Abstract
To prime local tissues for dealing with potential infection or injury, exposure to an acute, intense stressor evokes increases in circulating and local tissue inflammatory proteins. Regular physical activity facilitates stress-evoked innate reactivity and modulates the expression of inflammatory proteins in immuno-metabolic tissues such as white adipose tissue (WAT). The impact of regular physical activity on stress-evoked inflammatory protein expression in WAT, however, remains unclear. To investigate this question, lean male F344 rats (150–175 g) were allowed voluntary access to a running wheel for 6 weeks followed by exposure to an acute stressor (100, 1.5 mA-5 s inescapable tail shocks). Using ELISAs, corticosterone, heat shock protein 72 (Hsp72), macrophage chemoattractant protein (MCP-1), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, and IL-10 concentrations were measured in plasma and subcutaneous, intraperitoneal (epididymal and retroperitoneal WAT depots) and visceral (omental and mesenteric WAT depots) WAT compartments. Acute stress increased plasma concentrations of all proteins except TNF-α and, depending upon the compartment examined, WAT concentrations of MCP-1, IL-1β, IL-6, and IL-10. Exercise ubiquitously increased IL-1β within WAT, potentiated stress-evoked Hsp72 in plasma and WAT, and differentially increased stress-evoked MCP-1, IL-6, and IL-10 within WAT. These data suggest: (a) inflammatory proteins in non-obese WAT may serve compartment-specific immune and metabolic roles important to the acute stress response and; (b) voluntary habitual exercise may optimize stress-induced augmentation of innate immune function through increases in stress-evoked Hsp72, MCP-1, IL-6, and IL-10 and decreases in IL-1β/IL10 and TNF-α/IL10 ratios within white adipose tissue.
Keywords: Innate immunity, Acute stress, Visceral adipose tissue, Sterile inflammation, Cytokine, Interleukin-1beta, Interleukin-6, Interleukin-10, Macrophage chemoattractant protein-1, Heat shock protein 72
1. Introduction
Exposure to acute, non-pathogenic mental or physical stressors can significantly impact immune function. The immuno-modula-tory effects of stress are thought to be due, in part, to increases in immune-related proteins that occur as part of the inflammatory response to acute stress. In the absence of a pathogen, for example, acute stressor exposure increases the concentration of danger associated molecular patterns (DAMPs), inflammatory cytokines, and chemokines in the blood and peripheral organs such as the liver and spleen (Johnson et al., 2005; Maslanik et al., 2012). Along with other signals, these stress-evoked proteins help to facilitate innate reactivity by stimulating the release of nitric oxide and cytokines (Asea et al., 2000; Campisi and Fleshner, 2003; Campisi et al., 2012) which modulate the phagocytic activity of macrophages and neutrophils (Popi et al., 2004) while chemokines such as macrophage chemoattractant protein (MCP)-1 modulate leukocyte trafficking between the blood and tissues (Uchida et al., 2012; Yadav et al., 2010). Acute activation of the stress response, however, can be a double-edged sword as it can either augment or suppress immune function depending on the type, duration, and intensity of the acute stressor, the nature of the immune response, and the timing between the cessation of the stress and the injurious or pathogenic challenge. Rats exposed to 100 min of intermittent inescapable tail-shock followed immediately by a subcutaneous injection of Escherichia coli, for instance, resolve their inflammatory responses faster than non-stressed rats (Campisi et al., 2003a; Deak et al., 1999) whereas rats exposed to acute restraint stress demonstrate a suppressed acquired antibody response to both viral (Sheridan et al., 1991) and benign antigens (Fleshner et al., 1996; Moraska and Fleshner, 2001).
White adipose tissue (WAT) – an endocrine organ sensitive to stress – contains a multitude of cells that express and respond to inflammatory proteins suggesting that, in addition to energy metabolism, WAT plays an important role in immune function (Batra and Siegmund, 2012; Dempsey, 2012; Lago et al., 2007). WAT is capable of expressing stress-responsive DAMPS such as heat shock protein (Hsp)-72 and inflammatory proteins such as MCP-1, tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-10 (Altintas et al., 2011; Batra and Siegmund, 2012; Caspar-Bauguil et al., 2009). The bulk of WAT-related studies, however, currently investigate inflammatory protein expression from the perspective of obesity, leaving inflammatory protein expression and function in non-obese WAT relatively unexplored.
Unlike other endocrine organs, WAT exists throughout the body in compartments, or depots, distinguished both by their anatomical location and the circulatory system into which they drain (Abate and Garg, 1995). Subcutaneous WAT, for example, exists outside the body cavity and drains into the systemic circulation; whereas intraperitoneal WAT lay within the body cavity and drains either into the systemic or the portal circulation. Portal draining WAT – which is comprised of the omental and mesenteric depots – is considered visceral WAT whereas the epididymal and retroperitoneal depots comprise systemic draining intraperitoneal WAT (Frayn, 2000; Speaker and Fleshner, 2012). These compartmental distinctions are extremely important because the physiology, metabolism, and function of WAT vary in a compartment-specific manner and visceral WAT, specifically, contributes toward the pathophysiology of obesity (Lafontan and Berlan, 2003). Very few studies, however, investigate and distinguish between subcutaneous, intraperitoneal portal draining visceral WAT, confounding many of the previous conclusions regarding visceral vs. non-visceral WAT physiology and function.
Habitual exercise enhances the adaptive effect of acute stress on innate immunity (Fleshner et al., 2002) and brain DAMP expression (Campisi et al., 2003b), and modulates the inflammatory status of WAT in a compartment-specific manner (Lira et al., 2009) suggesting that stress- and WAT-immune pathways can be modulated by the physical activity status of an organism. No studies to date, however, have investigated the effect of habitual exercise on stress-evoked DAMP or inflammatory expression in WAT. The purpose of this study, therefore, is to investigate the effect of voluntary habitual exercise on the Hsp72 and inflammatory protein response to acute stress in non-obese subcutaneous, intraperitoneal, and visceral WAT (Speaker and Fleshner, 2012). Given the system-wide nature of the inflammatory protein response to acute stress (Maslanik et al., 2013; Rock et al., 2010), the immuno-metabolic nature and heterogeneity of WAT compartments and the effect of regular physical activity on innate reactivity (Campisi and Flesh-ner, 2003; Campisi et al., 2003a; Moraska and Fleshner, 2001), it is hypothesized that: (a) stress will evoke the expression of inflammatory proteins in non-obese WAT in a compartment-specific manner and (b) voluntary habitual exercise will increase stress-evoked DAMP and inflammatory protein concentrations in WAT. The results of this study suggest that physical activity modulates stress–WAT–immune responses and these changes may contribute to stress-induced augmentation of innate immune function.
2. Methods
2.1. Animals
Adult, inbred male Fischer 344 rats were purchased from Harlan Laboratories (Denver, CO) and used in all experiments. Rats were housed individually in Nalgene plexiglass cages (45 × 25.2 × 14.7 cm) in a temperature (22 °C) and humidity-controlled environment in the University of Colorado at Boulder's pathogen-free animal facility. Lights were maintained on a 12:12 h light/dark cycle (lights on at 0700 and off at 1900). For the exercise protocol, rats were 5–6 weeks old (~175 g) upon arrival and acclimatized to the facility for three days prior to the onset of voluntary wheel running. For the saline perfusion protocol, rats were 8–9 weeks old upon arrival and acclimatized to the facility for one week prior to stress as described below. All rats were fed a 2018 Teklad Global 18% protein diet (Harlan Laboratories Denver, CO) and had ad libitum access to food and water. All experimental protocols were approved by the University of Colorado Institutional Animal Care and Use Committee.
2.2. Exercise protocol
To determine the effect of habitual exercise on the sterile systemic and WAT inflammatory response to stress, rats (N = 48) were randomly divided into two equal groups: exercise (RUN) or sedentary (SED). Those assigned to the RUN group were given unlimited access to a running wheel (Mini Mitter, Bend, OR) for 6 weeks while sedentary rats remained in standard plexiglass cages without wheels (Barrientos et al., 2011). Upon arriving to the University of Colorado facility, RUN rats were acclimatized for a period of three days while being housed with running wheels that were rendered immobile with a metal stake. Using Vital View software (Mini Mitter), daily wheel revolutions were digitally recorded and daily running distance calculated as a product of wheel revolutions and wheel circumference (1.081 m). To assess the effect of voluntary wheel running on body weight, all rats were weighed weekly and on the day preceding the stress protocol.
2.3. Stress protocol
On the day of stress, rats assigned to the stressed groups were taken to a separate room, placed in Plexiglas restraining tubes (23.4 cm long and 7.0 cm in diameter) and exposed to 100, 5-s, 1.5 mA inescapable tail shocks (100IS). Shocks were delivered at random with an average interval of 60-s between shocks and occurred during the inactive (light) cycle between 0800 and 1100 h. Control (0IS) SED and RUN rats remained in their home cages during the stress procedure. The tail shock stress procedure used in these experiments was chosen because it consistently results in a sterile immune response as evidenced by increases in systemic cytokines (Johnson et al., 2005; Maslanik et al., 2013, 2012; Nguyen et al., 1998; O'Connor et al., 2003).
2.4. Saline tissue perfusion
Because inflammatory proteins are carried in the blood and WAT tissue is vascularized it could be argued that inflammatory proteins measured in WAT reflect blood rather tissue protein concentrations. To demonstrate that the contribution of blood cytokine (IL-1β) concentrations was minimal in our WAT samples, we conducted a separate experiment in which male F344 rats of the same age (11–12 weeks) were exposed to 100 tail shocks immediately followed by transcardial, saline tissue perfusion. Briefly, 24 sedentary rats were randomly divided into control (no shock, n = 12) or stressed (100 tail shocks, n = 12) groups; 6 stressed and 6 non-stressed rats were perfused with saline and compared to 6 stressed and 6 control non-perfused rats. For the saline perfusion procedure rats were placed under isofluorane anesthesia and perfused transcardially with approximately 100 mL of chilled physiological saline as described previously (Greenwood et al., 2003). Immediately following the perfusion procedure tissues were dissected, processed and comparatively analyzed for IL-1β protein expression as described below.
2.5. Tissue and plasma collection
To capture the acute physiological responses to acute stressor exposure, rats were rapidly decapitated for trunk blood collection and WAT dissections immediately following the tail shock procedure. Blood was collected in EDTA tubes (Greiner Vacuette, Monroe, NC) and spun at 3000 g in a chilled centrifuge (4 °C) for 15 min. Plasma was removed and stored in a Legaci™ refrigeration system at −80 °C (Kendro Laboratory Products, Asheville, NC) for later analysis. The entire omental depot and approximately 0.35 g of subcutaneous, epididymal, retroperitoneal and mesenteric WAT was excised, weighed and immediately spot frozen in liquid nitrogen. As depicted in Supplemental Fig. A, subcutaneous WAT was taken from the inguinal area just above the thigh; the epididymal depot was taken from the portion of the pad most central to the animal and distal from the epididymis while retroperitoneal depot WAT was harvested from the most caudal third of the depot. To reduce variability, all depots were taken from the right side of the animal (dissectors left) by the same dissector for all experiments.
As previously described (Lesniewski et al., 2009), tissue samples were added to an ice-cold radioimmunoprecipitation (RIPA) lysis buffer (0.5 M Tris–HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA, 1.0 mM NaF, 1 mM sodium ortovanadate, 1.0 mM phenylmethylsulfonylfluoride) containing protease and phosphatase inhibitors [protease inhibitor cocktail tablet (Roche, Indianapolis, IN) and 0.01% phosphatase inhibitor cocktail (Sigma, St. Louis, MO)]. WAT samples were homogenized by rapid shaking with ceramic beads (2 × 45 s @ 5000 rpm) using a Precellys 24 high-throughput tissue homogenizer (Bertin Coretroperitoneal USA, Rockville, MD). To minimize variability in total protein concentrations between samples, immediately prior to homogenization, each tissue sample was weighed using a Sartorius R200D digital scale (Bohemia, NY) and the volume of RIPA lysis buffer adjusted to a ratio of 3 mL of buffer per gram of WAT. Following homogenization, tissue lysates were stored at −80 °C (Kendro Laboratory Products, Asheville, NC) for later analysis.
2.6. Assessment of WAT and plasma protein concentrations
According to manufacturer instructions MCP-1, TNF-α, IL-1β, IL-6, and IL-10 cytokine concentrations were measured in tissue ly-sates and plasma by multiplex ELISA (Searchlight Rat Cytokine Assay; Aushon Biosystems, Billerica, MA) as described previously (Rippe et al., 2010). Notably, numerous inflammatory proteins are measurable in WAT using multiplex ELISA systems. A multiplex plate with MCP-1, TNF-α, IL-1β, IL-6, and IL-10 was used to assess these relevant inflammatory proteins which, importantly, were detectable in our homogenates at similar concentration ranges. The sensitivity of this multiplex assay was 1.5 pg/mL for MCP-1, 6.0 pg/mL for TNF-α, and 0.8 pg/mL for IL-1β, IL-6, and IL-10. Due to an irreparable error in the standard curve for the multiplex MCP-1 assay used to analyze the plasma, we measured the concentrations of plasma MCP-1 using singleplex ELISA (RnD Systems Minneapolis, MN, minimal detectable limit 8.0 pg/mL, Invitrogen, Camarillo, CA). Plasma corticosterone was assessed via EIA (Enzo Biosciences, Farmingdale, NY), extracellular plasma Hsp72 with a high sensitivity ELISA (Enzo Biosciences, Farmingdale, NY) and Hsp72 in WAT using a standard ELISA (Enzo Biosciences, Farming-dale, NY) as previously described (Campeau et al., 2010; Maslanik et al., 2012). To account for differences in total protein concentrations between WAT samples, cytokine protein expression is presented relative to the amount of total protein measured in the WAT sample (lg of cytokine/mg of total protein) (Rosa Neto et al., 2009). Protein concentrations of tissue lysates were determined by BCA colorimetric protein assay (Pierce, Rockford, IL) according the manufacturer's instructions.
2.7. Statistical analyses
To evaluate the intraperitoneal WAT compartment, results from the epididymal and retroperitoneal depots were averaged; to evaluate the visceral WAT compartment, results from omental and mestenteric WAT were averaged. A three-way analysis of variance (ANOVA) was used to evaluate the effect of voluntary habitual exercise (SED vs. RUN) on acute stress responses (0IS vs. 100IS) between WAT compartments (subcutaneous vs. intraperitoneal vs. visceral WAT). Significant two- or three-way interactions were followed by post-hoc pairwise comparisons using the Bonferroni/Dunn correction. A significant exercise × stress interaction, for example, was followed by unconfounded pairwise comparisons between the exercise and stress conditions, irrespective of WAT compartment: SED 0IS vs. RUN 0IS, SED 0IS vs. SED 100IS, RUN 0IS vs. RUN 100IS, SED 100IS vs. RUN 100IS. A two-factor ANOVA (exercise × stress) was used to analyze plasma data; post-hoc pairwise comparisons using the Bonferroni/Dunn correction were used to interpret significant exercise × stress interactions. All data are expressed as means ± SEM and were evaluated using Statview software (Statview, SAS Institute Cary, NC). Results were significant if P < 0.05.
3. Results
3.1. Effect of 6 weeks of voluntary wheel running on characteristic physical parameters
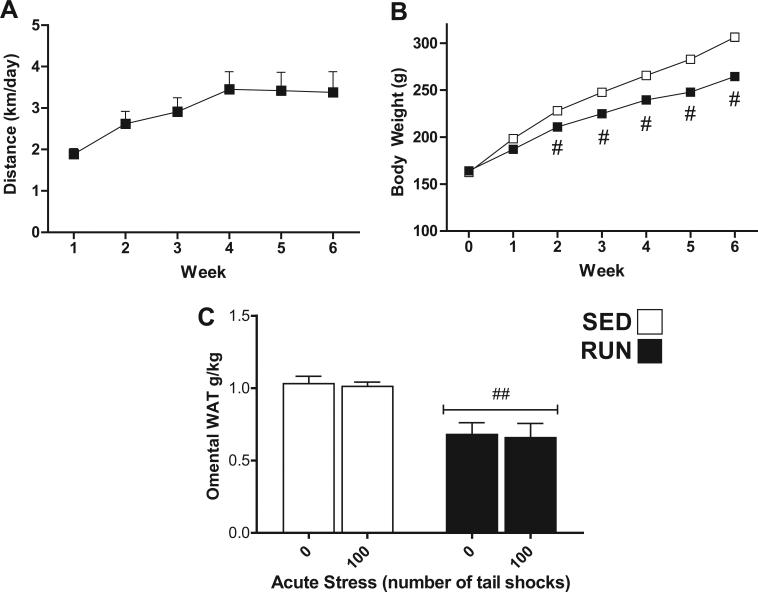
Rats were allowed access to a running wheel for 6 weeks before exposure to acute tail shock stress. Fig. 1(A) illustrates the average distances run per day per week (1.88 ± 0.134 to 3.42 ± 0.445 km/day) during 6 weeks of voluntary wheel running (RUN). Fig. 1(B) reflects the difference in mean body weight (g) ± SEM between sedentary (SED) and exercised (RUN) rats at the end of each week. Using repeated-measures ANOVA, a significant effect of wheel running on body weight was found. At the end of week 6, RUN rats had gained significantly less body weight than their sedentary counterparts (mean body weight, RUN = 264.6 g ± 3.3, SED = 306.7 g ± 4.6). As shown in Fig. 1(C), relative to their body weight, RUN rats had significantly less omental WAT than SED rats (F1,42 = 51.988, p < 0.0001).
Fig. 1.
Effect of 6 weeks of voluntary wheel running on physical parameters of non-obese F344 rats. Adult, male F344 rats remained sedentary in home cages (SED □) or were allowed voluntary access to a running wheel for six weeks (RUN ■). (A) Average distance (km) run per day (24 h) each week. (B) Body weight (g) at the end of each week. (C) Omental fat pad weight relative to body weight (g/kg) on day of sacrifice. Data represent mean values + SEM. ##P < 0.001 (SED vs. RUN). Note: For (B), symbol size exceeded the SEM value.
3.2. Effect of 6wk voluntary wheel running on plasma corticosterone, eHsp72, MCP-1, TNF-α, IL-1β, IL-6, and IL-10 response to acute tail shock stress
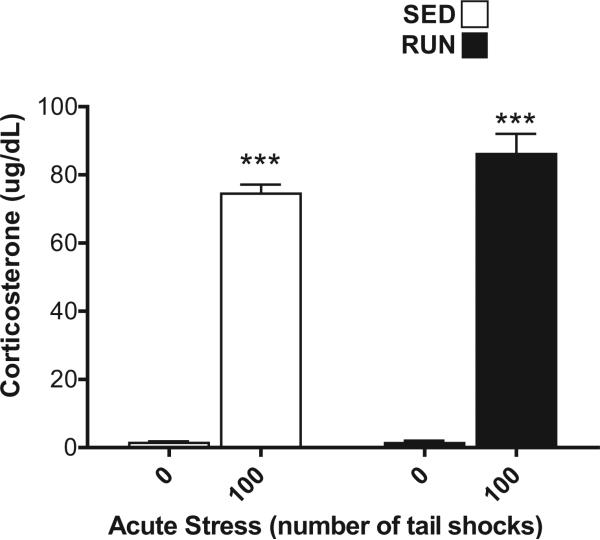
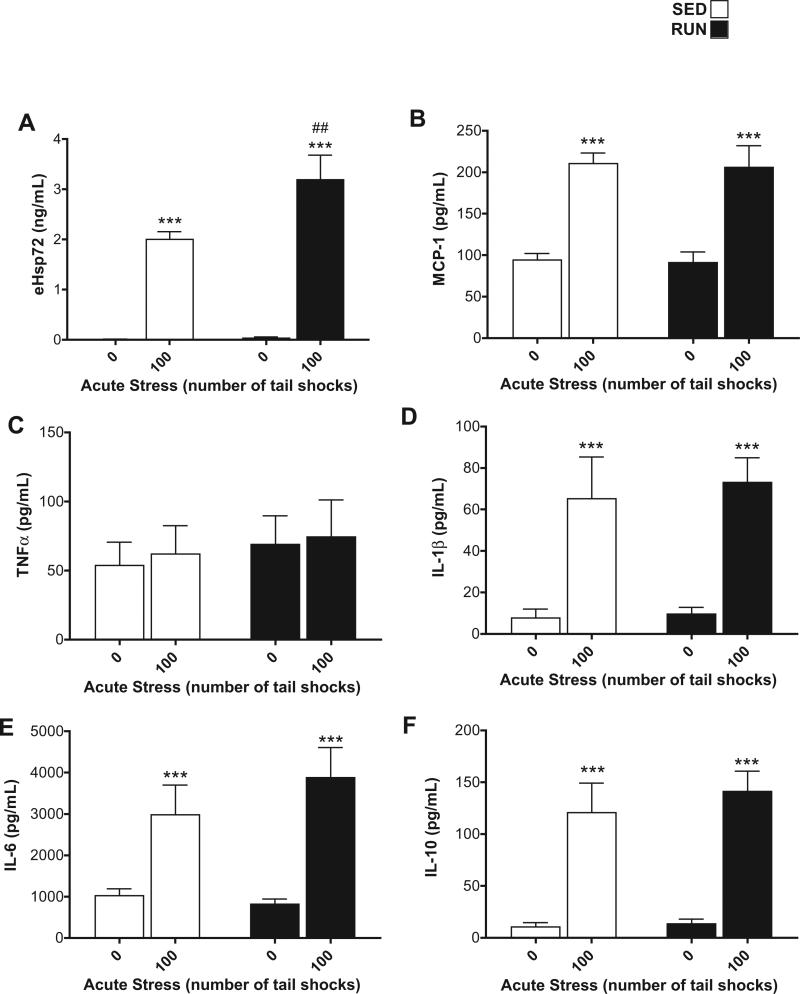
Corticosterone (CORT, Fig. 2), eHsp72 (Fig. 3(A)), and MCP-1, TNF-α, IL-1β, IL-6, IL-10 (Fig. 3(B)–(F)) were measured in the plasma from trunk blood collected from all rats to verify that our stressor protocol induced reliable hormone, DAMP and inflammatory protein responses.
Fig. 2.
Effect of 6 weeks of voluntary wheel running on the plasma corticosterone response to acute tail shock stress. Sedentary (SED □) and exercised (RUN ■) rats were exposed to 0 (control condition) or 100 acute, inescapable shocks (IS), immediately sacrificed, and trunk blood collected. Data represent mean values + SEM. ***P < 0.0001, (0IS vs. 100IS).
Fig. 3.
Effect of 6 weeks of voluntary wheel running on plasma eHsp72, TNF-α, IL-1β, IL-6, and IL-10 protein responses to acute tail shock stress. Sedentary (SED □) and exercised (RUN ■) rats were exposed to 0 (control condition) or 100 acute, inescapable tail shocks (IS), immediately sacrificed and trunk blood collected. Shown is the mean (+ SEM) plasma concentration of (A) extracellular heat shock protein 72 (eHsp72) (B) macrophage chemoattractant protein (MCP)-1 (C) tumor necrosis factor-alpha (TNF-α) (D) interleukin (IL)-1beta (IL-1β) (E) IL-6 and (F) IL-10. ***P < 0.0001, (0IS vs. 100IS), ##P < 0.01 (100IS SED vs. 100IS RUN); two factor ANOVA with Bonferroni post hoc analysis.
Both SED and RUN rats exposed to 100 shocks had higher levels of CORT than non-stressed controls demonstrating a main effect of stress (F1,28 = 592.44, p < 0.0001). 6 weeks of wheel running did not affect the CORT response to stress (F1,28 = 3.2, p = 0.08) (Fig. 2).
An interaction between exercise and stress (F1,28 = 5.1, p = 0.03) was found for plasma eHsp72. Post hoc analyses revealed that RUN rats demonstrated a greater increase in eHsp72 after 100 shocks relative to SED, 100 shock rats (Fig. 3(A)).
Stress increased plasma MCP-1 (F1,28 = 49.53, p < 0.0001), IL-1β (F1,28 = 25.26, p < 0.0001), IL-6 (F1,28 = 22.96, p < 0.0001), and IL-10 (F1,28 = 45.82, p < 0.0001); there were no main effects of exercise (MCP-1 (p = 0.82), IL-1β (p = 0.69), IL-6 (p = 0.51), IL-10 (p = 0.51) (Fig. 3(B), (D)–(F)). Neither stress (p = 0.75) nor exercise (p = 0.53) affected plasma TNF-α (Fig. 3(C)).
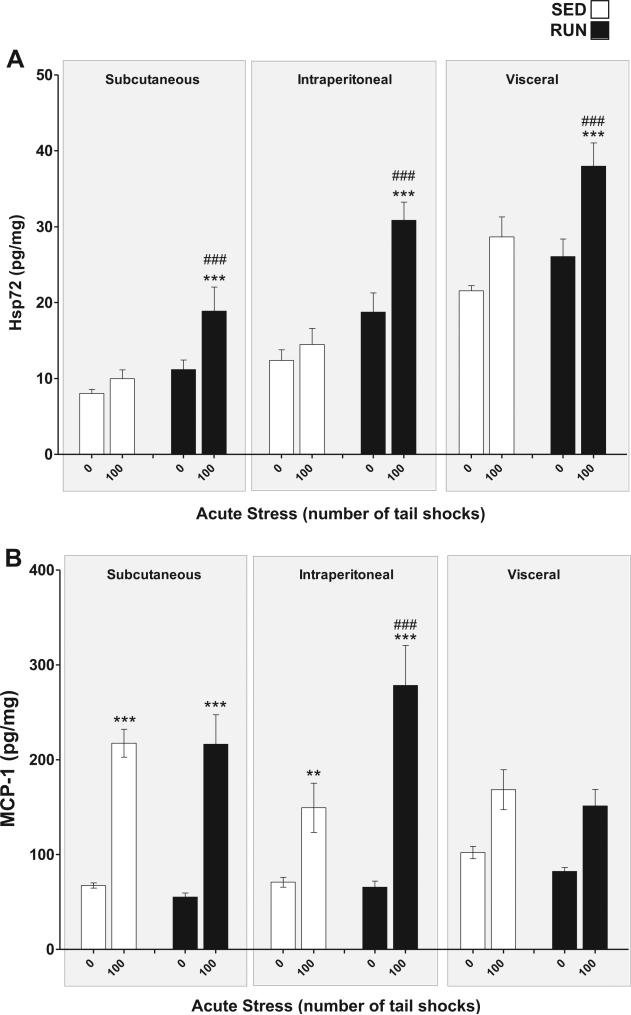
3.3. Effect of 6wk voluntary wheel running on the Hsp72, MCP-1, TNF-α, IL-1β, IL-6, and IL-10 response to tail shock in WAT of lean rats
Hsp72 (Fig. 4(A)): a significant exercise stress interaction was found for Hsp72 in WAT (F1,84 = 8.21, p = 0.001). Post hoc analyses revealed that RUN rats exposed to 100 shocks had elevated concentrations of Hsp72 relative to RUN, non-stressed controls (p = .0001); this response was absent in the WAT of SED rats (p = .16). Hsp72 was differentially expressed between WAT depots with the greatest concentration being found in visceral WAT (main effect of depot (F2,84 = 61.1, p < 0.0001)).
Fig. 4.
Effect of 6 weeks of voluntary wheel running on Hsp72 and MCP-1 response to acute tail shock stress in non-obese white adipose tissue (WAT). Sedentary (SED □) and exercised (RUN ■) rats were exposed to 0 (control condition) or 100 acute, inescapable tail shocks (IS), immediately sacrificed and subcutaneous, intraperitoneal and visceral WAT collected. Shown is the mean (+ SEM) concentration of (A) heat shock protein 72 (Hsp72) ***P < 0.0001 (0IS RUN vs. 100IS RUN) ###P < 0.0001 (100IS SED vs. 100IS RUN) (B) macrophage chemoattractant protein-1 (MCP-1) **P < 0.01, ***P < 0.0001 (0IS vs. 100IS within exercise condition and WAT compartment). ###P < 0.0001 (Intraperitoneal 100IS SED vs. Intraperitoneal 100IS RUN); three factor ANOVA with Bonferroni post hoc analysis.
MCP-1 (Fig. 4(B)): a significant WAT compartment × exercise stress interaction was found for MCP-1 (F2,84 = 3.59, p = 0.03). Post hoc analyses revealed that, for both SED and RUN rats, stress evoked an increase in MCP-1 in subcutaneous (SED p < 0.0001, RUN p < 0.0001) and intraperitoneal (SED p = 0.005, RUN p = <0.001) but not visceral WAT (SED = 0.018, RUN p = 0.014). RUN rats exposed to 100 shocks had higher concentrations of MCP-1 relative to their stressed, SED counterparts (p < 0.0001) within intraperitoneal WAT.
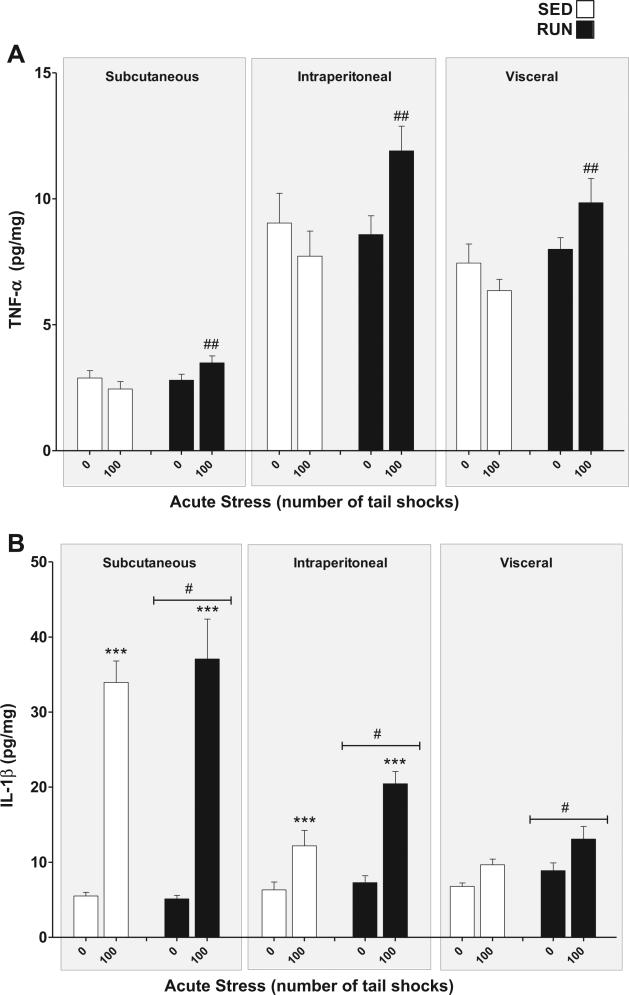
TNF-α (Fig. 5(A)): a significant exercise × stress interaction was found for TNF-α in WAT (F1,84 = 15.08, p = 0.0002). Post hoc analyses revealed that RUN rats exposed to stress had higher overall concentrations of TNF-α in their WAT than stressed, SED rats (p = 0.0016), however, neither SED nor RUN rats demonstrated significant increases in TNF-α expression in response to stress (SED p = .30, RUN p = 0.04). TNF-α was differentially expressed between WAT depots with concentrations being highest in intraperitoneal followed by visceral and subcutaneous WAT (main effect of compartment (F2,84 = 101.13, p < 0.0001)).
Fig. 5.
Effect of 6 weeks of voluntary wheel running on TNF-α and IL-1β response to acute tail shock stress in non-obese white adipose tissue (WAT). Sedentary (SED □) and exercised (RUN ■) rats were exposed to 0 (control condition) or 100 acute, inescapable tail shocks (IS), immediately sacrificed and subcutaneous, intraperitoneal and visceral WAT collected. Shown is the mean (+ SEM) concentration (A) tumor necrosis factor-alpha (TNF-α) ##P < 0.01 (100 IS SED vs. 100 IS RUN) and (B) interleukin (IL)-1beta (IL-1β) ***P < 0.0001 (0IS SQ vs. 100IS SQ, 0IS INTRA vs. 100 IS INTRA), #P < 0.05 SED vs. RUN; three factor ANOVA with Bonferroni post hoc analysis.
IL-1β (Fig. 5(B)): a significant WAT compartment × stress interaction was found for IL-1β (F2,84 = 47.89, p < 0.0001). Post hoc analyses revealed that rats exposed to 100 shocks had elevated concentrations of IL-1β relative to non-stressed controls in subcutaneous (p < 0.0001) and intraperitoneal (p < 0.000) but not visceral WAT (p = 0.09). As a whole, RUN rats demonstrated higher concentrations of IL-1β in their WAT than SED rats as reflected by a main effect of exercise (F1,84 = 6.25, p = 0.01).
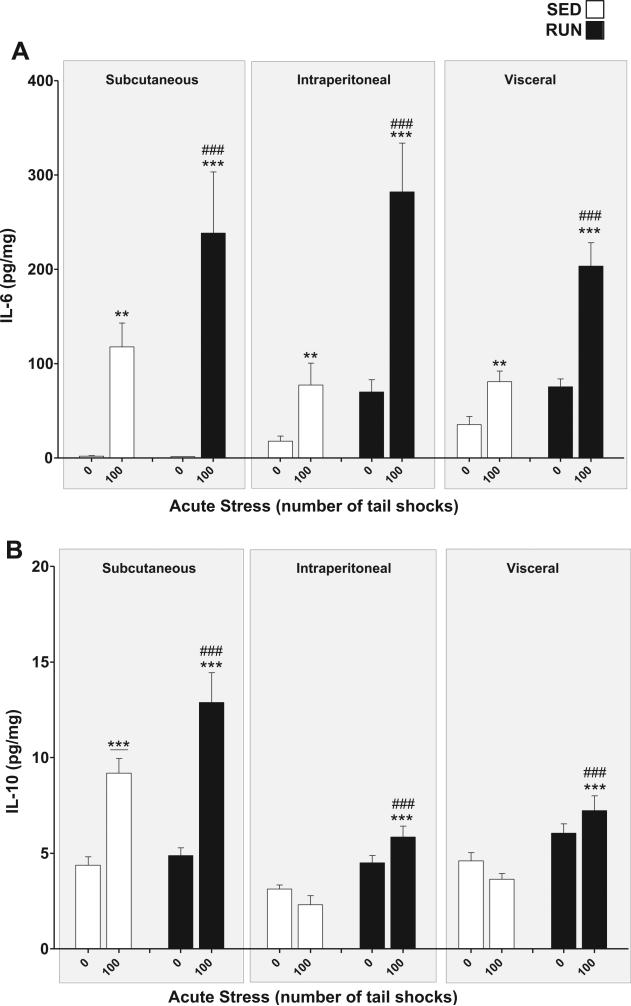
IL-6 (Fig. 6(A)): a significant exercise × stress interaction was found for IL-6 in WAT (F1,84 = 13.93, p = 0.0003). Post hoc analyses revealed that rats exposed to stress had higher concentrations of IL-6 in their WAT than non-stressed rats (SED, p = 0.0017, RUN p < 0.0001). RUN rats demonstrated greater increases in IL-6 expression in response to stress (p < 0.0001).
Fig. 6.
Effect of 6 weeks of voluntary wheel running on IL-6 and IL-10 response to acute tail shock stress in non-obese white adipose tissue (WAT). Sedentary (SED □) and exercised (RUN ■) rats were exposed to 0 (control condition) or 100 acute, inescapable tail shocks (IS), immediately sacrificed and subcutaneous, intraperitoneal and visceral WAT collected. Shown is the mean (+ SEM) concentration of (A) interleukin (IL)-6 (IL-6) **P < 0.01, ***P < 0.0001 (0IS SED vs. 100IS SED, 0IS RUN vs. 100IS RUN), ###P < 0.0001 (100IS SED vs. 100IS RUN). (B) IL-10, ***P < 0.0001 (0IS RUN vs. 100IS RUN), ***P < 0.0001 (Subcutaneous 0IS SED vs. Subcutaneous100IS SED), ###P < 0.0001 (100IS SED vs. 100IS RUN); three factor ANOVA with Bonferroni post hoc analysis.
IL-10 (Fig. 6(B)): significant compartment × stress (F2, 84 = 29.62, p < 0.0001) and exercise × stress (F1,84 = 10.75, p = 0.0015) interactions were found for IL-10 in WAT. Post hoc analyses revealed that, relative to non-stressed controls, only the sub-cutaneous WAT of rats exposed to stress had higher concentrations of IL-10 (p < 0.0001), however, RUN rats demonstrated significant stress-evoked increases in IL-10 in all WAT compartments (p < 0.0001) which were absent in the WAT of SED rats (p = 0.2254).
3.4. Effect of 6wk voluntary wheel running on stress-evoked shifts in the inflammatory status of WAT
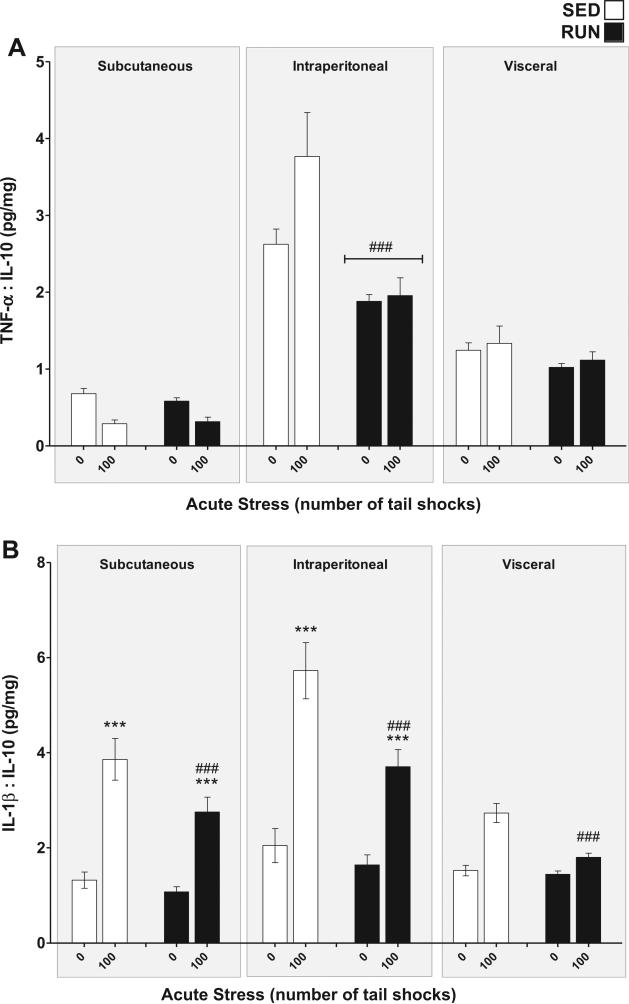
To determine the inflammatory status of WAT, pro-inflamma-tory TNF-α, and IL-1β concentrations were compared relative to the anti-inflammatory concentration of IL-10 for each sample (Gomes da Silva et al., 2013).
TNF-α:IL-10 (Fig. 7(A)): significant compartment × exercise (F2,84 = 10.53, p < 0.0001) and compartment × stress (F2,84 = 5.17, p = 0.0076) interactions were found for the TNF-α:IL-10 ratio in WAT. Post hoc analyses revealed the intraperitoneal WAT of RUN rats had a lower overall inflammatory status than the intraperito-neal WAT of SED rats (p < 0.0001) but exercise did not affect the TNF-α:IL-10 ratio of subcutaneous (p = 0.87) or visceral WAT (p = 0.32). 100 tailshocks did not reliably elevate the TNF-α:IL-10 ratio in any of the WAT compartments.
Fig. 7.
Effect of 6 weeks of voluntary wheel running on the inflammatory status response to acute tail shock stress in non-obese white adipose tissue (WAT). Sedentary (SED □) and exercised (RUN ■) rats were exposed to 0 (control condition) or 100 acute, inescapable tail shocks (IS), immediately sacrificed and subcutaneous, intraperitoneal and visceral WAT collected. Shown is the mean (+ SEM) ratio of (A) TNF-α relative to IL-10, ###P < 0.0001 (Intraperitoneal SED vs. Intraperitoneal RUN) and (B) IL-1β relative to IL-10, ***P < 0.0001 (Subcutaneous 0IS vs. Subcutaneous 100IS, Intraperitoneal 0IS vs. Intraperitoneal 100IS), ###P < 0.0001 (100IS SED vs. 100IS RUN); three factor ANOVA with Bonferroni post hoc analysis.
IL-1β:IL-10 (Fig. 7(B)): significant compartment ×stress (F2,84 = 8.89, p < 0.0001) and exercise × stress (F1,84 = 10.49, p = 0.0017) interactions were found for the IL-1β:IL-10 ratio in WAT. Post hoc analyses revealed that exposure to acute stress increased the inflammatory status of subcutaneous (p < 0.0001) and intraperitoneal (p < 0.0001) but not visceral (p = 0.02) WAT. RUN rats demonstrated less of a stress-evoked increase in inflammatory status than SED rats in all WAT compartments (p < 0.0001).
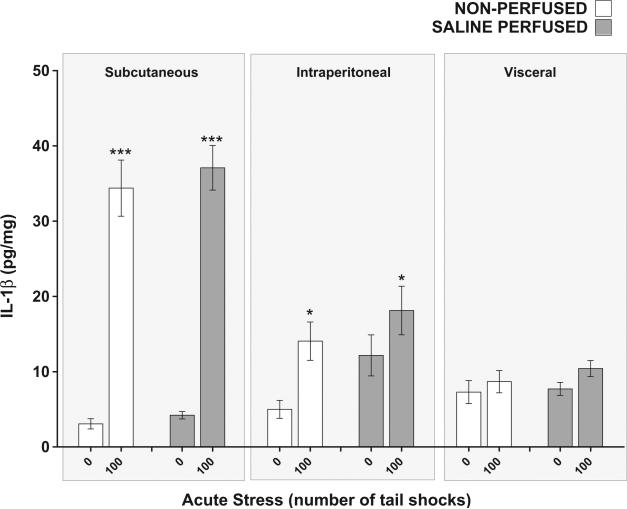
3.5. Effect of saline tissue perfusion on the IL-1β response to acute stress in WAT
Because blood contains inflammatory proteins and WAT tissue is vascularized, it could be argued that inflammatory proteins measured in WAT are the result of blood-related proteins, not tissue proteins. To determine what contribution if any, blood cytokines may have made to the cytokine (IL-1β) concentrations measured in WAT, a separate group of rats were perfused with saline solution after being exposed to 0 or 100 shocks and compared to non-per-fused counterparts (N = 24, 6 rats per group). A significant compartment × stress interaction was replicated for IL-1β (F2,60 = 31.28, p < 0.0001) and post hoc analyses revealed that rats exposed to 100 shocks had elevated concentrations of IL-1β relative to non-stressed controls in subcutaneous (p < 0.0001) but not visceral WAT (p = 0.54). Across compartments, saline perfused rats demonstrated higher concentrations of IL-1β in their WAT than non-perfused rats (main effect of perfusion F1,60 = 5.19, p = 0.03). Perfusion, however, did not affect the IL-1β response to acute stress (perfusion × stress, F1,60 = 0.57, p = 0.45) suggesting that circulating cytokines (IL-1β) did not significantly contribute to the concentration of cytokines measured in WAT. In contrast to Fig. 4(B), however, stress did not significantly affect intraperitoneal WAT IL-1β concentrations due to an abnormally high concentration of IL-1β found in the intraperitoneal WAT of the perfused, non-stressed group. We hypothesize that the aberrantly high basal concentrations of IL-1β in the non-stressed per-fused may be the result of tissue damage or cellular stress to the intraperitoneal WAT during the transcardial perfusion procedure.
4. Discussion
The purpose of the present study was to investigate the impact of voluntary habitual exercise on stress-evoked DAMP and inflammatory protein responses in subcutaneous, intraperitoneal, and visceral WAT compartments of non-obese rats. The current study demonstrates that DAMP and inflammatory protein responses to acute stress and the impact of wheel running on these responses are unique to the inflammatory protein measured and the anatomical compartment of WAT. In addition, WAT compartments of physically active rats expressed greater pro- and anti-inflamma-tory protein responses to acute stress, resulting in an overall reduction in the inflammatory status of WAT. These changes could function in concert to optimize stress-induced augmentation of innate immune function (Fleshner et al., 2002).
Physically active rats have facilitated stress-evoked innate immune function via increased expression of Hsp72, a DAMP reported to promote immune responses and improve host defense (Campisi et al., 2003a; Fleshner et al., 2002, 2003). Physically active animals also release more nitric oxide and inflammatory cytokines following eHsp72 stimulation compared to sedentary rats (Campisi and Fleshner, 2003). The results presented here corroborate that rats given voluntary access to a running wheel for 6 weeks (RUN) prior to 100 inescapable tail shocks (100IS) have higher plasma concentrations of extracellular Hsp72 (eHsp72, Fig 3(A)) than sedentary rats exposed to the same stressor. In addition we report for the first time that physically active rats exposed to acute stress have increases in WAT-concentrations of Hsp72 compared to stressed, sedentary (SED) rats (Fig. 4(A)) suggesting a novel organ through which habitual exercise facilitates stress-induced augmentation of innate immune function. Moreover, the WAT of RUN rats compared to SED rats had greater cytokine responses to acute stress (Figs. 5 and 6) consistent with the hypothesis that Hsp72 may be a key regulator of cytokine expression in stressed WAT.
Studies by Dhabhar et al. have established that one important immunoprotective function of the acute stress response involves the expression of proteins that promote leukocyte trafficking and cytokine gene expression at the site of antigen entry (Dhabhar, 2002, 2003). Fig. 4(B) establishes that acute stress evokes the expression of MCP-1, a key leukocyte attractant in subcutaneous and intraperitoneal WAT and voluntary habitual exercise increases stress-evoked MCP-1 within intraperitoneal WAT. Considering the anatomical locations of subcutaneous and intraperitoneal WAT (intraperitoneal WAT is comprised of the epididymal depot within the peritoneal cavity and the retroperitoneal WAT depots that lay just under the muscular layer of the abdomen), these compartments are highly prone to injury during an attack. Rats challenged subcutaneously with bacteria immediately following acute stress clear the pathogen faster than their unstressed counterparts (Deak et al., 1999) and physically active stressed rats resolve their bacterial inflammation 2 days faster than sedentary stressed rats (Flesh-ner et al., 2002). Thus, physically active animals may be able to better attract leukocytes to potential sites of injury and infection because they have an even greater MCP-1 response than sedentary, stressed rats. Compartment-specific increases in stress-evoked MCP-1 may therefore contribute to the augmented innate responses (i.e. bacterial clearance) demonstrated in physically active and/or acutely stressed rats (Campisi et al., 2012; Fleshner et al., 2002; Nickerson et al., 2005).
While the inflammatory response to intense acute stress can potentiate host defense and facilitate the response to- and recovery from injury and infection, if it lacks resolution or is repeatedly induced, stress-evoked inflammatory responses can result in tissue destruction and cellular impairments that have severe health consequences (Black, 2006; Fleshner et al., 2011; Rock et al., 2010; Uchida et al., 2012). IL-1β and TNF-α act as pro-inflammatory cytokines while IL-10 is a master anti-inflammatory cytokine that encourages inflammatory resolution by blocking signals that initiate the synthesis of pro-inflammatory proteins (Schottelius et al., 1999). Interestingly, compared to SED rats, WAT IL-1β protein expression is ubiquitously increased (Fig. 5(B)), stress-evoked TNF-α is elevated (Fig. 5(A)) and basal and stress-evoked WAT IL-10 is markedly increased in all of the WAT compartments of RUN rats (Fig. 6(B)). As Fig. 7(A) and (B) demonstrate, through increases in IL-10, voluntary habitual exercise reduces the degree to which stress increases the inflammatory status of WAT (as determined by the ratio of pro-/anti-inflammatory proteins). Voluntary habitual exercise may therefore optimize the inflammatory response to acute stress by constraining increases in inflammatory status leading to more rapid resolution of either bacterial (Fleshner et al., 2002) or a DAMP-evoked inflammation. Higher concentrations of WAT IL-10 found in RUN rats (Fig. 6(B)) may also help to protect against the negative inflammatory consequences of repeated stressor exposure linked to the development of visceral obesity (Speaker and Fleshner, 2012).
In addition to priming innate immunity an important feature of the acute stress response involves the mobilization of energy stores. Inflammatory proteins not only participate in the regulation of immunity but also in many other physiological responses, especially those related to adipose metabolism. TNF-α, IL-1β, and IL-6, for example, promote the release of free fatty acids from WAT (e.g. lipolysis)(Doerrler et al., 1994; Hardardottir et al., 1992; van Hall et al., 2003). Traditionally WAT-related inflammatory proteins have been investigated in the context of obesity where they have been reported to induce macrophage infiltration, dysregulate adi-pose metabolism and contribute to a state of chronic inflammation (Balistreri et al., 2010). In the case of expression during acute stress, however, these proteins may serve adaptive or beneficial functions. Figs. 5(B) and 6(A) reveal that exercise increases IL-1β and potentiates stress-evoked IL-6. Reports have demonstrated that sustained activation of the sympathetic nervous system results in a down regulation of beta-adrenergic receptors (Hadcock and Malbon, 1988; Jockers et al., 1999) and norepinephrine depletion (Kennedy et al., 2005). Keeping in mind that lipolysis is predominantly regulated by catecholamines (Bartness and Song, 2007), rats exposed to 100 min of tail-shock stress may suffer from a reduced ability to meet the lipolytic demands of stress. Because IL-1β and IL-6 can promote lipolysis, the expression of these cytokines in stressed WAT, therefore, may help to sustain lipolysis in the face of stress-induced reductions in catecholamine concentrations and beta-receptor down regulation. Physically active rats therefore may be better suited to meet the energy demands of sustained acute stress. Given the importance of mobilizing energy stores throughout the stress response and the role of WAT as an energy reservoir, this study suggests that stress-evoked inflamma-tory protein expression in non-obese WAT serves adaptive roles that facilitate both immune and metabolic stress responses.
Finally, Fig. 8 demonstrates that perfusion of WAT with saline did not impact the increase in IL-1β concentrations after exposure to acute stress suggesting circulating cytokines (IL-1β) do not significantly contribute to the concentration of cytokines measured in WAT. While these data are sufficient to conclude that circulating cytokines (IL-1β) do not significantly contribute toward WAT values, our work cannot establish the directional relationship between WAT and circulating IL-1β or any of the other plasma cytokines for that matter. Moreover, these results enforce the concept that blood/plasma cytokines are not conclusive biomarkers of inflammation because inflammation is indeed a tissue response and tissue responses do not necessarily mirror blood/plasma responses. Additionally, body weight gain and omental fat volume were reduced in rats given access to a running wheel suggesting RUN rats had less total body fat than sedentary rats yet stress-evoked inflammatory protein expression was increased in the WAT of RUN rats (Fig. 1(B,C)). This observation suggests that the WAT of RUN rats may either be more densely populated with stress-sensitive, inflammatory protein producing cells or contain cells that are more sensitive to stress-associated signals that evoke the expression of inflammatory proteins. Further studies, however, are required to elucidate cellular adaptations that occur in the WAT of physically active animals.
Fig. 8.
Effect of saline tissue perfusion on interleukin-1beta (IL-1β) response to acute tail shock stress in non-obese white adipose tissue (WAT). Shown is the mean (+ SEM) concentration of interleukin-1beta (IL-1β) in the WAT of rats exposed to 100 inescapable tail shocks (IS) followed immediately by saline tissue perfusion or rapid decapitation (non-perfused). ***P < 0.0001 (Subcutaneous 0IS vs. Subcutaneous 100IS), *P < 0.05 (Intraperitoneal 0IS vs. Intraperitoneal 100IS); two factor ANOVA with Bonferroni post hoc analysis.
In summary, the findings of this study demonstrate for the first time that exposure to acute tail shock increases Hsp72, cytokines, and chemokine expression in WAT of non-obese rats. The DAMP and inflammatory protein responses to acute stress within WAT are increased after 6 weeks of voluntary habitual exercise and occur in a compartment-specific manner. Because stress augments innate immune function more in physically active rats than sedentary rats, increases in stress evoked DAMPs and inflammatory proteins within WAT may optimize the adaptive features of the acute stress response for host defense. Given the functions of stress-induced inflammatory proteins in non-obese WAT remain unclear, future investigation into the functions of transient (adaptive) and sustained (maladaptive) cytokine expression as well as the mechanisms responsible for the modulation of these responses after voluntary habitual exercise are needed.
Supplementary Material
Acknowledgments
This research was supported by grants awarded to MF by the Army Research Office (#W911NF-10-1-0050) and the National Science Foundation (#IOS 1022451).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbi.2013.10.028.
References
- Abate N, Garg A. Heterogeneity in adipose tissue metabolism: causes, implications and management of regional adiposity. Prog. Lipid. Res. 1995;34:53–70. doi: 10.1016/0163-7827(94)00006-8. [DOI] [PubMed] [Google Scholar]
- Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 2011;52:480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J. Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J. Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- Batra A, Siegmund B. The role of visceral fat. Dig. Dis. 2012;30:70–74. doi: 10.1159/000335722. [DOI] [PubMed] [Google Scholar]
- Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med. Hypotheses. 2006;67:879–891. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L, Masini CV, Babb JA, Greenwood BN, Fleshner M, Day HE. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J. Neuroendocrinol. 2010;22:872–888. doi: 10.1111/j.1365-2826.2010.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Fleshner M. Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. J. Appl. Physiol. 2003;94:43–52. doi: 10.1152/japplphysiol.00681.2002. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003a;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, Smith TP, Fleshner M. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003b;284:R520–530. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- Campisi J, Sharkey C, Johnson JD, Asea A, Maslanik T, Bernstein-Hanley I, Fleshner M. Stress-induced facilitation of host response to bacterial challenge in F344 rats is dependent on extracellular heat shock protein 72 and independent of alpha beta T cells. Stress. 2012;15:637–646. doi: 10.3109/10253890.2011.653596. [DOI] [PubMed] [Google Scholar]
- Caspar-Bauguil S, Cousin B, Bour S, Castiella L, Penicaud L, Carpene C. Adipose tissue lymphocytes: types and roles. J. Physiol. Biochem. 2009;65:423–436. doi: 10.1007/BF03185938. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Fleshner M, Watkins LR, Maier SF. Acute stress may facilitate recovery from a subcutaneous bacterial challenge. Neuroimmunomodulation. 1999;6:344–354. doi: 10.1159/000026394. [DOI] [PubMed] [Google Scholar]
- Dempsey LA. Leptin and mTor. Nat. Immunol. 2012;13:939. [Google Scholar]
- Dhabhar FS. Stress-induced augmentation of immune function – the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav. Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Stress, leukocyte trafficking, and the augmentation of skin immune function. Ann. N. Y. Acad. Sci. 2003;992:205–217. doi: 10.1111/j.1749-6632.2003.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Doerrler W, Feingold KR, Grunfeld C. Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine. 1994;6:478–484. doi: 10.1016/1043-4666(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Brennan FX, Nguyen K, Watkins LR, Maier SF. RU-486 blocks differentially suppressive effect of stress on in vivo anti-KLH immunoglobulin response. Am. J. Physiol. 1996;271:R1344–1352. doi: 10.1152/ajpregu.1996.271.5.R1344. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Campisi J, Deak T, Greenwood BN, Kintzel JA, Leem TH, Smith TP, Sorensen B. Acute stressor exposure facilitates innate immunity more in physically active than in sedentary rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1680–1686. doi: 10.1152/ajpregu.00661.2001. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Campisi J, Johnson JD. Can exercise stress facilitate innate immunity? A functional role for stress-induced extracellular Hsp72. Exerc. Immunol. Rev. 2003;9:6–24. [PubMed] [Google Scholar]
- Fleshner M, Maier SF, Lyons DM, Raskind MA. The neurobiology of the stress-resistant brain. Stress. 2011;14:498–502. doi: 10.3109/10253890.2011.596865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn KN. Visceral fat and insulin resistance – causative or correlative? Br. J. Nutr. 2000;83(Suppl 1):S71–77. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Simoes PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graca Naffah-Mazzacoratti M, Arida RM. Exercise-induced hippocampal anti-inflammatory response in aged rats. J. Neuroinflammation. 2013;10:61. doi: 10.1186/1742-2094-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120:269–281. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Hadcock JR, Malbon CC. Down-regulation of beta-adrenergic receptors: agonist-induced reduction in receptor mRNA levels. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5021–5025. doi: 10.1073/pnas.85.14.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardardottir I, Doerrler W, Feingold KR, Grunfeld C. Cytokines stimulate lipolysis and decrease lipoprotein lipase activity in cultured fat cells by a prostaglandin independent mechanism. Biochem. Biophys. Res. Commun. 1992;186:237–243. doi: 10.1016/s0006-291x(05)80798-3. [DOI] [PubMed] [Google Scholar]
- Jockers R, Angers S, Da Silva A, Benaroch P, Strosberg AD, Bouvier M, Marullo S. Beta(2)-adrenergic receptor down-regulation. Evidence for a pathway that does not require endocytosis. J. Biol. Chem. 1999;274:28900–28908. doi: 10.1074/jbc.274.41.28900. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Kennedy SL, Smith TP, Fleshner M. Resting cellular and physiological effects of freewheel running. Med. Sci. Sports Exerc. 2005;37:79–83. doi: 10.1249/01.mss.0000150080.60101.25. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends. Pharmacol. Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira FS, Rosa JC, Yamashita AS, Koyama CH, Batista ML, Jr., Seelaender M. Endurance training induces depot-specific changes in IL-10/TNF-alpha ratio in rat adipose tissue. Cytokine. 2009;45:80–85. doi: 10.1016/j.cyto.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Mahaffey L, Tannura K, Beninson LA, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav. Immun. 2013;28:54–62. doi: 10.1016/j.bbi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Benninson LA, Ursell L, Greenwood BN, Knight R, Fleshner M. Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1b and IL-18 but not IL-6, IL-10 or MCP-1. PLoS One. 2012;7:e50636. doi: 10.1371/journal.pone.0050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R484–489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J. Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson M, Elphick GF, Campisi J, Greenwood BN, Fleshner M. Physical activity alters the brain Hsp72 and IL-1beta responses to peripheral E. coli challenge. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1665–1674. doi: 10.1152/ajpregu.00601.2004. [DOI] [PubMed] [Google Scholar]
- O'Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Pond CM. Adipose tissue: quartermaster to the lymph node garrisons. Biologist. 2000;47:147–150. [PubMed] [Google Scholar]
- Popi AF, Lopes JD, Mariano M. Interleukin-10 secreted by B-1 cells modulates the phagocytic activity of murine macrophages in vitro. Immunology. 2004;113:348–354. doi: 10.1111/j.1365-2567.2004.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu. Rev. Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa Neto JC, Lira FS, Oyama LM, Zanchi NE, Yamashita AS, Batista ML, Jr., Oller do Nascimento CM, Seelaender M. Exhaustive exercise causes an anti-inflammatory effect in skeletal muscle and a pro-inflammatory effect in adipose tissue in rats. Eur. J. Appl. Physiol. 2009;106:697–704. doi: 10.1007/s00421-009-1070-1. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr. Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J. Biol. Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Feng NG, Bonneau RH, Allen CM, Huneycutt BS, Glaser R. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J. Neuroimmunol. 1991;31:245–255. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- Speaker KJ, Fleshner M. Interleukin-1 beta: a potential link between stress and the development of visceral obesity. BMC Physiol. 2012;12:8. doi: 10.1186/1472-6793-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Takeshita K, Yamamoto K, Kikuchi R, Nakayama T, Nomura M, Cheng XW, Egashira K, Matsushita T, Nakamura H, Murohara T. Stress augments insulin resistance and prothrombotic state: role of visceral adipose-derived monocyte chemoattractant protein-1. Diabetes. 2012;61:1552–1561. doi: 10.2337/db11-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J. Clin. Endocrinol. Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin. Chim. Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.