Background
While in search of a tissue source of catalase that would lack the biliverdin that contaminated liver preparations, Kjell Agner discovered in tuberculous empyema a green enzyme that was structurally different from other proteins known at that time (Agner, 1941). Agner described many of the structural and enzymatic properties of the green enzyme, verdoperoxidase, in his doctoral thesis for medical school (Agner, 1941). Two years later, in 1943, Theorell and Ǻkeson purified a brown-green peroxidase from cow’s milk and demonstrated that it had spectral properties distinct from those of Agner’s leukocyte-derived verdoperoxidase (Theorell et al., 1943). Recognizing that despite both enzymes being green, they originated from different sources, Theorell and Ǻkeson proposed the names myeloperoxidase for Agner’s enzyme and lacto-peroxidase for the milk enzyme.
Whereas Agner’s subsequent studies detailed how myeloperoxidase (MPO) inactivated bacterial toxins (Agner, 1941; Agner, 1950; Agner, 1955), Schultz initiated seminal studies to define the biological relevance of MPO in a broader context. Noting that MPO represented >5% of the dry weight of human neutrophils (Schultz et al., 1962), Schultz purified the protein from neutrophils isolated from human blood and defined many of the structural and enzymatic features of MPO. Pioneering studies by Zgliczynski, Schultz and others elucidated much of the chemistry supported by MPO and speculated how it might participate in human host defense (Schultz et al., 1962; Zgliczynski et al., 1968; Zgliczynski et al., 1971; Harrison, et al., 1976; Zgliczynski et al., 1980). Seminal studies by Klebanoff and colleagues established the critical links between the presence of MPO in neutrophils, halogenation of ingested bacteria in phagosomes, and the microbicidal action of the MPO-H2O2-Cl system (Klebanoff, 1967; Klebanoff, 1968; Klebanoff, 1970a; Klebanoff, 1970b; Klebanoff et al., 1972; Klebanoff, 2005).
Based on a comprehensive analysis of the evolutionary relationships among sequences of peroxidase proteins in the plant and animal kingdoms, the Obinger laboratory has recently categorized MPO and other proteins previously called animal peroxidases as members of Chordata peroxidase subfamily (Zamocky et al., 2008). All members expressed in mammals, including MPO, EPO, LPO, and TPO, contain conserved motifs on both proximal and distal sides of the essential heme prosthetic group, a calcium-binding site, and at least two covalent bonds linking the heme group to the protein backbone. In addition to the conserved ester linkages in the heme pocket, MPO contains a third, a sulfonium linkage between the 2-vinyl group and methionine 409. The presence of the sulfonium linkage empowers MPO with unique spectral qualities as well as greater oxidizing potential, rendering MPO the only member of the protein superfamily able to oxidize Cl− to Cl+ and thereby generate HOCl at physiologic pH (Furtmuller et al., 2003; Arnhold et al., 2006; Zederbauer et al., 2007).
Collectively, all peroxidases support the same general chemistry; they catalyze one- and two-electron oxidations of susceptible substrates, both organic (AH2) and inorganic, such as halides (X−), to promote structural changes with physiological consequences:
| (reaction 1) |
| (reaction 2) |
Peroxidase-mediated post-translational modifications underlie a diverse set of biological phenomena, from thyroid hormone synthesis (Ruf et al., 2006.) to pathophysiologic lipoprotein modification (Undurti et al., 2009), to crosslinking of fertilized sea urchin eggs to block entry of sperm arriving too late (Foerder et al. 1977). In all situations, the principles remain the same, only the substrate and context differ. This review focuses attention on MPO-mediated events in neutrophil phagosomes, the highly specialized space where ingested microbes are confined, and in the context of their contribution to host defense against infection. Extensive and elegant studies have examined MPO-dependent biochemistry in other inflammatory diseases, most notably cardiovascular disease, and the interested reader will be richly rewarded by reading any of several recent and excellent reviews (Malle et al., 2003; Nicholls et al., 2009; Shao et al., 2012; Nussbaum et al., 2013).
Players
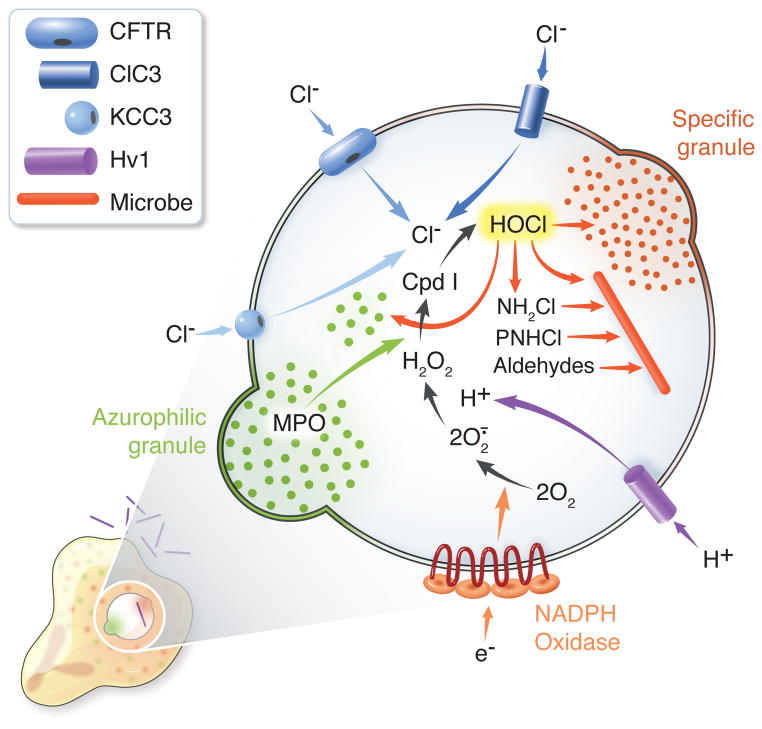
The essential elements of heme peroxidase-based biochemistry include the peroxidase, a source for H2O2, and substrates that can undergo one or two electron oxidation (Dunford, 1999). In the context of events within the phagosome of human neutrophils, the identities of the peroxidase and the H2O2 source, MPO and the phagocyte NADPH oxidase, respectively, are well established (Figure). In addition, the voltage-gated proton channel, Hv1, provides the charge compensation required to sustain NADPH oxidase activity (DeCoursey, 2013). Less well characterized is the source of chloride to support HOCl production. The MPO-H2O2-Cl system requires a continuous supply of Cl, as the small amounts internalized incidentally during phagocytosis are rapidly consumed as degranulation and NADPH oxidase activation occur (Winterbourn et al., 2006). The unusually high chloride concentration in neutrophil cytoplasm provides a reservoir to meet intraphagosomal needs, and several different transporters serve to deliver the needed chloride. Cystic fibrosis transmembrane conductance regulator (CFTR) transports the bulk of the chloride from cytoplasm into the phagosomal lumen (Aiken et al., 2012; Zhou et al, 2013). CFTR, a constituent of secretory vesicle, but not plasma, membrane in resting neutrophils, reaches the nascent phagosomes as granules fuse during phagocytosis, thereby providing the means to transfer chloride from the cytoplasmic reserves (Painter et al., 2006; Zhou et al., 2013). The steady state concentrations of chloride in phagosomes of patients with cystic fibrosis [25.5 ± 3.3 mM, n = 5] or normal neutrophils treated with the CFTR inhibitor GlyH-101 [27.7 ± 4.4 mM, n = 5] are significantly lower than that in normal neutrophil phagosomes [67.7 ± 7.3 mM, n = 5] (Painter et al., 2010). Although the measured values may be higher than the bona fide steady state concentration due to technical reasons1, optimal MPO activity in phagosomes requires chloride transport via CFTR; phagosomes containing genetically or pharmacologically dysfunctional CFTR exhibit reduced MPO-dependent modification of targets (vide infra) and killing of ingested Pseudomonas aeruginosa (Painter et al., 2008). In addition to CFTR, the anion transporter ClC3 (Moreland et al., 2006) and the K+/Cl− co-transporter KCC3 (Sun et al., 2012.) also contribute to chloride transport into neutrophil phagosomes.
Figure. MPO-dependent events in human neutrophil phagosomes.
Concomitant with phagocytosis, human neutrophils assemble and activate the NADPH oxidase and recruit granules to fuse with nascent phagosomes. The NADPH oxidase transfers electrons into the phagosome, generating superoxide anion (O2·−) and hydrogen peroxide (H2O2) from molecular oxygen. The voltage-gated proton channel Hv1 provides the bulk of charge compensation necessitated by electron transfer from cytoplasm, thereby allowing sustained generation of oxidants in close proximity to the ingested microbe. Chloride (Cl−) from the neutrophil cytoplasm enters phagosomes predominantly via cystic fibrosis conductance regulator (CFTR), although ClC3 and KCC3 support some Cl− influx. MPO, provided by azurophilic granules, and H2O2 react to yield Compound I (Cpd I), which catalyzes the two electron oxidation of Cl− to Cl+ to produce hypochlorous acid (HOCl) or bleach. HOCl reacts with peptides and proteins from host and microbe to generate an array of products, including monochloramines (NH2Cl), protein chloramines (PNHCl), which can then decompose to form aldehydes. Granule proteins, both native and oxidant-modified, HOCl and its derivatives attack microbial targets, with the outcome dependent both on the number and accessibility of vulnerable targets as well on the defensive responses specific to the ingested organism.
Products of the MPO-H2O2-Cl system
Compound I, the Fe(IV) cation radical produced by the reaction between H2O2 and native MPO (Marquez et al., 1994; Furtmüller et al., 1998), can support one-and two-electron oxidations of an array of substrates, including halides, the pseudohalide thiocyanate, amino acids, proteins, lipids, and nucleic acids [reviewed comprehensively in (Pattison et al., 2006; Summers et al., 2008)]. Even after restricting consideration to the chemistry plausible in the phagosome, the number of potential reactions exceeds facile comprehension. Rather than catalogue the multiple and diverse possibilities, I will summarize key reactions likely to occur in human neutrophils and contribute, directly or indirectly, to killing ingested organisms. Many of the details of reactions and their kinetics will be omitted here, instead directing the interested reader to recently published excellent reviews (Hurst, 2012; Klebanoff et al., 2013; Winterbourn et al., 2013).
For the sake of simplifying the presentation, consider HOCl as the most proximal effector product of the MPO-H2O2-Cl system. The product of the two-electron oxidation of Cl−, HOCl exists in equilibrium with OCl− at the near neutral pH in neutrophil phagosomes, as the pKa is 7.53 at 30°C. Under conditions that include substrates that would compete with Cl− for oxidation by Compound I, the number of potential reaction substrates multiplies. The reactions of HOCl with amino acids, peptides, and proteins have been extensively studied [review (Hawkins et al. 2003)] and generate products, including chloramines and eventually aldehydes (Zgliczynski et al., 1971) that can exert antimicrobial activity as well (Thomas et al., 1986), and can generate tyrosyl radicals that prompt protein dimerization and aggregation (Vissers et al., 1991). HOCl generated by MPO produces mono- and dichloramines of primary amines, including those on neutrophil granule proteins present within phagosomes (Thomas et al., 1982). Although these represent quantitatively minor reactions, they can be long-lived and, depending on the concentration and structural features, can contribute to antimicrobial action. Some products, such as the monochloramine NH2Cl generated by the reaction of HOCl and NH4+, can penetrate the hydrophobic surfaces of particular microbes and thereby gain access to vulnerable intracellular targets (Thomas, 1979). In the case of E.coli, MPO-dependent generation of long-lived chloramines and chloramide derivatives correlates with oxidation of critical bacterial sulfhydryl groups and loss of microbial viability (Thomas, 1979a; Thomas, 1979b). The magnitude of the contribution of chloramines and other HOCl-derived agents to antimicrobial action varies as a function of the size, charge, and polarity of the toxin produced, the chemical composition of the surface of the ingested microbe, and the specific vulnerable targets in the organism.
The abundant supply of MPO ideally equips neutrophils for service in innate immune defense against microbes, converting the H2O2 produced by the NADPH oxidase into the potent microbicide HOCl. H2O2 possesses greater redox potential than does HOCl (1.776V vs 1.482V) but exerts much less antimicrobial action (Klebanoff, 1978; Hurst et al., 1989; Elzanowska et al., 1995). For example, E.coli washed with EDTA to remove adventitious metal cations that might support Fenton chemistry can tolerate 50 mM H2O2 for more than 40 minutes without a loss in viability (Elzanowska et al., 1995). In part, this tolerance reflects the detoxifying action of peroxide-consuming enzymes present in aerobic organisms, as without such enzymes, even endogenously produced H2O2 can exert substantial DNA damage (Park et al., 2005). H2O2 oxidizes cysteine and methionine residues most readily but at relatively slow rates compared to the activity of HOCl. For example, the rate constant for thiol oxidation by H2O2 is 2.9 M−1s−1 (Imlay, 2003), whereas that for HOCl is ~ a million-fold faster, 3.0 × 107 M−1s−1 (Pattison et al., 2001). The rapid reaction kinetics of HOCl enhances its capacity to attack microbial targets that may be accessible only briefly. Supporting that prediction, HOCl, but not H2O2, prompts protein aggregation in vitro (Winter et al., 2008), presumably by irreversibly modifying vulnerable targets that are exposed only transiently during the continuous folding and unfolding of protein conformations.
Targets of the MPO-H2O2-Cl system
The specific target and the HOCl-mediated changes that culminate in microbial death have not been defined and vary among organisms (Hurst, 2012). All things being equal with respect to inherent susceptibility of microbial components to modification, spatial arrangements dictate that surface structures would be the first to encounter MPO-derived toxins. However, in many cases, HOCl attacks surface structures but with little consequence on bacterial viability. Instead, loss of function supported by proteins in bacterial inner membranes correlates with microbial death [reviewed in (Hurst, 2012)], as does HOCl-dependent oxidation of methionine residues in cytosolic and inner membrane proteins of E.coli (Rosen et al., 2009). Whatever the underlying chemistry, HOCl kills within milliseconds (Albrich et al., 1982) and more efficiently than does H2O2 in a variety of experimental settings. Based on the number of molecules of oxidant/organism needed to kill E.coli, HOCl is ~1000-fold more potent than is H2O2 (0.4–5.0 × 108 vs >3 × 1011)(Lymar et al., 1995). Similarly, the relative dose-dependent killing of K.pneumoniae demonstrates 50% reduction in viability at 60 min for 1 μM HOCl vs 1 mM H2O2 (Hirche et al., 2005). Like many experimental systems, both of these examples utilized buffer systems without protein and were thus free of competing substrates for the oxidants. As discussed later, teasing out “lethal hits” from the array of competing reactions with inadvertant targets that constitute complex biological systems, as in neutrophil phagosomes, represents a formidable challenge to constructing in vitro conditions that accurately mirror event in neutrophils and be used to assess how neutrophils kill.
Diversity among the microbes with respect to surface composition (e.g. presence or absence of capsule, composition of capsule if present, lipopolysaccharides in enteric Gram negative rods vs lipoteichoic acid in Gram positive bacteria) undermine the notion of the existence of a single microbial target. Furthermore, microbes sense attack by antimicrobial agents and respond to threats to their viability by evading toxins, enduring the modifications to structure or function, repairing host-induced damage, or all of the aforementioned actions. In some cases, the bacterial responses match predictions based on current understanding of events in phagosomes. The different transcriptional responses of E.coli ingested by neutrophils from normal individuals vs those with chronic granulomatous disease (CGD), an inherited deficiency in NADPH oxidase activity, illustrate the remarkable specificity and speed with which an organism can respond to its environment. For example, E.coli fed to normal neutrophils modulate expression of > 70 genes, including upregulation of genes such as OxyRS that respond to H2O2 (Staudinger et al., 2002). In contrast, the same E.coli fed to CGD neutrophils failed to upregulate H2O2-responsive genes, as anticipated given the absence of oxidants in CGD phagosomes. In other settings, bacterial responses affirm the complexity of biochemistry in normal neutrophils. MPO-mediated HOCl oxidizes methionine residues, and methionine sulfoxide (MeO) production correlates with loss of viability of E.coli (Rosen et al., 2009). Deletion of methionine sulfoxide reductase (msr) from E.coli eliminates the capacity to regenerate methionine from MeO and increases susceptibility to HOCl. Likewise, msr-deletion renders S.aureus more susceptible to oxidant and neutrophil killing (Pang et al., 2014.). These data jibe with expectations, given the recognized importance of oxidants in killing and the role of msr in the repair of oxidant damage. Whereas S.aureus ingested by normal neutrophils increase expression of msr, upregulation is far greater in neutrophils without NADPH oxidase activity. Furthermore, purified neutrophil granules alone, without added oxidants, promote msr upregulation in S.aureus. Collectively, these data demonstrate that ingested bacteria react promptly and selectively to neutrophil-derived toxins but often in ways not readily explained by current models of antimicrobial activity. However, the unexpected observation that S.aureus upregulates msr under conditions free of exogenous oxidants illustrates the complex interactions among phagosomal contents.
In situ
A significant fraction of the oxygen consumed by activated neutrophils can be recovered as HOCl, and a sensitive, specific, and readily performed assay can be used to quantitate extracellular HOCl production by stimulated neutrophils (Dypbukt et al., 2005). HOCl represents from 28 to 72% of the oxygen consumed by phorbol myristate acetate (PMA)-stimulated neutrophils (Weiss et al., 1982; Foote et al., 1983; Thomas et al., 1983; Chapman et al., 2002). The susceptibility of the fluorophores green fluorescent protein (GFP) to bleaching by chlorination of Y66 (Espey et al. 2002) provides a specific and selective tool to monitor HOCl generation with neutrophil phagosomes. Both E.coli (Palazzolo et al. 2005) and S.aureus (Schwartz et al., 2009) stably expressing GFP have served as probes for neutrophil HOCl production. In addition to HOCl being generated within phagosomes, the loss of viability directly correlates with MPO-dependent modification of ingested bacteria.
Compartmentalization of ingested microorganisms within phagosomes not only limits their access to vital nutrients but also creates conditions that enhance toxicity of antimicrobial agents present. From the perspective of chemistry, the close approximation of phagosomal membrane to the microbial surface creates a relatively small reaction zone for oxidants to attack soluble substrates in phagosomal fluid. Consequently, the tight quarters favor reactions between oxidants and microbe even in an environment rich in competing substrates (Lymar et al. 1995). Additionally, the very small volume of the phagosome, estimated to be 1.2 fl (Winterbourn et al., 2006), allows high concentrations of oxidants to be achieved, and the ability of MPO to adhere to some bacteria enhances the effective concentration of toxic agents at the site of attack.
Adopting a reductionist approach to examine in vitro the antimicrobial activity of MPO, most investigators utilize the MPO-H2O2-Cl system (Klebanoff, 1968) free of other neutrophil granule proteins. However, during phagocytosis, fusion of neutrophil granules delivers agents, including proteolytic enzymes and proteins with direct antimicrobial activity [reviewed in (Rorvig et al., 2013)], that contribute to the antimicrobial activity and can serve as substrate for HOCl. Accessibility and inherent susceptibility determine sites for attack by the MPO-H2O2-Cl system, rendering both exogenous substrates originating from ingested microbes and endogenous neutrophil-derived molecules released into phagosomes during degranulation equally eligible targets.
The complex mix of synergistic as well as antagonistic interactions dictates the fate and overall survival of bacteria in neutrophils (Nauseef, 2007; Hurst, 2012; Winterbourn et al., 2013). Neutrophil elastase (NE) enhances the killing of S.aureus as well as E.coli by MPO-H2O2-Cl by a mechanism that is independent of protease activity, as heating NE does not compromise its synergistic influence (Odeberg et al., 1976). NE augments cathepsin G-mediated killing as well, but cathepsin G does not synergize with the MPO-H2O2-Cl system. Adding to the complexity of so many interactions among effector molecules, MPO-mediated modifications can inactivate many of the granule proteins in neutrophils (Voetman et al., 1981). Although only some have been examined, many granule proteins undergo irreversible inactivation in the presence of HOCl or MPO-H2O2-Cl, including acid phosphatase, N-acetyl-β-glucosaminidase, β-glucuronidase, α-fucosidase, α-mannosidase, lysozyme, vitamin B12-binding protein, MMP-7, -8, and -9, cathepsin G, and NE (Kobayashi et al., 1982; Clark et al., 1985; Fu et al., 2001; Fu et al., 2003; Hawkins et al., 2005; Hirche et al., 2005). Whereas HOCl or the MPO-H2O2-Cl system inactivates NE, cathepsin G, and MMP-7, H2O2 alone does not (Fu et al., 2003; Hirche et al., 2005; Shao et al., 2005). Furthermore, HOCl at low concentrations inactivates NE without prompting changes in apparent size after SDS-PAGE, whereas at high concentrations of HOCl NE undergoes complete degradation (Hirche et al., 2005), suggesting that extensive oxidation of NE by HOCl may induce its autodegradation.
MPO can mediate the same inactivation of lysosomal enzymes in more complex biological settings, as in supernatants from stimulated neutrophils. Supernatants from stimulated neutrophils from patients with chronic granulomatous disease or MPO deficiency, cells lacking essential elements for MPO-mediated chemistry, contain more lysosomal enzyme activity than do secretions from normal neutrophils, and the activity of lysosomal enzymes released by normal neutrophils are increased under anaerobic conditions, in the presence of inhibitors of MPO or the NADPH oxidase, or by adding agents that scavenge oxidants (Kobayashi et al., 1982; Clark et al., 1985; Weiss et al., 1985; Fu et al., 2003; Hirche et al., 2005). Not directly relevant to host-pathogen interactions but pertinent to inflammation in general, MPO can oxidatively inactivate plasma protease inhibitors and thereby modulate extracellular tissue damage at inflammatory sites (Clark et al., 1979; Clark et al., 1980; Clark et al., 1981; Clark, 1983; Hawkins et al., 2005). As a result of egalitarian attack of the MPO-H2O2-Cl system on substrates whether from man or microbe, the granule proteins released into phagosomes can be chemically modified and functionally modulated, enhanced as well as reduced, depending on the particular agent and the specific modification. Recognition of such interactions and their consequences figures into the final analysis of overall events in the neutrophil phagosome.
Role for MPO in the antimicrobial activity of intact neutrophils
Neutrophils possess MPO and can generate HOCl, which can exert rapid microbicidal action on a wide variety of microorganisms, but do they rely on MPO to kill ingested microbes trapped in phagosomes?
Studied in vitro and compared to normal neutrophils, those deficient in MPO have defective killing of bacteria as well as fungi, with variation among organisms in the degree of the defect. For example, killing of S.aureus after 30 minutes by MPO-deficient neutrophils is ~30% of that by normal neutrophils but ~80% of normal when E.coli are fed to neutrophils (Klebanoff et al., 1972; Klebanoff et al., 2013). Furthermore, MPO-deficient neutrophils kill susceptible bacteria more slowly than do normal neutrophils, but in some cases achieve the same success in the end (Lehrer et al., 1969; Hampton et al., 1996; Decleva et al., 2006). In contrast to the slow but eventually effective killing of selected bacteria, MPO-deficient neutrophils lack the capacity to kill Candida albicans (Decleva et al., 2006), a defect that parallels the clinical picture of some individuals with MPO deficiency (vide infra). The variation in antimicrobial activity as a function of the specific organism is not unique to MPO deficiency but seen as well in chronic granulomatous disease (Holland, 2013). In fact, normal, CGD, and MPO-deficient neutrophils kill E.coli equally well (Rosen et al., 1997).
Experimental infection models using MPO knock-out mice have demonstrated increased susceptibility of deficient mice to challenges with a variety of organisms, including Klebsiella and Candida (Aratani et al., 1999; Aratani et al., 2000; Hirche et al., 2005; Aratani et al., 2006), and to the cecal ligation and puncture model (Gaut et al., 2001). Data from some of the animal studies highlight a relationship between organism load and the impact of MPO on outcome of infection. When the inoculum of infection with fungi is high, eradication of infection and survival require MPO. In contrast, oxidants generated by the NADPH oxidase suffice in responding effectively to challenges with low inocula (Aratani et al., 2002a; Aratani et al., 2002b). Although the validity of extrapolations from MPO knock-out mice to humans can be undermined by inherent differences between phagocytes from the two species with respect to NADPH oxidase activity, MPO content, and the repertoire of MPO-independent antimicrobial agents such as defensins (Rausch et al., 1975; Eisenhauer et al., 1992; Mestas et al., 2004; Rittirsch et al., 2007), the contributions of MPO to the inflammatory process per se, independent of direct antimicrobial action, also obfuscate interpretation of the results of experimental models. MPO knock-out animals express higher levels of induced nitric oxide synthase, generate more nitric oxide, and exhibit less lung injury in response to sepsis or endotoxin (Brovkovych et al., 2008; Haegens et al., 2009; Takeuchi et al., 2012), demonstrating that overall survival from infection reflects important factors in addition to the eradication of viable organisms.
As reviewed recently (Klebanoff et al., 2013), widespread application of flow cytometry in clinical laboratories upended appreciation of the prevalence and clinical phenotype of inherited MPO deficiency in humans. Only two patients with MPO deficiency had been reported (Grignaschi et al., 1963; Undritz, 1966) prior 1969 when Lehrer and Cline described a young diabetic male with MPO deficiency and disseminated candidiasis (Lehrer et al., 1969) and reports of MPO deficiency were infrequent until hematology laboratories adopted peroxidase staining and flow cytometry to identify and enumerate cells in peripheral blood. Subsequently, studies demonstrate that MPO deficiency occurs relatively commonly, affecting 1 in 2,000 to 4,000 healthy individuals in North America and Europe (Kitahara et al., 1981; Kutter et al., 1994; Kutter, 1998) and 1 in 57,000 in Japan (Nunoi et al., 2003). With the exception of systemic candidiasis in the presence of diabetes mellitus, few MPO-deficient patients with increased or unusual infections have been reported. Although the paucity of infectious complications in MPO-deficient subjects could be offered as evidence against the importance of MPO in host response to infection, that would be a superficial interpretation of the data, as MPO-deficient neutrophils are not equivalent to neutrophils that lack only MPO (Klebanoff, 1970. Klebanoff demonstrated that MPO-deficient neutrophils kill test microbes more efficiently than do normal neutrophils treated with azide (to inhibit MPO) and that killing by MPO-deficient neutrophils is not inhibited by azide treatment, together suggesting that myeloid cells in MPO-deficient subjects may adapt to the deficiency by enlisting antimicrobial systems that compensate. However, the absence of MPO has consequences for antimicrobial action that reflect the loss of HOCl-dependent modification of neutrophil granule proteins.
The many synergies among antimicrobial toxins acting in phagosomes sabotage reaching a straightforward answer to questions about the role of MPO in human host defense. The absence of MPO eliminates not only the capacity to deliver lethal damage to target microbes but also to modify the structure and activity of other antimicrobial elements present in phagosomes. Without MPO, granule proteins such as NE, cathepsin G, and MMP-7, agents otherwise inactivated by the MPO-H2O2-chloride system, will be functional and exert antimicrobial action not present in the phagosomes of normal neutrophils. Furthermore, the ambient H2O2 concentration in phagosomes lacking MPO will be higher than that in normal neutrophils for two reasons. MPO terminates activity of the NADPH oxidase (Jandl et al., 1978) and MPO-deficient neutrophils have prolonged oxidase activity and generate more H2O2 than do normal neutrophils (Klebanoff et al., 1971; Klebanoff et al., 1972; Rosen et al., 1976; Nauseef et al., 1983). In addition, the absence of MPO, which normally rapidly consumes H2O2 to generate HOCl, will further increase the concentration of H2O2 (Winterbourn et al., 2006). The altered oxidant composition and tone in MPO-deficient phagosomes modifies not only the available effector molecules but also substrates on and in microbes that constitute vulnerable targets. Microbial sites may be more or less susceptible to attack after modification by H2O2 vs HOCl. Taken together, these factors suggest that contrasting the prevalence of infection in vivo or antimicrobial action in vitro of normal and MPO-deficient neutrophils overlooks the downstream effects of MPO-H2O2-Cl on the collective antimicrobial activity of other oxidants and granule proteins in phagosomes and offers only limited insight into the contribution of MPO to normal neutrophil function.
Take Home
As noted nearly four decades ago, initial neutrophil-dependent killing of microbes depends on a system that can be inhibited by azide (Koch, 1974). The most rapid and complete antimicrobial action by human neutrophils against many organisms relies on H2O2 from the phagocyte oxidase and MPO from azurophilic granules. MPO supports production of lethal amounts of HOCl in human neutrophils, bacteria recovered from phagosomes have MPO-specific posttranslational modifications, MPO-mediated changes in bacteria correlate with loss of their viability, and MPO-deficient neutrophils kill many species of microorganisms more slowly than do normal cells. Collectively, these data suggest that efficient antimicrobial activity by neutrophils depends on the MPO-H2O2-Cl system.
The strikingly different clinical phenotypes of humans lacking the NADPH oxidase or MPO highlights the complexity of interactions within phagosomes that culminate in the death of ingested microbes. The dominant oxidant present in normal (HOCl), MPO-deficient (H2O2), and CGD (none) neutrophils react differently with both microbial targets as well as vulnerable sites on granule proteins, thereby altering the overall antimicrobial “tone” in phagosomes in the distinctly different settings.
Acknowledgments
Supported by grants AI070958 and AI044642 from the National Institutes of Health (WMN). The Nauseef lab is also supported by a Merit Review award and use of facilities at the Iowa City Department of Veterans Affairs (VA) Medical Center, Iowa City, IA, USA 52246.
Footnotes
Note that these measurements likely overestimate the true chloride concentration, because the intrinsic susceptibility of the fluorescent probe used to detect chloride requires inclusion of sodium azide in experimental conditions. Consequently, chloride consumption by MPO is inhibited under the experimental conditions.
Disclosures: The author has nothing to disclose.
References
- Agner K. Verdoperoxidase: a ferment isolated from leukocytes. Acta Physiol Scand. 1941;2:1–62. [Google Scholar]
- Agner K. Detoxicating effect of verdoperoxidase on toxins. Nature. 1947;159:271–272. doi: 10.1038/159271a0. [DOI] [PubMed] [Google Scholar]
- Agner K. Studies on peroxidative detoxification of purified diphtheria toxin. J Exp Med. 1950;92:337–347. doi: 10.1084/jem.92.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agner K. Peroxidative detoxification of diphtheria toxin studied by using I 131. Rec Trav Chim. 1955;74:373–376. [Google Scholar]
- Aiken ML, Painter RG, Zhou Y, Wang G. Chloride transport in functionally active phagosomes isolated from Human neutrophils. Free Radic Biol Med. 2012;53:2308–2317. doi: 10.1016/j.freeradbiomed.2012.10.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrich JM, Hurst JK. Oxidative inactivation of Escherichia coli by hypochlorous acid. Rates and differentiation of respiratory from other reaction sites. FEBS Lett. 1982;144:157–161. doi: 10.1016/0014-5793(82)80591-7. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Koyama H, Nyui SI, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Ishida-Okawara A, et al. Contribution of the myeloperoxidase-dependent oxidative system to host defense against Cryptococcus neoformans. J Med Microbiol. 2006;55:1291–1299. doi: 10.1099/jmm.0.46620-0. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J Infect Dis. 2002a;185:1833–1837. doi: 10.1086/340635. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, et al. Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J Infect Dis. 2000;182:1276–1279. doi: 10.1086/315843. [DOI] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa Y, Takano Y, Suzuki K, et al. Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus. Med Mycol. 2002b;40:557–563. doi: 10.1080/mmy.40.6.557.563. [DOI] [PubMed] [Google Scholar]
- Arnhold J, Monzani E, Furtmüller PG, Zederbauer M, Casella L, Obinger C. Kinetics and thermodynamics of halide and nitrite oxidation by mammalian heme peroxidases. Eur J Inorg Chem. 2006;19:3801–3811. [Google Scholar]
- Brovkovych V, Gao XP, Ong E, Brovkovych S, Brennan ML, Su X, et al. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L96–L103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ALP, Hampton MB, Senthilmohan R, Winterbourn CC, Kettle AJ. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J Biol Chem. 2002;277:9757–9762. doi: 10.1074/jbc.M106134200. [DOI] [PubMed] [Google Scholar]
- Clark RA. Extracellular effects of the myeloperoxidase-hydrogen peroxide-halide system. Advances in Inflammation Research. 1983;5:107–146. [Google Scholar]
- Clark RA, Borregaard N. Neutrophils autoinactivate secretory products by myeloperoxidase-catalyzed oxidation. Blood. 1985;65:375–381. [PubMed] [Google Scholar]
- Clark RA, Klebanoff SJ. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. An inflammatory control mechanism. J Clin Invest. 1979;64:913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Stone PJ, El Hag A, Calore JD, Franzblau C. Myeloperoxidase-catalyzed inactivation of α1-protease inhibitor by human neutrophils. J Biol Chem. 1981;256:3348–3353. [PubMed] [Google Scholar]
- Clark RA, Szot S, Venkatasubramanian K, Schiffmann E. Chemotactic factor inactivation by myeloperoxidase-mediated oxidation of methionine. J Immunol. 1980;124:2020–2026. [PubMed] [Google Scholar]
- Decleva E, Menegazzi R, Busetto S, Patriarca P, Dri P. Common methodology is inadequate for studies on the microbicidal activity of neutrophils. J Leuk Biol. 2006;79:87–94. doi: 10.1189/jlb.0605338. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE. Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family. Physiol Rev. 2013;93:599–652. doi: 10.1152/physrev.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford HB. Heme Peroxidases. New York: Wiley-VCH; 1999. [Google Scholar]
- Dypbukt JM, Bishop C, Brooks WM, Thong B, Eriksson H, Kettle AJ. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic Biol Med. 2005;39:1468–1477. doi: 10.1016/j.freeradbiomed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzanowska H, Wolcott RG, Hannum DM, Hurst JK. Bactericidal properties of hydrogen peroxide and copper or iron-containing complexes in relation to leukocyte function. Free Radic Biol Med. 1995;18:437–449. doi: 10.1016/0891-5849(94)00150-i. [DOI] [PubMed] [Google Scholar]
- Espey MG, Xavier S, Thomas DD, Miranda KM, Wink DA. Direct real-time evaluation of nitration with green fluorescent protein in solution and within human cells reveals the impact of nitrogen dioxide vs. peroxynitrite mechanisms. Proc Nat Acad Sci USA. 2002;99:3481–3486. doi: 10.1073/pnas.062604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerder CA, Shapiro BM. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc Natl Acad Sci U S A. 1977;74:4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote CS, Goyne TE, Lehrer RI. Assessment of chlorination by human neutrophils. Nature. 1983;301:715–726. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7) J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2003;278:28403–28409. doi: 10.1074/jbc.M304739200. [DOI] [PubMed] [Google Scholar]
- Furtmuller PG, Arnhold J, Jantschko W, Pichler H, Obinger C. Redox properties of the couples compound I/compound II and compound II/native enzyme of human myeloperoxidase. Biochem Biophys Res Commun. 2003;301:551–557. doi: 10.1016/s0006-291x(02)03075-9. [DOI] [PubMed] [Google Scholar]
- Furtmüller PG, Burner U, Obinger C. Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate. Biochemistry. 1998;37:17923–17930. doi: 10.1021/bi9818772. [DOI] [PubMed] [Google Scholar]
- Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Nat Acad Sci USA. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignaschi VJ, Sperperato AM, Etcheverry MJ, Macario AJ. An nuevo cuadro citoquimico: Negativad espontanea de as reacciones de peroxidisas, oxidas y lipido en la progenia neutrofilia y en los monocitos de dos hermanos. Rev Assoc Med Argent. 1963;77:218–221. [Google Scholar]
- Haegens A, Heeringa P, van Suylen RJ, Steele C, Aratani Y, O’Donoghue RJJ, et al. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. J Immunol. 2009;182:7990–7996. doi: 10.4049/jimmunol.0800377. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun. 1996;64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- Hawkins CL, Davies MJ. Inactivation of protease inhibitors and lysozyme by hypochlorous acid: role of side-chain oxidation and protein unfolding in loss of biological function. Chem Res Toxicol. 2005;18:1600–1610. doi: 10.1021/tx050207b. [DOI] [PubMed] [Google Scholar]
- Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides, and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- Hirche TO, Gaut JP, Heinecke JW, Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J Immunol. 2005;174:1557–1565. doi: 10.4049/jimmunol.174.3.1557. [DOI] [PubMed] [Google Scholar]
- Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am. 2013;27:89–99. doi: 10.1016/j.hoc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JK. What really happens in the neutrophil phagosome? Free Radic Biol Med. 2012;53:508–520. doi: 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JK, Barrette WC., Jr Leukocyte oxygen activation and microbicidal oxidative toxins. CRC Crit Rev Biochem Mol Biol. 1989;24:271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Jandl RC, Andre S, Borges-DuBois JL, Kipnes RS, McMurrich BJ, Babior BM. Termination of the respiratory burst in human neutrophils. J Clin Invest. 1978;61:1176–1185. doi: 10.1172/JCI109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara M, Eyre HJ, Simonian Y, Atkin CL, Hasstedt SJ. Hereditary myeloperoxidase deficiency. Blood. 1981;57:888–893. [PubMed] [Google Scholar]
- Klebanoff SJ. Iodination of bacteria; a bactericidal mechanism. J Exp Med. 1967;126:1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968;95:2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase-mediated antimicrobial systems and their role in leukocyte function. In: Schultz J, editor. Biochemistry of the Phagocytic Process. North-Holland Publishing Company; 1970a. pp. 89–110. [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970b;169:1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leuk Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ, Hamon CB. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Retic Soc. 1972;12:170–196. [PubMed] [Google Scholar]
- Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leuk Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ, Pincus SH. Hydrogen peroxide utilization in myeloperoxidase-deficient leukocytes: a possible microbicidal control mechanism. J Clin Invest. 1971;50:2226–2229. doi: 10.1172/JCI106718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJC, RA . The Neutrophil: Function and Clinical Disorders. Amsterdam: Elsevier/North Holland Biomedical Press; 1978. p. 810. [Google Scholar]
- Kobayashi M, Tanaka T, Usui T. Inactivation of lysosomal enzymes by the respiratory burst of polymorphonuclear leukocytes. J Lab Clin Med. 1982;100:896–907. [PubMed] [Google Scholar]
- Koch C. Neutrophil granulocyte functionin vitro. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974;82:127–135. [PubMed] [Google Scholar]
- Kutter D. Prevalence of myeloperoxidase deficiency: population studies using Bayer-Technicon automated hematology. J Mol Med. 1998;76:669–675. doi: 10.1007/s001090050266. [DOI] [PubMed] [Google Scholar]
- Kutter D, Al-Haidari K, Thoma J. Myeloperoxidase deficiency: simple methods for its diagnosis and significance of different forms. Klinisches Labor. 1994;40:342–346. [Google Scholar]
- Lehrer RI, Cline MJ. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969a;48:1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Hanifin J, Cline MJ. Defective bactericidal activity in myeloperoxidase-deficient human neutrophils. Nature. 1969b;223:78–79. doi: 10.1038/223078a0. [DOI] [PubMed] [Google Scholar]
- Lymar SV, Hurst JK. Role of compartmentation in promoting toxicity of leukocyte-generated strong oxidants. Chem Res Toxicol. 1995;8:833–840. doi: 10.1021/tx00048a003. [DOI] [PubMed] [Google Scholar]
- Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int. 2003;64:1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- Marquez LA, Huang JT, Dunford HB. Spectral and kinetic studies on the formation of myeloperoxidase compounds I and II: Roles of hydrogen peroxide and superoxide. Biochemistry. 1994;33:1447–1454. doi: 10.1021/bi00172a022. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Moreland JG, Davis AP, Bailey G, Nauseef WM, Lamb FS. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Nauseef WM, Metcalf JA, Root RK. Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood. 1983;61:483–491. [PubMed] [Google Scholar]
- Nicholls SJ, Hazen SL. Myeloperoxidase, modified lipoproteins, and atherogenesis. J Lipid Res. 2009;50:S346–S351. doi: 10.1194/jlr.R800086-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoi H, Kohi F, Kajiwara H, Suzuki K. Prevalence of inherited myeloperoxidase deficiency in Japan. Microbiol Immunol. 2003;47:527–531. doi: 10.1111/j.1348-0421.2003.tb03414.x. [DOI] [PubMed] [Google Scholar]
- Nussbaum C, Klinke A, Adam M, Baldus S, Sperandio M. Myeloperoxidase - a leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxidants & Redox Signaling. 2013;18:692–713. doi: 10.1089/ars.2012.4783. [DOI] [PubMed] [Google Scholar]
- Odeberg H, Olsson I. Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect Immun. 1976;14:1276–1283. doi: 10.1128/iai.14.6.1276-1283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RG, Bonvillain RW, Valentine VG, Laombard GA, Laplace SG, Nauseef WM, Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leuk Biol. 2008;83:1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RG, Marrero L, Lombard GA, Valentine VG, Nauseef WM, Wang G. CFTR-mediated halide transport in phagosomes of human neutrophils. J Leuk Biol. 2010;87:933–942. doi: 10.1189/jlb.1009655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RG, Valentine VG, Lanson NA, Jr, Leidal K, Zhang O, Lombard G, et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo AM, Suquet C, Konkel ME, Hurst JK. Green fluorescent protein-expressing Escherichia coli as a selective probe for HOCl generation within neutrophils. Biochemistry. 2005;44:6910–6919. doi: 10.1021/bi047342s. [DOI] [PubMed] [Google Scholar]
- Pang YY, Schwartz J, Bloomberg S, Boyd JM, Horswill AR, Nauseef WM. Methionine Sulfoxide Reductases Protect against Oxidative Stress in Staphylococcus aureus Encountering Exogenous Oxidants and Human Neutrophils. J Innate Immun. 2014;6:353–364. doi: 10.1159/000355915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc Nat Acad Sci USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison DI, Davies MJ. Absolute rate constants for the reactions of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- Pattison DI, Davies MJ. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem. 2006;13:3271–3290. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- Rausch PG, Moore TG. Granule enzymes of polymorphonuclear neutrophils: a phylogenetic comparison. Blood. 1975;46:913–919. [PubMed] [Google Scholar]
- Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leuk Biol. 2007;81:137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- Rorvig S, Ostergaard O, Heegaard NH, Borregaard N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J Leukoc Biol. 2013;94:711–721. doi: 10.1189/jlb.1212619. [DOI] [PubMed] [Google Scholar]
- Rosen H, Klebanoff SJ. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976;58:50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc Nat Acad Sci USA. 2009;106:18686–18691. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Michel BR. Redundant contribution of myeloperoxidase-dependent systems to neutrophil-mediated killing of Escherichia coli. Infect Immun. 1997;65:4173–4178. doi: 10.1128/iai.65.10.4173-4178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf J, Carayon P. Structural and functional aspects of thyroid peroxidase. Arch Biochem Biophys. 2006;445:269–277. doi: 10.1016/j.abb.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. Arch Biochem Biophys. 1962;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Leidal KG, Femling JK, Weiss JP, Nauseef WM. Neutrophil bleaching of GFP-expressing staphylococci:probing the intraphagosomal fate of individual bacteria. J Immunology. 2009;183:2632–2641. doi: 10.4049/jimmunol.0804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B, Belaaouaj A, Verlinde CLMJ, Fu X, Heinecke JW. Methionine sulfoxide and proteolytic cleavage contribute to the inactivation of cathepsin G by hypochlorous acid. An oxidative mechanism for regulation of serine proteinases by myeloperoxidase. J Biol Chem. 2005;280:29311–29321. doi: 10.1074/jbc.M504040200. [DOI] [PubMed] [Google Scholar]
- Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A01, the major high density lipoprotein protein, for site-specific oxidation in human athersclerotic lesions. J Biol Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger BJ, Oberdoerster MA, Lewis PJ, Rosen H. mRNA expression profiles for Escherichia coli ingested by normal and phagocyte oxidase-deficient human neutrophils. J Clin Invest. 2002;110:1151–1163. doi: 10.1172/JCI15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers FA, Morgan PE, Davies MJ, Hawkins CL. Identification of plasma proteins that are susceptible to thiol oxidation by hypochlorous acid and N -chloramines. Chem Res Toxicol. 2008;21:1832–1840. doi: 10.1021/tx8001719. [DOI] [PubMed] [Google Scholar]
- Sun YT, Shieh CC, Delpire E, Shen MR. K+-Cl− cotransport mediates the bactericidal activity of neutrophils by regulating NADPH oxidase activation. J Physiol. 2012;590:3231–3243. doi: 10.1113/jphysiol.2011.225300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Umeki Y, Matsumoto N, Yamamoto K, Yoshida M, Suzuki K, Aratani Y. Severe neutrophil-mediated lung inflammation in myeloperoxidase-deficient mice exposed to zymosan. Inflamm Res. 2012;61:197–205. doi: 10.1007/s00011-011-0401-y. [DOI] [PubMed] [Google Scholar]
- Theorell H, Åkeson Highly purified milk peroxidase. Arkiv för Kemi, Minerologi, och Geologi. 1943;17:1–6. [Google Scholar]
- Thomas EL. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: effect of exogenous amines on antibacterial action against Escherichia coli. Infect Immun. 1979a;25:110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979b;23:522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL, Grisham MB, Jefferson MM. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest. 1983;72:441–454. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL, Grisham MB, Jefferson MM. Cytotoxicity of chloramines. Methods Enzymol. 1986;132:585–593. doi: 10.1016/s0076-6879(86)32043-3. [DOI] [PubMed] [Google Scholar]
- Thomas EL, Jefferson MM, Grisham MB. Myeloperoxidase-catalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochemistry. 1982;21:6299–6308. doi: 10.1021/bi00267a040. [DOI] [PubMed] [Google Scholar]
- Undritz E. DIe Alius-Grignaschi-Anomalie: der erblich konstitutionelle Peroxydasedefekt der Neutrophilien und Monozyten. Blut. 1966;14:129–136. doi: 10.1007/BF01631534. [DOI] [PubMed] [Google Scholar]
- Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009;284:30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers MCM, Winterbourn CC. Oxidative damage to fibronectin. I The effects of the neutrophil myeloperoxidase system and HOCl. Arch Biochem Biophys. 1991;285:53–59. doi: 10.1016/0003-9861(91)90327-f. [DOI] [PubMed] [Google Scholar]
- Voetman AA, Weening RS, Hamers MN, Meerhof LJ, Bot AA, Roos D. Phagocytosing human neutrophils inactivate their own granular enzymes. J Clin Invest. 1981;67:1541–1549. doi: 10.1172/JCI110185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Peppin G, Ortiz X, Ragsdale C, Test ST. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Winter J, Ilbert M, Graf PCF, Özcelik D, Jakob U. Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell. 2008;135:691–701. doi: 10.1016/j.cell.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- Zamocky M, Jakopitsch C, Furtmüller PG, Dunand C, Obinger C. The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins:structure, function, and genetics. 2008;72:589–605. doi: 10.1002/prot.21950. [DOI] [PubMed] [Google Scholar]
- Zederbauer M, Furtmüller PG, Brogioni S, Jakopitsch C, Smulevich G, Obinger C. Heme to protein linkages in mammalian peroxidases: impact on spectroscopic, redox, and catalytic properties. Nature Product Reports. 2007;24:571–584. doi: 10.1039/b604178g. [DOI] [PubMed] [Google Scholar]
- Zgliczynski JM. Characteristics of MPO from neutrophils and other peroxidases from different cells. In: Sbarra AJ, Strauss RR, editors. The Reticuloendothelial System. A Comprehensive Treatise. Volume 2: Biochemistry and Metabolism. New York: Plenum Press; 1980. pp. 255–278. [Google Scholar]
- Zgliczynski JM, Stelmaszynska T, Domanski J, Ostrowski W. Chloramines as intermediates of oxidative reaction of amino acids by myeloperoxidase. Biochem Biophys Acta. 1971;235:419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Zgliczynski JM, Stelmaszynska T, Ostrowiski W, Naskalski J, Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968;4:540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Zgliczynski JM, Stelmaxzynska T. Hydrogen cyanide and cyanogen chloride formation by the myeloperoxidase-H2O2-Cl− system. Biochim Biophys Acta. 1979;567:309–314. doi: 10.1016/0005-2744(79)90116-5. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Song K, Painter RG, Aiken M, Reiser J, Stanton BA, et al. Cystic fibrosis transmembrane conductance regulator recruitment to phagosomes in neutrophils. J Innate Immun. 2013;5:219–230. doi: 10.1159/000346568. [DOI] [PMC free article] [PubMed] [Google Scholar]