10.1093/emboj/16.16.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoilov P, Daoud R, Nayler O, Stamm S. 2004. Human tra2-beta1 autoregulates its protein concentration by influencing alternative splicing of its pre-mRNA. Hum Mol Genet 13:509–524. doi: 10.1093/hmg/ddh051. [DOI] [PubMed] [Google Scholar]
- 16.Dai W, Zhang G, Makeyev EV. 2012. RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res 40:787–800. doi: 10.1093/nar/gkr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala YM, De Conti L, Avendano-Vazquez SE, Dhir A, Romano M, D'Ambrogio A, Tollervey J, Ule J, Baralle M, Buratti E, Baralle FE. 2011. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J 30:277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Melo Neto OP, Standart N, Martins de Sa C. 1995. Autoregulation of poly(A)-binding protein synthesis in vitro. Nucleic Acids Res 23:2198–2205. doi: 10.1093/nar/23.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S, Zhang Z, Sinha R, Karni R, Krainer AR. 2010. SF2/ASF autoregulation involves multiple layers of post-transcriptional and translational control. Nat Struct Mol Biol 17:306–312. doi: 10.1038/nsmb.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap K, Lim ZQ, Khandelia P, Friedman B, Makeyev EV. 2012. Coordinated regulation of neuronal mRNA steady-state levels through developmentally controlled intron retention. Genes Dev 26:1209–1223. doi: 10.1101/gad.188037.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng S, Gray EE, Chawla G, Porse BT, O'Dell TJ, Black DL. 2012. PSD-95 is post-transcriptionally repressed during early neural development by PTBP1 and PTBP2. Nat Neurosci 15:381–388. doi: 10.1038/nn.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, Jiang L, Cai Z, Sun H, Zhang K, Zhang Y, Chen J, Fu XD. 2013. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 152:82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid R, Grellscheid SN, Ehrmann I, Dalgliesh C, Danilenko M, Paronetto MP, Pedrotti S, Grellscheid D, Dixon RJ, Sette C, Eperon IC, Elliott DJ. 2013. The splicing landscape is globally reprogrammed during male meiosis. Nucleic Acids Res 41:10170–10184. doi: 10.1093/nar/gkt811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noiret M, Audic Y, Hardy S. 2012. Expression analysis of the polypyrimidine tract binding protein (PTBP1) and its paralogs PTBP2 and PTBP3 during Xenopus tropicalis embryogenesis. Int J Dev Biol 56:747–753. doi: 10.1387/ijdb.120017sh. [DOI] [PubMed] [Google Scholar]
- 25.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. 2009. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell 33:591–601. doi: 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Revil T, Jerome-Majewska LA. 2013. During embryogenesis, Esrp1 expression is restricted to a subset of epithelial cells and is associated with splicing of a number of developmentally important genes. Dev Dyn 242:281–290. doi: 10.1002/dvdy.23918. [DOI] [PubMed] [Google Scholar]
- 27.Heyd F, Lynch KW. 2009. Getting under the skin of alternative splicing: identification of epithelial-specific splicing factors. Mol Cell 33:674–676. doi: 10.1016/j.molcel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Hamon S, Le Sommer C, Mereau A, Allo MR, Hardy S. 2004. Polypyrimidine tract-binding protein is involved in vivo in repression of a composite internal/3′-terminal exon of the Xenopus alpha-tropomyosin pre-mRNA. J Biol Chem 279:22166–22175. doi: 10.1074/jbc.M313809200. [DOI] [PubMed] [Google Scholar]
- 29.Le Sommer C, Lesimple M, Mereau A, Menoret S, Allo MR, Hardy S. 2005. PTB regulates the processing of a 3′-terminal exon by repressing both splicing and polyadenylation. Mol Cell Biol 25:9595–9607. doi: 10.1128/MCB.25.21.9595-9607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duriez P, Lesimple M, Allo MR, Hardy S. 2000. Alternative splicing of Xenopus alphafast-tropomyosin pre-mRNA during development: identification of determining sequences. DNA Cell Biol 19:365–376. doi: 10.1089/10445490050043335. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwkoop S, Faber J. 1967. Normal table of Xenopus laevis. North-Holland Publishing Co, Amsterdam, Netherlands. [Google Scholar]
- 32.Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P. 2002. Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev Dyn 225:384–391. doi: 10.1002/dvdy.10174. [DOI] [PubMed] [Google Scholar]
- 33.Hardy S, Fiszman MY, Osborne HB, Thiebaud P. 1991. Characterization of muscle and non muscle Xenopus laevis tropomyosin mRNAs transcribed from the same gene. Eur J Biochem 202:431–440. doi: 10.1111/j.1432-1033.1991.tb16392.x. [DOI] [PubMed] [Google Scholar]
- 34.Hardy S, Hamon S, Cooper B, Mohun T, Thiebaud P. 1999. Two skeletal alpha-tropomyosin transcripts with distinct 3′UTR have different temporal and spatial patterns of expression in the striated muscle lineages of Xenopus laevis. Mech Dev 87:199–202. doi: 10.1016/S0925-4773(99)00148-3. [DOI] [PubMed] [Google Scholar]
- 35.Anquetil V, Le Sommer C, Mereau A, Hamon S, Lerivray H, Hardy S. 2009. Polypyrimidine tract binding protein prevents activity of an intronic regulatory element that promotes usage of a composite 3′-terminal exon. J Biol Chem 284:32370–32383. doi: 10.1074/jbc.M109.029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spellman R, Llorian M, Smith CW. 2007. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol Cell 27:420–434. doi: 10.1016/j.molcel.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, Guo W, Xing Y, Carstens RP. 2010. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J 29:3286–3300. doi: 10.1038/emboj.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin JC, Tarn WY. 2011. RBM4 down-regulates PTB and antagonizes its activity in muscle cell-specific alternative splicing. J Cell Biol 193:509–520. doi: 10.1083/jcb.201007131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin JC, Tarn WY. 2005. Exon selection in alpha-tropomyosin mRNA is regulated by the antagonistic action of RBM4 and PTB. Mol Cell Biol 25:10111–10121. doi: 10.1128/MCB.25.22.10111-10121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue Y, Zhou Y, Wu T, Zhu T, Ji X, Kwon YS, Zhang C, Yeo G, Black DL, Sun H, Fu XD, Zhang Y. 2009. Genome-wide analysis of PTB-RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Mol Cell 36:996–1006. doi: 10.1016/j.molcel.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou MY, Underwood JG, Nikolic J, Luu MH, Black DL. 2000. Multisite RNA binding and release of polypyrimidine tract binding protein during the regulation of c-src neural-specific splicing. Mol Cell 5:949–957. doi: 10.1016/S1097-2765(00)80260-9. [DOI] [PubMed] [Google Scholar]
- 42.Wagner EJ, Garcia-Blanco MA. 2001. Polypyrimidine tract binding protein antagonizes exon definition. Mol Cell Biol 21:3281–3288. doi: 10.1128/MCB.21.10.3281-3288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. 2007. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castelo-Branco P, Furger A, Wollerton M, Smith C, Moreira A, Proudfoot N. 2004. Polypyrimidine tract binding protein modulates efficiency of polyadenylation. Mol Cell Biol 24:4174–4183. doi: 10.1128/MCB.24.10.4174-4183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llorian M, Schwartz S, Clark TA, Hollander D, Tan LY, Spellman R, Gordon A, Schweitzer AC, de la Grange P, Ast G, Smith CW. 2010. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nat Struct Mol Biol 17:1114–1123. doi: 10.1038/nsmb.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye J, Llorian M, Cardona M, Rongvaux A, Moubarak RS, Comella JX, Bassel-Duby R, Flavell RA, Olson EN, Smith CW, Sanchis D. 2013. A pathway involving HDAC5, cFLIP and caspases regulates expression of the splicing regulator polypyrimidine tract binding protein in the heart. J Cell Sci 126:1682–1691. doi: 10.1242/jcs.121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dittmar KA, Jiang P, Park JW, Amirikian K, Wan J, Shen S, Xing Y, Carstens RP. 2012. Genome-wide determination of a broad ESRP-regulated posttranscriptional network by high-throughput sequencing. Mol Cell Biol 32:1468–1482. doi: 10.1128/MCB.06536-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horiguchi K, Sakamoto K, Koinuma D, Semba K, Inoue A, Inoue S, Fujii H, Yamaguchi A, Miyazawa K, Miyazono K, Saitoh M. 2012. TGF-beta drives epithelial-mesenchymal transition through deltaEF1-mediated downregulation of ESRP. Oncogene 31:3190–3201. doi: 10.1038/onc.2011.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB. 2011. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet 7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu H, Liu J, Liu S, Zeng J, Ding D, Carstens RP, Cong Y, Xu X, Guo W. 2013. Exo70 isoform switching upon epithelial-mesenchymal transition mediates cancer cell invasion. Dev Cell 27:560–573. doi: 10.1016/j.devcel.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H, Osawa T, Kanki Y, Minami T, Aburatani H, Ohmura M, Kubo A, Suematsu M, Takahashi K, Saya H, Nagano O. 2012. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun 3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- 52.Ueda J, Matsuda Y, Yamahatsu K, Uchida E, Naito Z, Korc M, Ishiwata T. 2014. Epithelial splicing regulatory protein 1 is a favorable prognostic factor in pancreatic cancer that attenuates pancreatic metastases. Oncogene 33:4485–4495. doi: 10.1038/onc.2013.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carstens RP, Wagner EJ, Garcia-Blanco MA. 2000. An intronic splicing silencer causes skipping of the IIIb exon of fibroblast growth factor receptor 2 through involvement of polypyrimidine tract binding protein. Mol Cell Biol 20:7388–7400. doi: 10.1128/MCB.20.19.7388-7400.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner EJ, Baraniak AP, Sessions OM, Mauger D, Moskowitz E, Garcia-Blanco MA. 2005. Characterization of the intronic splicing silencers flanking FGFR2 exon IIIb. J Biol Chem 280:14017–14027. doi: 10.1074/jbc.M414492200. [DOI] [PubMed] [Google Scholar]