Abstract
Globin-coupled sensors (GCS) are heme-binding signal transducers in Bacteria and Archaea where an N-terminal globin controls the activity of a variable C-terminal domain. Here we report that BpeGReg, a globin-coupled diguanylate cyclase (GCDC) from the whooping-cough pathogen Bordetella pertussis, synthesizes the second messenger bis-(3’–5’)-cyclic diguanosine monophosphate (c-di-GMP) upon oxygen binding. Expression of BpeGReg in Salmonella typhimurium enhances biofilm formation, while knockout of the BpeGReg gene of B. pertussis results in decreased biofilm formation. These results represent the first identification of a gaseous ligand for any diguanylate cyclase and provide definitive experimental evidence that a globin-coupled sensor regulates c-di-GMP synthesis and biofilm formation. We propose that the synthesis of c-di-GMP by globin sensors is a widespread phenomenon in bacteria.
Keywords: globin, oxygen sensor, c-di-GMP, diguanylate cyclase, biofilm
Introduction
Globins are heme-containing proteins found in all three domains of life, Eukarya, Archaea, and Bacteria.1 The discovery of microbial globins showed that globins participate in many more roles than O2 metabolism in cells, including sensing of gaseous ligands. The first globin-coupled sensors (GCS) discovered were the HemAT aerotactic transducers that couple a globin-based O2-sensing domain to a chemotaxis methyl-carrier protein (MCP) domain.2–4 The GCS proteins form a broad family of sensors with variable transmitter modules, including the GGDEF domain.3,4 GGDEF domains are widespread in bacteria and possess diguanylate cyclase (DGC) activity.5–10 DGCs synthesize cyclic dimeric guanosine monophosphate (c-di-GMP), a global second messenger implicated in regulating bacterial motility, exopolysaccharide (EPS) production, and biofilm formation at both translational and post-translational levels.8,11–14 Given that biofilm formation is important for virulence of many bacterial pathogens,9,15–17 including Bordetella pertussis,18 these data suggest a role of O2 sensing in regulating pathogenicity.
Results and Discussion
Our in silico analysis of microbial genome sequences identified BpeGReg (Bordetella pertussis globin-coupled regulator) from the whooping-cough pathogen B. pertussis as a potential globin-coupled diguanylate cyclase (GCDC) (Fig. S1). The conserved domain search initially identified two domains in BpeGReg (475 residues): a globin domain (residues 1–155) and a GGDEF domain (residues 297–475) (Fig. 1a). A hybrid threading/homology modeling technique identified the highest-scoring template for the region spanning residues 167 to 289 as the receiver domain of response regulator PhoB from Escherichia coli.19 Thus, BpeGReg consists of three domains: an N-terminal globin domain, a middle domain, and a C-terminal DGC domain. We created a homology model of BpeGReg based on the crystal structures of HemAT-Bs from Bacillus subtilis and PleD from Caulobacter crescentus (Fig. 1a). In this model BpeGReg is a dimer, with an active site (A-site) in each monomer for binding to one GTP molecule and an inhibitory site (I-site) in each monomer20,21 for binding to a pair of intercalated c-di-GMP molecules. We therefore postulated that BpeGReg is a heme-binding protein that can synthesize c-di-GMP and influence biofilm formation based on O2 tension.
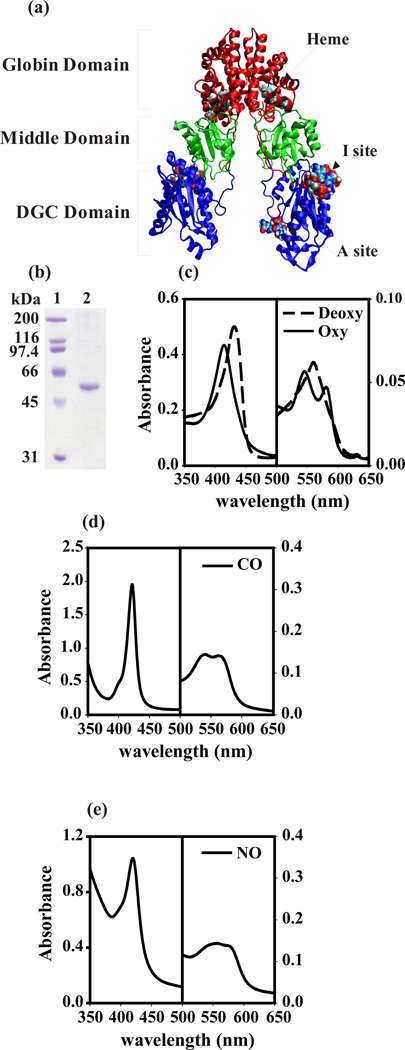
Figure 1.
Identification of BpeGReg from B. pertussis as a globin. (a) Model of a possible complete BpeGReg dimer in ribbon representation with globin (red), middle (green), and DGC (blue) domains. (b) Coomassie-stained SDS-PAGE of purified BpeGReg. Lane 1, molecular weight markers; lane 2, BpeGReg6×-His. (c–e) Absorption spectra of BpeGReg in ferrous state and bound to various ligands. All measurements were done at 25°C and pH 7.5 in 100 mM sodium phosphate buffers.
To characterize BpeGReg, we purified recombinant His-tagged BpeGReg by metal-affinity chromatography. The recombinant protein had the expected molecular mass of 53.7 kDa (Fig. 1b) and showed characteristic heme protein absorption spectra in the near UV and visible regions (Fig. 1c–e and Fig. S2a and b). Addition of ligands to the ferrous BpeGReg caused the Soret absorption band to shift from 431 nm (deoxy, unliganded FeII state) to 416 nm for the O2-bound, 422 nm for the carbon-monoxide bound, and 420 nm for the nitric-oxide bound states. We further measured the O2, CO and NO binding affinities and kinetics of BpeGReg (Table 1). BpeGReg binds O2 and CO (Kd = 0.64 and 0.055 µM, respectively) with affinities comparable to those of sperm-whale myoglobin (Table 1). The association rate constant for NO binding to BpeGReg was 16 µM−1s−1, a value similar to that reported for sperm-whale myoglobin (Table 1). We concluded that BpeGReg is a heme-binding protein.
Table 1.
Ligand-binding parameters of BpeGRega compared to HemAT-Bs and sperm-whale myoglobin
| O2 | CO | NO | |||||
|---|---|---|---|---|---|---|---|
|
kon µM−1 s−1 |
koff s−1 |
Kd µM |
kon µM−1 s−1 |
koff s−1 |
Kd µM |
kon µM−1 s−1 |
|
| BpeGReg | 7.0 | 4.5 | 0.64 | 1.03 | 0.056 | 0.055 | 16 |
| 0.12 | 0.45 | ||||||
| HemAT-Bsb | 19 | 1,900 | 100 | 0.34 | 0.067 | 0.20 | |
| 87 | 4.6 | ||||||
| SWMbc | 17 | 15 | 0.88 | 0.51 | 0.019 | 0.037 | 22 |
Binding parameters for BpeGReg were measured at 25°C in 100 mM NaPi, pH 7.5. The association of CO to BpeGReg was bimodal and manifested two different on-rate constants.
HemAT-Bs data were from Zhang et al..22 The dissociation of O2 from HemAT-Bs was bimodal and manifested two-different rate constants.
To test whether BpeGReg functions as a globin-regulated diguanylate cyclase, we measured the conversion of GTP to c-di-GMP using two independent methods: reversephase liquid chromatography coupled with mass spectrometry (LC-MS) and a thin layer chromatography (TLC) radioactive assay using [α-32P]-GTP as the substrate. The data showed that ferrous BpeGReg can synthesize c-di-GMP from GTP (Fig. 2). We then measured the production of c-di-GMP by different states of BpeGReg, unliganded and liganded. The ferrous O2-bound form of BpeGReg produced the highest amount of c-di-GMP per mole of protein compared to the unliganded form, and the CO- and NO-bound forms (Fig. 2c). We concluded that BpeGReg is an O2-switched DGC that cycles between a low-activity unliganded ferrous state and a highly active O2-bound state.
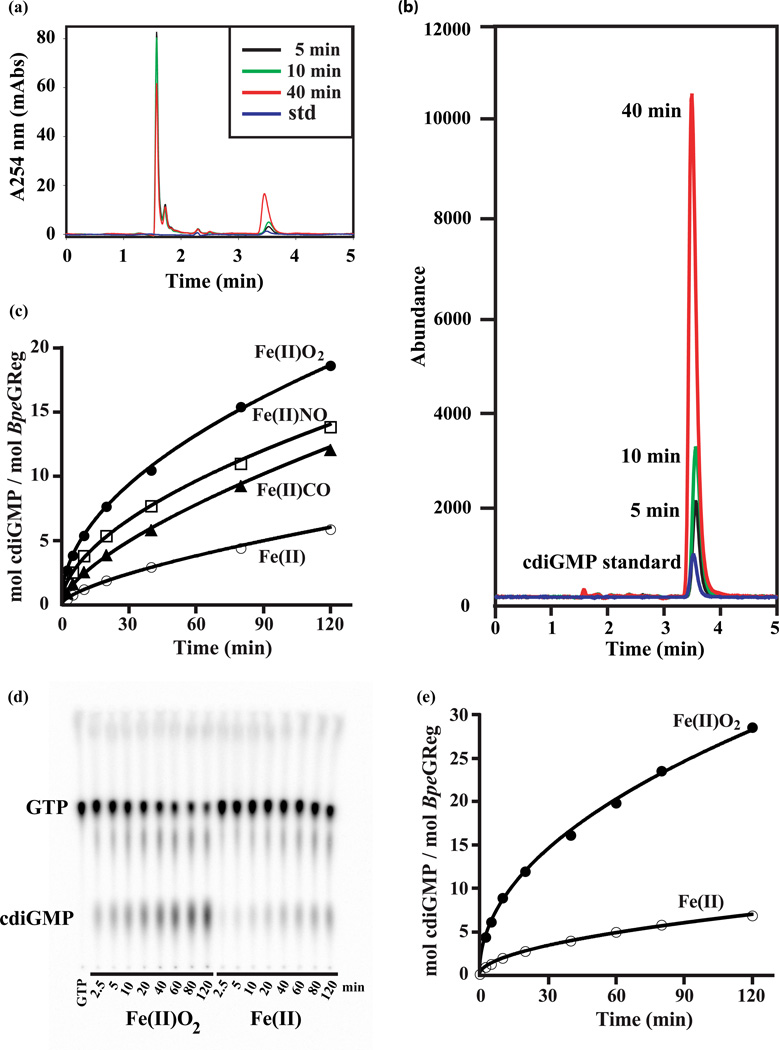
Figure 2.
Identification and quantification of c-di-GMP production in vitro by different liganded states of BpeGReg. All the experiments shown here measure the conversion of GTP (100–500 µM) to c-di-GMP. (a) Activity of BpeGReg at 5 min (black), 10 min (green) and 40 min (red), as assayed by reverse-phase HPLC. The GTP elutes at 1.8 min and the c-di-GMP at 3.6 min; a c-di-GMP standard is shown (blue). (b) Mass spectra coupled to the HPLC shown in panel A; the c-di-GMP is detected with single-ion mode (SIM, negative) at m/z 689. (c) Influence of heme ligands on BpeGReg activity; activity data for the deoxy, O2-bound, CO-bound, and NO-bound states are based on HPLC-MS and TLC assays that gave identical results. Levels of c-di-GMP were measured for reactions stopped at 2.5, 5, 10, 20, 40, 80, and 120 min. (d) Influence of O2 on BpeGReg activity; levels of c-di-GMP were measured by TLC for reactions stopped at 2.5, 5, 10, 20, 40, 60, 80, and 120 min. The reactions correspond to the curves shown in panel (e). The differences between the respective oxy and deoxy curves in panels (c) and (e) are due to the different degrees of feedback inhibition in the starting preparations of enzyme when the DGC assay is not coupled to a PDE (see Fig. 3).
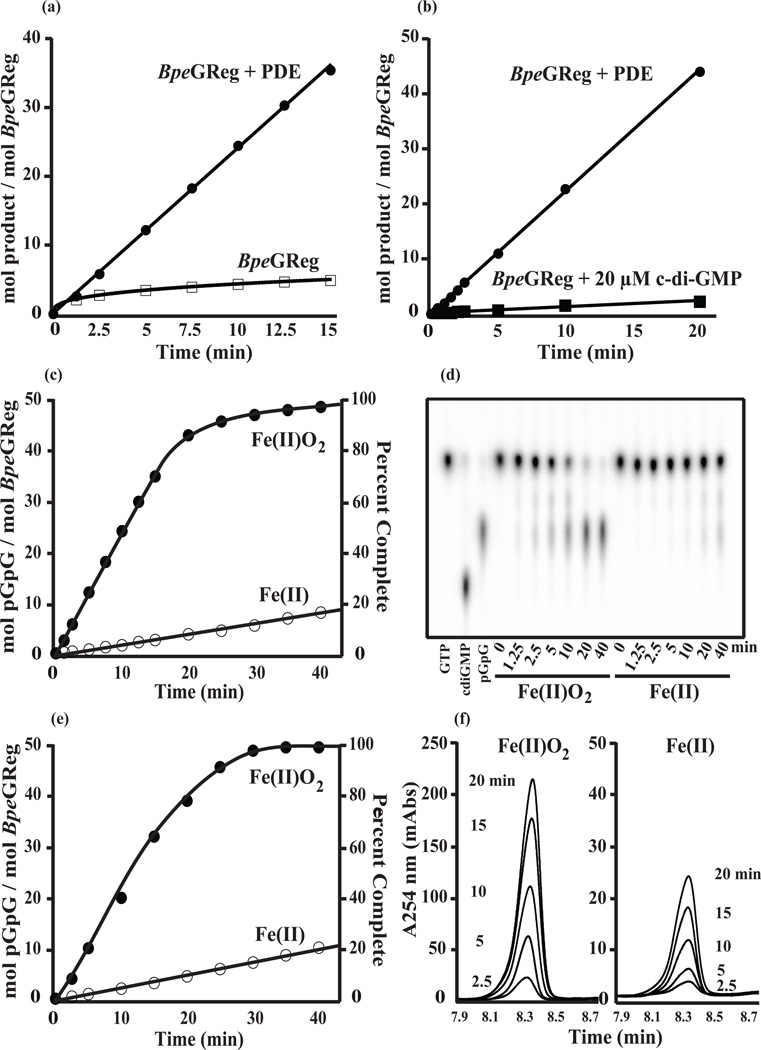
Our BpeGReg model predicted that c-di-GMP can bind to I-sites, causing feedback inhibition of the enzyme activity as initially noted for PleD.20 To test the inhibition activity of BpeGReg, we coupled the cyclase reaction with a phosphodiesterase (PDE) that continuously linearized the cyclic nucleotide product to pGpG. Comparison of the reactions with and without the PDE clearly indicates product inhibition (Fig. 3a). The reaction quickly slowed without removal of the c-di-GMP product and stopped long before the GTP substrate was exhausted. By contrast, the reaction was linear and proceeded to completion when it was coupled to the PDE (Fig. 3a). Furthermore, the addition of 20 µM c-di-GMP at the start of a BpeGReg-catalyzed reaction strongly inhibited the reaction (Fig. 3b). The use of a coupled assay allowed us to measure the reaction rates of the liganded and unliganded forms of BpeGReg without the complications of feedback inhibition. Thus we determined that the O2-bound BpeGReg produces c-di-GMP at an initial rate of 2.5 min−1, a rate 10 times faster than that of the unliganded ferrous form (Fig. 3c–f). In conclusion, O2 enhances the DGC activity of BpeGReg, but accumulation of the c-di-GMP product inhibits this activity.
Figure 3.
Activation of BpeGReg by O2 and feedback inhibition of the enzyme by c-di-GMP. Rates of conversion of 500 µM GTP to c-di-GMP were followed either by measuring c-di-GMP directly or by measuring formation of pGpG from a PDE-coupled reaction. (a) Activity of 5 µM oxy-BpeGReg without coupling to a PDE, or with coupling to a PDE. (b) Activity of 1 µM oxy-BpeGReg in a reaction started in the presence of 20 µM c-di-GMP, or coupled to a PDE. (c) Activity of 5 µM BpeGReg in air (Fe(II)O2), or under anaerobic conditions (Fe(II)). Note the 13-fold drop in the activity in the absence of O2, and note that in air the reaction is essentially complete within 30 min. (d) TLC showing representative time points from the reactions shown in panel (c); the first three lanes are shown as references, with the Rf values being: GTP (0.58), c-di-GMP (0.18), and pGpG (0.34). (e) Repeat of the reactions shown in (c) for analysis by HPLC. (f) HPLC traces of representative pGpG peaks from the reactions shown in panel (e); the peaks are from the 2.5, 5.0, 10, 15, and 20 min time points.
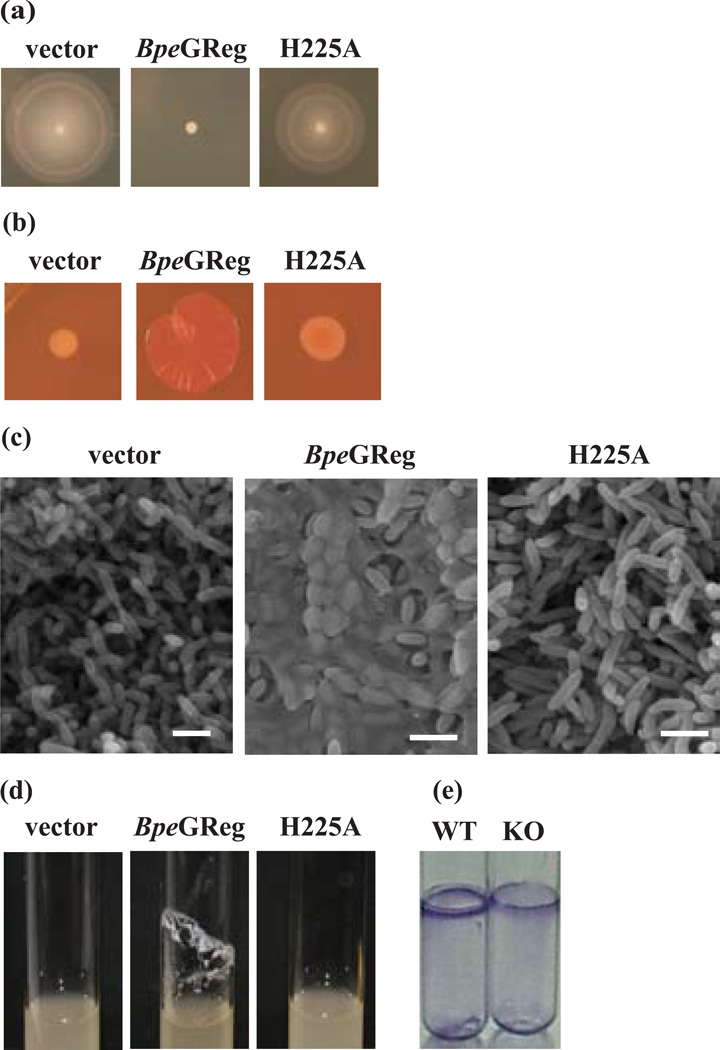
It has been shown that in motile bacterial cells, high levels of c-di-GMP suppress motility in favor of EPS production and biofilm formation.8,25 Salmonella typhimurium strain ATCC 14028 produces an rdar (red, dry, and rough) colony morphology on Congo red agar (as indicators of cellulose or biofilm formation) at 28°C, but not at 37°C.26 However, when the DGC AdrA is overexpressed in this strain at 37°C (leading to high levels of c-di-GMP), the temperature regulation is overcome and the rdar morphotype develops.25,27 Accordingly, we tested whether the expression of BpeGReg in S. typhimurium at 37°C would affect motility, EPS production and biofilm formation by this bacterium. We found that, in cells expressing BpeGReg, motility was suppressed (Fig. 4a) and the rdar morphotype was observed (Fig. 4b). Cells carrying only the vector did not develop the rdar morphotype (Fig. 4b). In parallel, we examined the colonies by scanning electron microscopy (SEM). The SEM micrographs showed that the cells expressing BpeGReg produced layers of biofilm in contrast to the control strain (Fig. 4c). An alternative biofilm assay (liquid culture in glass tubes) showed that BpeGReg also enhances biofilm formation in liquid culture (Fig. 4d). These physiological data demonstrate that BpeGReg, when expressed in S. typhimurium, inhibits motility and enhances EPS production and biofilm formation.
Figure 4.
Phenotypic effects of BpeGReg and its H225A mutant. (a–d) Phenotypes of S. typhimurium cells either harboring an empty vector or expressing BpeGReg and the H225A mutant. (a) Swimming motility on 0.3% tryptone agar plates. (b) rdar morphotype development on Congo red plates. (c) SEM micrographs of the colonies shown in (b). Scale bars, 2 µm. (d) Biofilm formation in liquid culture. (e) Biofilm assay of wild-type (WT) B. pertussis and the BpeGReg knockout strain (KO). Adherent cells were stained with crystal violet.
Next we investigated the function of the middle domain (Fig. 1a). Bioinformatic analysis of this domain identified one highly conserved residue, His225 (Fig. S1). Mutation of His225 to alanine resulted in a protein with normal globin absorption spectra (Fig. S4). Neither the unliganded nor the O2-bound states, however, produced any detectable c-di-GMP. In addition, physiological assays showed a failure of the BpeGReg H225A mutant to confer an rdar morphotype or support biofilm formation (Fig. 4a–d). These data suggest that the middle domain might be required for proper folding of the enzymatic domain, but not the heme binding domain.
To examine whether BpeGReg is involved in biofilm formation in its native B. pertussis host, we constructed a bpeGReg knockout mutant of B. pertussis strain ATCC 9340. A transcriptional fusion suicide vector pFUS228 was used to inactivate the target bpeGReg gene. Compared to wild-type B. pertussis 9340, the knockout strain formed less biofilm (Fig. 4e). The residual biofilm formation could be due to the fact that, besides BpeGReg, the B. pertussis genome encodes four other predicted DGCs and four c-di-GMP PDEs.7 These proteins likely respond to physiological signals other than O2 and probably modulate a variety of processes via the second messenger c-di-GMP, including biofilm and virulence-factor production.
It has been reported that B. pertussis does not grow anaerobically but will grow in O2 tensions as low as 6% of atmospheric O2.29 Thus the lowest concentration of dissolved O2 to allow growth, about 78 µM, would be expected to keep BpeGReg (Kd = 0.64 µM) in an O2-bound active state. Considering that B. pertussis colonizes the upper respiratory tract, BpeGReg could serve as a key O2 sensor for directing B. pertussis to colonize or not.
We provided experimental evidence that a globin-based O2 sensor regulates DGC activity and controls bacterial biofilm formation. Our working hypothesis for BpeGReg is that O2 binding to the globin domain will reorient the BpeGReg dimer so as to enhance the BpeGReg activity. Alternatively, it is also possible that O2 binding to the globin domain of BpeGReg will enhance its dimerization to an active enzyme, analogously to the phosphorylation-dependent dimerization needed for the activation of PleD.30 GCDCs are found in a variety of bacteria besides B. pertussis, including several other pathogens and a number of free-living soil and marine organisms (Fig. S1). Analysis of the GCDCs from Azotobacter vinelandii and Chromobacterium violaceum shows that, like BpeGReg, they also regulate biofilm formation and motility (Fig. S5), indicating that O2 sensing by globins to enhance biofilm production is a widespread phenomenon that was previously overlooked.
Materials and Methods
Sequence alignment and homology modeling
The multiple sequence alignment of selected GCDCs was created in ClustalX and manually refined in DNAstar before final presentation using Adobe Illustrator. Homology modeling was performed using the Prime package (Schrödinger). A threaded model of the middle domain (residues 156–266) of BpeGReg was produced using Threader 3.5 from University College London. The alignment of the middle domain with pdb 1b00 was modeled using MODELLER Release 8v2 from the Sali Lab at UCSF. Analyses of hydrophobicity and hydrophilicity in the active and inhibitory sites in the GGDEF domain were calculated and illustrated with Site Map 2.0 (Schrödinger).
Bacterial strains and culture conditions
E. coli TOP10 cells (Invitrogen) were used for routine cloning. Rosetta2(DE3)pLysS cells (Novagen) were used for expression of the His-tagged proteins. S. typhimurium ATCC 14028, donated by Dr. Susan Ayin (University of Hawaii, Manoa), was used for physiological studies. S. typhimurium was cultured in LB without salt. B. pertussis ATCC 9340 was grown at 37°C on Bordet-Gengou (BG) agar plates with 15% defibrinated sheep blood or Stainer-Scholte broth (SSB) supplemented with heptakis (2, 6-di-O-methyl)-β-cyclodextrin (Sigma). When appropriate, the antibiotics used were ampicillin (100 µg/ml), chloramphenicol (34 µg/ml), and gentamycin (20 µg/ml). Genomic DNA from B. pertussis ATCC 9797D, A. vinelandii ATCC 12518, and C. violaceum ATCC 12472 were purchased from American Type Culture Collection. Genomic DNA from B. pertussis 9340 was extracted using the GNOME DNA Isolation Kit (QBiogene).
Plasmid construction
For protein expression and purification, BpeGReg (GenBank accession no. NP_882025) was engineered with an N-terminal hexahistidine tag by PCR (primers listed in Table S1). The PCR product was cloned into the pCR4Blunt-TOPO vector (Invitrogen) and subcloned into the pET-3a expression vector (Novagen). AvGReg (GenBank accession no. ZP_00415257) and CvGReg (GenBank accession no. NP_899909) were engineered with C-terminal hexahistidine tags and cloned into pET-25b and pET-27b, respectively. For expression in S. typhimurium, non-tagged versions of BpeGReg, AvGReg, and CvGReg were cloned into the pTrc99A vector (Table S1). The QuikChange Site-Directed Mutagenesis protocol (Stratagene) was used to construct the H225A mutant (Table S1).
To inactivate BpeGReg in B. pertussis 9340, a 600 bp region (nucleotides 289–888) of the bpeGReg gene was amplified by PCR (Table S1) and cloned into the suicide vector pFUS2 (provided by Dr. Camille Locht). Gene inactivation was performed by one-step homologous recombination28 and confirmed by PCR.
Expression and purification
His-tagged BpeGReg, AvGReg, and CvGReg were overexpressed in E. coli Rosetta2(DE3)pLysS cells induced with 0.05 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at room temperature for 6–8 hours and purified by Co2+-affinity chromatography according to Piatibratov et al..31 Purified proteins were analyzed by SDS-PAGE and the concentration was determined by the Bradford protein assay (BioRad) using a bovine serum albumin standard.32 The purified proteins were stored at −70°C until use.
Absorption spectra and ligand binding parameters
Unless otherwise noted, all determinations of UV-Vis absorption and ligand binding were for 2–5 µM protein in 0.10 M sodium phosphate, pH 7.5 at 25°C. Absorption spectra were monitored with a Cary 4000 UV-Vis spectrophotometer (Varian). Laser-flash photolysis and stopped-flow measurements were done with an LKS.60 laser kinetic spectrometer fitted with a PiStar stopped-flow drive unit (Applied Photophysics). For sample excitation, the LKS.60 spectrometer was coupled to a Quantel Brilliant B Nd: YAG laser with second-harmonic generation. Ligand-binding kinetics were followed for 2–5 µM protein at a wavelength of maximum difference between the starting and final species. Each rate constant was calculated from a linear plot of kobs versus ligand concentration including at least four ligand concentrations.
The BpeGReg O2 association rate constant (kon (O2)) was measured by laser-flash photolysis at 436 or 414 nm using 64–1024 µM O2. The BpeGReg O2 dissociation rate constant (koff (O2)) was measured by stopped-flow at 436 nm by mixing 2–5 µM oxy-BpeGReg with 1 mM sodium dithionite. The BpeGReg CO association rate constant (kon (CO)) was measured by laser-flash photolysis at 419 nm using 30–480 µM CO. The BpeGReg CO dissociation rate constant (koff (CO)) was measured by ligand displacement in the stopped-flow at 423 nm by mixing 3.5 µM carbonmonoxy-BpeGReg (in 20 µM CO) with 500 µM nitric oxide. The BpeGReg NO association rate constant (kon (NO)) was measured by laser-flash photolysis at 435 nm using 15–60 µM nitric oxide.
Enzymatic diguanylate cyclase assays
Deoxy-BpeGReg was produced by reduction of the purified protein with 10 mM DTT in an anaerobic chamber (Coy). Oxy-BpeGReg was prepared by diluting deoxy-BpeGReg into air-saturated reaction buffer. Generally, reactions contained 1–5 µM BpeGReg in 20 mM sodium phosphate, pH 7.5, 10 mM MgCl2, and 2 mM DTT at 23°C. For the DGC-PDE coupling assays, EcDos was added to a three-fold molar excess to the BpeGReg. Reactions were started by adding 500 µM GTP ([α-32P], Perkin-Elmer). Aliquots were removed from the reaction at the indicated times and mixed with one-fourth volume of 0.50 M EDTA, pH 8.0. The stopped reactions were then heated at 100°C for 5 minutes, and precipitated proteins were removed by centrifugation at 10,000 rpm for 10 minutes. The supernatant was analyzed by thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), or liquid chromatography coupled with mass spectrometry (LC-MS). For TLC analysis, 2 µl of the supernatant was spotted onto PEI-cellulose F TLC plates (Merck KGaA). The plates were developed in a 1.5:1 solution of KH2PO4 (1.5 M, pH 3.6): (NH4)2SO4 (4 M, pH 3.6). Under these conditions GTP migrates with Rf = 0.58, c-di-GMP with Rf = 0.18, and pGpG with Rf = 0.34. Data acquisition was by phosphorimaging with a storage phosphor screen (Kodak K-HD) and a Typhoon 9200 variable mode imager (Amersham Pharmacia Biotech). Data analysis was with Image Quant 5.2 software (Molecular Dynamics). Known amounts of [α-32P]-GTP were used for standardization. For HPLC analysis shown in Fig. 3f, 10 µl of the stopped reactions were injected onto an Adsorbosphere Nucleotide-Nucleoside reverse-phase C18 HPLC column (Alltech, 250 × 4.6 mm) equipped with an Adsorbosphere Nucleotide-Nucleoside guard column (Alltech/Grace, 7.5 × 4.6 mm). The mobile phase consisted of (A) 0.15 M NaH2PO4, pH 5.2 and (B) 40% acetonitrile (balance A eluent). A linear gradient from 0–35% B for 10 min at 1 ml/min was used to separate GTP, c-di-GMP, and pGpG. Known quantities of all three molecules were used for standardization. For LC-MS analyses shown in Fig. 2a and b, the samples were injected into a Dionex C-18 column (150 × 4.6 mm) in an Agilent 1100 series LC/MS system and separated with a gradient from 2% acetonitrile/98% trifluoroacetic acid (vol/vol) to 95% aceotonitrile/5% trifluoroacetic acid at a flow rate of 1 ml/min. Nucleotides were detected at a wavelength of 254 nm for LC, as shown in Fig. 2a; c-di-GMP was detected in negative single-ion monitoring mode at m/z 689 with MS, as shown in Fig. 2b.
Phenotypic assays
To detect cellulose biosynthesis, S. typhimurium was grown on LB without salt plates containing Congo red (40 µg/ml) for 40 hours at 37°C. Swimming motility was assayed on 0.3% agar plates (1% tryptone, 0.5% NaCl, 1 µM thiamine) at 28°C for 6 hours. Biofilm formation was observed in glass tubes for S. typhimurium and B. pertussis. S. typhimurium strains were diluted to an OD600 of 0.01 in LB without salt. Five milliliters were aliquoted into glass tubes and sealed with parafilm. The tubes were incubated at 37°C with shaking (250 rpm) for 36 hours. Adherence to the glass and cell clumping were visually compared. B. pertussis strains were incubated at 37°C without shaking for 7 days and adherent cells were stained with 0.1% crystal violet. S. typhimurium colonies grown on Congo red plates were observed by scanning electron microscopy (SEM). Sections of colonies were fixed with 4% glutaraldehyde and 0.05% ruthenium red in 0.1 M cacodylate buffer (pH 7.4), post fixed with 1% osmium tetroxide, dehydrated in ethanol, and critical point dried. Samples were coated with gold/palladium and examined with a Hitachi S-800 field emission scanning electron microscope at 15 kV.
Supplementary Material
Acknowledgements
We thank Dr. C. Locht for providing the pFUS2 vector; Dr. G. Erdem for providing clinical lab facility; Dr. D. Raze and C. Mizumoto for technical assistance; Dr. H. Wahab and N. Bahiyahn for initial homology modeling; Dr. A. C. Whelen for providing the clinical strain of B. pertussis; and Dr. G. Hazelbauer, Dr. C. Appleby, Dr. P. Patek, and Dr. D. Hunt for critical reading of the manuscript. This work was supported by National Science Foundation grant MCB0446431 (M.A.) and US Army Award TATRC #W81XWH0520013 (M.A.); the NIH Intramural Research Program at NCBI, NLM (M.Y.G.); and by National Science Foundation grant 620531 (M.A.G.G.) and Welch Foundation grant I-1575 (M.A.G.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vinogradov SN, Hoogewijs D, Bailly X, Arredondo-Peter R, Guertin M, Gough J, Dewilde S, Moens L, Vanfleteren JR. Three globin lineages belonging to two structural classes in genomes from the three kingdoms of life. Proc. Natl. Acad. Sci. USA. 2005;102:11385–11389. doi: 10.1073/pnas.0502103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou S, Larsen RW, Boudko D, Riley CW, Karatan E, Zimmer M, Ordal GW, Alam M. Myoglobin-like aerotaxis transducers in Archaea and Bacteria. Nature. 2000;403:540–544. doi: 10.1038/35000570. [DOI] [PubMed] [Google Scholar]
- 3.Hou S, Freitas T, Larsen RW, Piatibratov M, Sivozhelezov V, Yamamoto A, Meleshkevitch EA, Zimmer M, Ordal GW, Alam M. Globin-coupled sensors: a class of heme-containing sensors in Archaea and Bacteria. Proc. Natl. Acad. Sci. USA. 2001;98:9353–9358. doi: 10.1073/pnas.161185598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freitas TA, Hou S, Alam M. The diversity of globin-coupled sensors. FEBS Lett. 2003;552:99–104. doi: 10.1016/s0014-5793(03)00923-2. [DOI] [PubMed] [Google Scholar]
- 5.Ross P, Weinhouse H, Aloni Y, Michaeli D, Ohana P, Mayer R, Braun S, de Vroom E, van der Marel GA, van Boom JH, Benziman M. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature. 1987;325:279–281. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 6.Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- 7.Galperin MY. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 2005;5:35. doi: 10.1186/1471-2180-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Römling U, Gomelsky M, Galperin MY. C-di-GMP: the dawning of a novel bacterial signaling system. Mol. Microbiol. 2005;57:629–639. doi: 10.1111/j.1365-2958.2005.04697.x. [DOI] [PubMed] [Google Scholar]
- 9.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenal U, Malone J. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 2006;40:385–407. doi: 10.1146/annurev.genet.40.110405.090423. [DOI] [PubMed] [Google Scholar]
- 11.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross P, Mayer R, Weinhouse H, Amikam D, Huggirat Y, Benziman M, de Vroom E, Fidder A, de Paus P, Sliedregt LA, van der Marel GA, van Boom JH. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. J. Biol. Chem. 1990;265:18933–18943. [PubMed] [Google Scholar]
- 13.Ryjenkov DA, Simm R, Römling U, Gomelsky M. The PilZ domain is a receptor for the second messenger c-di-GMP. J. Biol. Chem. 2006;281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- 14.Lee VT, Matewish JM, Kessler JL, Hyodo M, Hayakawa Y, Lory S. A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol. Microbiol. 2007;65:1474–1484. doi: 10.1111/j.1365-2958.2007.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 16.Kolter R, Greenberg EP. Microbial sciences: the superficial life of microbes. Nature. 2006;441:300–302. doi: 10.1038/441300a. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz FH. Processes controlling the transmission of bacterial pathogens in the environment. Res. Microbiol. 2007;158:195–202. doi: 10.1016/j.resmic.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Sloan GP, Love CF, Sukumar N, Mishra M, Deora R. The Bordetella Bps polysaccharide is critical for biofilm development in the mouse respiratory tract. J. Bacteriol. 2007;189:8270–8276. doi: 10.1128/JB.00785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sola M, Gomis-Ruth FX, Serrano L, Gonzalez A, Coll M. Three-dimensional crystal structure of the transcription factor PhoB receiver domain. J. Mol. Biol. 1999;285:675–687. doi: 10.1006/jmbi.1998.2326. [DOI] [PubMed] [Google Scholar]
- 20.Chan C, Paul R, Samoray D, Amiot NC, Giese B, Jenal U, Schirmer T. Structural basis of activity and allosteric control of diguanylate cyclase. Proc. Natl. Acad. Sci. USA. 2004;101:17084–17089. doi: 10.1073/pnas.0406134101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christen B, Christen M, Paul R, Schmid F, Folcher M, Jenoe P, Meuwly M, Jenal U. Allosteric control of cyclic di-GMP signaling. J. Biol. Chem. 2006;281:32015–32024. doi: 10.1074/jbc.M603589200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Olson JS, Phillips GN., Jr Biophysical and kinetic characterization of HemAT, an aerotaxis receptor from Bacillus subtilis. Biophys. J. 2005;88:2801–2814. doi: 10.1529/biophysj.104.047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer BA, Sligar SG, Olson JS, Phillips GN., Jr Mechanisms of ligand recognition in myoglobin. Chem. Rev. 1994;94:699–714. [Google Scholar]
- 24.Brucker EA, Olson JS, Ikeda-Saito M, Phillips GN., Jr Nitric oxide myoglobin: crystal structure and analysis of ligand geometry. Proteins. 1998;>30:352–356. [PubMed] [Google Scholar]
- 25.Simm R, Morr M, Kader A, Nimtz M, Römling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 2004;53:1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- 26.Gerstel U, Römling U. The csgD promoter, a control unit for biofilm formation in Salmonella typhimurium. Res. Microbiol. 2003;154:659–667. doi: 10.1016/j.resmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Kader A, Simm R, Gerstel U, Morr M, Römling U. Hierarchical involvement of various GGDEF domain proteins in rdar morphotype development of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2006;60:602–616. doi: 10.1111/j.1365-2958.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- 28.Antoine R, Alonso S, Raze D, Coutte L, Lesjean S, Willery E, Locht C, Jacob-Dubuisson F. New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J. Bacteriol. 2000;182:5902–5905. doi: 10.1128/jb.182.20.5902-5905.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood GE, Khelef N, Guiso N, Friedman RL. Identification of Btr-regulated genes using a titration assay. Search for a role for this transcriptional regulator in the growth and virulence of Bordetella pertussis. Gene. 1998;209:51–58. doi: 10.1016/s0378-1119(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 30.Paul R, Abel S, Wassmann P, Beck A, Heerklotz H, Jenal U. Activation of the diguanylate cyclase PleD by phosphorylation-mediated dimerization. J. Biol. Chem. 2007;282:29170–29177. doi: 10.1074/jbc.M704702200. [DOI] [PubMed] [Google Scholar]
- 31.Piatibratov M, Hou S, Brooun A, Yang J, Chen H, Alam M. Expression and fast-flow purification of a polyhistidine-tagged myoglobin-like aerotaxis transducer. Biochim. Biophys. Acta. 2000;1524:149–154. doi: 10.1016/s0304-4165(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.