Abstract
Objective
The aim of this guideline is to assist FPs and other primary care providers with recognizing features that should raise their suspicion about the presence of prostate cancer in their patients.
Composition of the committee
Committee members were selected from among the regional primary care leads from the Cancer Care Ontario Provincial Primary Care and Cancer Network and from among the members of the Cancer Care Ontario Genitourinary Cancer Disease Site Group.
Methods
This guideline was developed through systematic review of the evidence base, synthesis of the evidence, and formal external review involving Canadian stakeholders to validate the relevance of recommendations.
Report
Evidence-based guidelines were developed to improve the management of patients presenting with clinical features of prostate cancer within the Canadian context.
Conclusion
These guidelines might lead to more timely and appropriate referrals and might also be of value for informing the development of prostate cancer diagnostic programs and for helping policy makers to ensure appropriate resources are in place.
Résumé
Objectif
Ces lignes directrices ont pour but d’aider les médecins de famille et les autres professionnels des soins primaires à reconnaître les caractéristiques qui devraient les faire soupçonner la présence d’un cancer de la prostate chez leurs patients.
Composition du comité
Les membres du comité ont été choisis parmi les directeurs des soins primaires régionaux du Réseau provincial des soins primaires et de la lutte contre le cancer d’Action Cancer Ontario et les membres du Groupe sur le siège de la maladie - Cancers génito-urinaires d’Action Cancer Ontario.
Méthode
L’élaboration de ces lignes directrices est fondée sur une recension systématique des données probantes, une synthèse des données scientifiques et un examen externe formel impliquant des intervenants canadiens pour valider la pertinence des recommandations.
Rapport
Des lignes directrices fondées sur des données probantes ont été élaborées pour améliorer la prise en charge des patients présentant des caractéristiques cliniques de cancer de la prostate, et ce, dans le contexte canadien.
Conclusion
Ces lignes directrices pourraient entraîner des demandes de consultation plus appropriées et en temps plus opportuns. Elles pourraient aussi être utiles pour éclairer l’élaboration de programmes de diagnostic du cancer de la prostate et aider les décideurs à assurer la mise en place des ressources nécessaires.
Prostate cancer is the most common cancer diagnosed in men and it is the third leading cause of death due to cancer in men in Canada.1 However, in most men the disease progresses slowly over time, and the 5-year survival rate is 96% in Canada.1 Because some men are diagnosed with prostate cancer but survive unaffected, the challenge for FPs and other primary care providers (PCPs) is not only to determine when to suspect prostate cancer but also to decide, in consultation with their patients, when to refer patients for further testing.
In healthy asymptomatic men, screening for prostate cancer using prostate-specific antigen (PSA) testing might lead to diagnosis of prostate cancer disease that does not affect overall survival. Prostate cancer includes a spectrum of malignancy that ranges from low-grade indolent disease to high-grade cancers that have a propensity to spread. Organized screening programs for prostate cancer have been discouraged by experts in Canada, but despite this, opportunistic screening is very common, and current PSA testing practices have already led to large numbers of men being screened.2 Depending on the province, 35% to 75% of men aged 50 to 70 years have had at least 1 PSA test.2 The merits of screening asymptomatic men for prostate cancer are beyond the scope of this report, but what to do when there is a consequential positive result of a PSA test is included, as it is considered as a sign that raises suspicion of prostate cancer.
The aim of this guideline is to assist primary care clinicians with recognizing and managing clinical features that should raise their suspicion of prostate cancer and ultimately lead to more timely and appropriate referrals. There are no Canadian or provincial guidelines that address this. The recommendations are targeted to patients presenting in primary care settings. They are also aimed at policy makers to help ensure that resources such as prostate cancer diagnostic assessment programs (DAPs) are in place so that target wait times are achieved.
Composition of committee
In order to provide evidence-based guidance for the launch of prostate cancer DAPs in Ontario, Cancer Care Ontario’s (CCO’s) Provincial Primary Care and Cancer Network collaborated with the Program in Evidence-based Care (PEBC) to form the Prostate Cancer Referral Working Group. The working group consisted of 3 FPs (S.M.Y., P.B., C.L.), 1 radiation oncologist (A.L.), 1 urologist (A.F.), and 1 methodologist (E.T.V.). Committee members were selected from among the regional primary care leads from the Provincial Primary Care and Cancer Network and from among members of the CCO Genitourinary Cancer Disease Site Group. The work of the PEBC is supported by the Ontario Ministry of Health and Long-Term Care through CCO, and the PEBC is editorially independent from its funding source.
Methods
The guideline was developed using the methods of the practice guideline development cycle, including an environmental scan of existing guidelines, systematic review of the evidence base,3 evidence synthesis, and input from internal and external reviewers across Canada.4 Recommendations from the 2009 New Zealand Guideline Group (NZGG) and the 2005 National Institute for Health and Care Excellence (NICE) guidelines formed the foundation of our systematic review and subsequent guideline.5,6 An updated literature search of the MEDLINE and EMBASE databases using search strategies from the NZGG 2009 and NICE 2005 guidelines was conducted. Guidelines were assessed for quality using the AGREE II (Appraisal of Guidelines Research and Evaluation) tool.7,8 Systematic reviews and meta-analyses were assessed for quality using the AMSTAR (Assessment of Multiple Systematic Reviews) tool.9 Further details of the methods and findings of the systematic review are published elsewhere.3,10
The recommendations from the NZGG 2009 and NICE 2005 guidelines were considered during the development of our recommendations.5,6 The updated evidence base was also considered. The evidence base consisted mainly of cohort studies and case series. The working group held a teleconference to develop the recommendations through informal consensus. Each of the recommendations was discussed, taking into consideration any evidence found in the systematic review. The recommendations were written and approved by all members during the meeting.
Report
The following recommendations (Table 1 and Figure 1)2,5,11–13 reflect the integration of the NZGG 2009 and NICE 2005 recommendations with evidence from level I systematic reviews, level II case-control and cohort studies, and level III expert opinion by the PEBC Prostate Cancer Referral Working Group, as described below.5,6
Table 1.
Recommendations for referral of patients with suspected prostate cancer by FPs and other PCPs
| PATIENT GROUP | RECOMMENDATION | GUIDANCE FOR REFERRAL |
|---|---|---|
| Recommendation 1: actions for patients with unexplained symptoms of metastatic prostate cancer | A man ≥ 40 y should have a DRE and a PSA test if he has any unexplained symptoms suggestive of metastatic prostate cancer:
|
|
| Recommendation 2: actions for patients with LUTS | For a man presenting with LUTS (irritative and obstructive voiding symptoms), a DRE should be performed and a discussion about the benefits and risks of PSA testing should occur with the patient.* Lower urinary tract infection should be excluded before PSA testing, especially in men presenting with LUTS. The PSA test should be postponed for at least 1 mo after treatment of a proven urinary tract infection |
|
| Recommendation 3: actions for patients with incidental elevated PSA results | For incidental elevated age-based PSA findings, DRE should be performed for all patients. Rule out other reasons for elevated PSA values (age-related hypertrophy [BPH], infection, inflammation, prostatitis, recent sexual activity, etc). Repeat PSA testing if unsure | Guidance for referral b through f of recommendation 2 should be followed |
BPH—benign prostatic hypertrophy, DAP—diagnostic assessment program, DRE—digital rectal examination, LUTS-lower urinary tract symptoms, NZGG—New Zealand Guidelines Group, PCP—primary care provider, PSA—prostate-specific antigen.
For information of discussing the benefits and risks of PSA testing with patients, refer to the individual risk assessment from the Canadian Partnership Against Cancer PSA Toolkit.2
An example of age-based PSA values (upper limit of normal) from the NZGG is as follows: 40–50 y, 2.5 µg/L; 50–60 y, 3.5 µg/L; 60–70 y, 4.5 µg/L; ≥ 70 y, 6.5 µg/L.5 Differences in PSA assay can lead to differences in age-based ranges reported by laboratories.
Nomograms are available from http://sunnybrook.ca/content/?page=OCC_prostateCalc (includes the ratio of free PSA, unbound to serum proteins, to total PSA because this ratio is decreased in men with prostate cancer; in some cases patients might be charged a laboratory fee for this value; if this ratio is not determined, then a value of 0.1 can be entered into the risk calculator)11; http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp12; or www.prostatecancer-riskcalculator.com/assess-your-risk-of-prostate-cancer.13 If a nomogram is not used, then the patient should receive a nonurgent referral and expect a consultation with a urologist or a prostate cancer DAP within 4 wk.
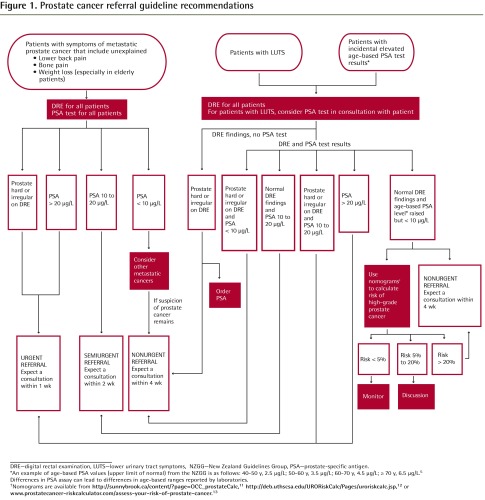
Figure 1.
Prostate cancer referral guideline recommendations
DRE—digital rectal examination, LUTS—lower urinary tract symptoms, NZGG—New Zealand Guidelines Group, PSA—prostate-specific antigen.
*An example of age-based PSA values (upper limit of normal) from the NZGG is as follows: 40–50 y, 2.5 μ/L; 50–60 y, 3.5 μ/L; 60–70 y, 4.5 μ/L; ≥ 70 y, 6.5 μ/L.5
Differences in PSA assay can lead to differences in age-based ranges reported by laboratories.
†Nomograms are available from http://sunnybrook.ca/content/?page=OCC_prostateCalc,11http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp,12 or www.prostatecancer-riskcalculator.com/assess-your-risk-of-prostate-cancer.13
Target population
Adult male patients presenting in primary care settings with signs, including incidental PSA test results (defined as results not ordered by the attending FP or other PCP), or symptoms suggestive of prostate cancer comprise the target population. This guideline does not provide recommendations for screening healthy patients or for opportunistic PSA testing.
Intended users
This guideline is targeted to FPs, GPs, emergency department physicians, other PCPs (nurse practitioners, registered nurses, and physician assistants), and urologists. For the purposes of this document, we have referred to FPs, GPs, emergency department physicians, and other PCPs as FPs and other PCPs. The guidelines are also intended for policy makers to help ensure that resources are in place so that target wait times are achieved. They are intended to coincide with the introduction of prostate cancer DAPs in Ontario. The DAPs provide a single point of referral, coordination of care using a clinical navigator, fast tracking of diagnostic tests, and a multidisciplinary team approach. They are an Ontario-wide strategic priority designed to improve patient access and outcomes, as outlined in the Ontario Cancer Plan, 2005 to 2011, and 2011 to 2014.14
Key evidence and justification
All recommended wait times were based on consensus of the working group. The Canadian Association of Radiation Oncology recommended a wait time from referral to consultation with a radiation oncologist of no longer than 10 working days.15 This was taken into consideration when developing the wait times in this guideline.
Recommendation 1: actions for patients with unexplained symptoms of metastatic prostate cancer
Two studies suggested that digital rectal examinations (DREs) performed by FPs might be useful in identifying patients who should be referred,16,17 and 4 studies suggested that PSA values were the strongest predictors of prostate cancer, compared with lower urinary tract symptoms (LUTS) and DRE findings, with positive predictive values ranging from 34.3% to 47%.16,18–20 The working group chose to endorse the recommendations from the NICE 2005 and NZGG 2009 guidelines to recommend DRE and PSA testing for all patients with symptoms of metastatic prostate cancer.5,6 An age threshold of 40 years was included at the suggestion of the internal reviewers owing to the few cases of prostate cancer in men younger than 40 years of age in Canada.1 The working group did not think it necessary for men with erectile dysfunction to undergo DRE and PSA testing and therefore excluded it as a symptom of metastatic prostate cancer. This is consistent with the NZGG 2009 guideline but in contrast to the NICE 2005 guideline.5,6 The working group also excluded unexplained hematuria as a symptom of metastatic prostate cancer because, although it can be associated with advanced prostate cancer, the working group believed that by far most men with gross hematuria usually have different underlying causes such as benign prostate hyperplasia, bladder or renal cancer, stones, or infections. The working group believed hematuria required urologic assessment but that it should not be part of a prostate cancer care algorithm.
The cutoff values, listed for guidance for referral recommendations a through c in Table 1,2,5,11–13 of 10 and 20 µg/L were taken from the D’Amico classification system for categorizing patients at low risk (clinical stage T1 to T2a, Gleason score < 7, and PSA level ≤ 10 µg/L), intermediate risk (clinical stage T2b, Gleason score of 7, or PSA level > 10 and ≤ 20 µg/L) or high risk (clinical stage T2c, PSA level > 20 µg/L, or Gleason score > 7) of prostate cancer.21,22 Although this classification system was not developed in the primary care population, the working group chose to include it owing to its wide use in classifying the risk of prostate cancer; also, these thresholds provide guidance for FPs in determining their course of action.
Recommendation 2: actions for patients with LUTS
The NICE 2005 guidelines recommended performing DRE and PSA testing for all men with LUTS, and the NZGG 2009 guidelines recommended these tests only for older men with LUTS.5,6 The working group chose to recommend DRE for all men with LUTS and PSA testing for selected patients with LUTS, following discussion with the patient about the benefits and harms of PSA testing. The limited evidence from the systematic review suggested that men with LUTS might not be at any higher risk of prostate cancer or have a poorer prognosis than asymptomatic men.18,23 The Canadian Urological Association’s benign prostatic hyperplasia guideline for men presenting with LUTS recommended DRE for all men and PSA testing for selected patients.24 The working group chose to be consistent with this guideline. The working group referred to the individual risk assessment developed by the Canadian Partnership Against Cancer as a guide to which patients should be given PSA testing; the assessment includes criteria similar to those of the Canadian Urological Association, and the document describes the benefits and harms of PSA testing.2 The working group also endorsed the recommendations from the NICE 2005 and NZGG 2009 guidelines to exclude urinary tract infection before PSA testing and to postpone PSA testing for at least 1 month after treatment of a confirmed urinary tract infection.5,6
Recommendation a in Table 12,5,11–13 was endorsed from the NICE 2005 guideline.6 The age-based PSA values in recommendation b were endorsed from the NZGG 2009 guidelines.5 For recommendation f, number i, a cutoff risk value of 5% was chosen because in Ontario the hospital admission rate for urological complications within 30 days of transrectal ultrasound–guided biopsy was found to be 4.1% in 2005.25 The working group decided to use 5% as a cutoff to separate patients into a higher-risk category because for these patients the risk of high-grade prostate cancer would be higher than the risk of complications from transrectal ultrasound–guided biopsy.
The working group thought that nomograms might be useful in the primary care setting to assist FPs and other PCPs in their management of patients. Nomograms are prediction tools that incorporate numerous factors and they can be used to enhance PSA and DRE results and help decide which treatment approaches will result in the greatest benefit for men at various stages of prostate cancer. The prostate risk calculator developed at Sunnybrook Hospital in Toronto, Ont,11 showed a net benefit (the relative value of false-positive versus false-negative results) when a risk of 15% for aggressive prostate cancer was chosen as a threshold to agree to a biopsy.26,27 Based on the consensus of the working group a conservative cutoff risk value of 20% was chosen and included in recommendation f, numbers ii and iii.
Recommendation 3: actions for patients with incidental elevated PSA test results
Although this guideline excludes patients in screening programs, the working group thought that FPs and other PCPs needed guidance on how to manage patients with incidental PSA test results, a frequently encountered occurrence in practice. Opportunistic screening has been excluded because it is beyond the scope of this guideline.
The working group believed that if an incidental PSA test result was abnormal, then standard practice would be to perform a DRE. A hard or irregular prostate on DRE might increase the urgency of referral.
Qualifying statements
Individuals with enlarged, smooth prostates were excluded as beyond the scope of this guideline because such enlargement was not considered to be a sign of prostate cancer. Also, although a rising PSA level could be considered a sign of prostate cancer, the working group believed the guideline was sufficiently thorough to include most possible scenarios for prostate cancer using the absolute PSA values.
Conclusion
There was no strong evidence to suggest that LUTS were good predictors of prostate cancer. Nor was there strong evidence examining the factors that predict metastatic prostate cancer. There was some evidence that suggested DRE performed by FPs and PSA levels were good predictors of prostate cancer. However, most recommendations were based on evidence from retrospective case series and on the expert opinions of the NICE 2005 and NZGG 2009 guideline groups and the Prostate Cancer Referral Working Group.5,6
This guideline might guide program development for DAPs for patients with suspicious prostate cancer and help policy makers ensure that resources are in place so that target wait times can be achieved.
EDITOR’S KEY POINTS
Prostate cancer is the most common cancer diagnosed in men and it is the third leading cause of death due to cancer in men in Canada. These guidelines, based on a systematic review of the literature and expert opinion, aimed to update previous guidelines and assist primary care providers with providing appropriate referrals for their patients with suspected prostate cancer.
A man 40 years of age or older should have a digital rectal examination (DRE) and a prostate-specific antigen (PSA) test if he has any unexplained symptoms suggestive of metastatic prostate cancer. For a man presenting with lower urinary tract symptoms, a DRE should be performed and a discussion about the benefits and risks of PSA testing should occur with the patient, and urinary tract infection should be ruled out. For patients with incidental elevated age-based PSA findings, DRE should be performed for all patients. Other reasons for elevated PSA values should be ruled out.
The guidelines provide recommendations for referral based on DRE findings and PSA test results. All referred patients should expect to be seen by a urologist or prostate cancer diagnostic assessment program within 1 to 4 weeks, depending on the urgency of referral.
POINTS DE REPÈRE DU RÉDACTEUR
Le cancer de la prostate est le cancer le plus souvent diagnostiqué chez les hommes et il vient au troisième rang des causes de décès dus au cancer chez les hommes au Canada. Ces lignes directrices, fondées sur un examen systématique de la littérature médicale et des opinions d’experts, visaient à mettre à jour les lignes directrices antérieures et à aider les professionnels des soins primaires à présenter des demandes de consultation appropriées pour leurs patients soupçonnés d’avoir un cancer de la prostate.
Les hommes de 40 ans et plus devraient subir un examen rectal digital (ERD) et une analyse de l’antigène spécifique de la prostate (ASP) s’ils souffrent d’un symptôme inexpliqué suggérant un cancer métastasique de la prostate. Chez les hommes qui présentent des symptômes aux voies urinaires inférieures, il faut procéder à un ERD et discuter avec le patient des avantages et des risques d’une analyse de l’ASP, et exclure la possibilité d’une infection des voies urinaires. Pour tous les patients dont les résultats du test de l’ASP sont fortuitement élevés en fonction de l’âge, il faut procéder à un ERD. Il faut exclure la possibilité d’autres causes de valeurs d’ASP élevées.
Les lignes directrices présentent des recommandations concernant les demandes de consultation en fonction des constatations à l’ERD et des résultats de l’analyse de l’ASP. Tous les patients référés devraient s’attendre à être vus par un urologue ou un programme d’évaluation diagnostique du cancer de la prostate dans un délai de 1 à 4 semaines selon l’urgence de la consultation.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the literature review and interpretation, and to preparing the report for submission.
Competing interests
None declared
References
- 1.What is cancer? Toronto, ON: Canadian Cancer Society; 2011. Cancer.ca [website] Available from: www.cancer.ca/Canada-wide/About%20cancer/Cancer%20statistics.aspc?sc_lang=en. Accessed 2011 Dec 2. [Google Scholar]
- 2.Canadian Partnership Against Cancer, Screening Action Group. PSA toolkit: PSA screening and testing for prostate cancer. Toronto, ON: Canadian Partnership Against Cancer; 2009. Available from: www.cancerview.ca/idc/groups/public/documents/webcontent/screen_panel_psa_tool.pdf. Accessed 2011 Feb 8. [Google Scholar]
- 3.Young SM, Bansal P, Vella ET, Finelli A, Levitt C, Loblaw A. Systematic review of clinical features of suspected prostate cancer in primary care. Can Fam Physician. 2015;61:e26–35. [PMC free article] [PubMed] [Google Scholar]
- 4.Browman GP, Levine MN, Mohide EA, Hayward RS, Pritchard KI, Gafni A, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13(2):502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 5.New Zealand Guidelines Group. Suspected cancer in primary care: guidelines for investigation, referral and reducing ethnic disparities. Wellington, NZ: New Zealand Guidelines Group; 2009. [Google Scholar]
- 6.National Institute for Health and Care Excellence. Referral guidelines for suspected cancer. NICE guideline CG27. London, UK: National Institute for Health and Care Excellence; 2005. [Google Scholar]
- 7.SAGE: standards and guidelines evidence. Toronto, ON: Partnership Against Cancer; 2012. Cancerview.ca [website] Available from: www.cancerview.ca. Accessed 2012 Feb 23. [Google Scholar]
- 8.The AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young S, Bansal P, Vella E, Finelli A, Levitt C, Loblaw A, Prostate Cancer Referral Expert Panel . Referral of suspected prostate cancer by family physicians and other primary care providers. Program in Evidence-based Care Evidence-based guideline No. 24-3. Toronto, ON: Cancer Care Ontario; 2012. [Google Scholar]
- 11.Sunnybrook Health Sciences Centre [website] Prostate risk calculator. Toronto, ON: Sunnybrook Health Sciences Centre; Available from: http://sunnybrook.ca/content/?page=OCC_prostateCalc. Accessed 2014 Dec 8. [Google Scholar]
- 12.UT Health Science Center San Antonio [website] Individualized risk assessment of prostate cancer. San Antonio, TX: UT Health Science Center San Antonio; 2014. Available from: http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp. Accessed 2014 Dec 8. [Google Scholar]
- 13.Kranse R, Roobol MJ, Schröder FH, Steyerberg EW. Your prostate cancer risk calculator. Rotterdam, Netherlands: SWOP—The Prostate Cancer Research Foundation; 2014. Available from: www.prostatecancer-riskcalculator.com/assess-your-risk-of-prostate-cancer. Accessed 2014 Dec 8. [Google Scholar]
- 14.Cancer Care Ontario [website] Diagnostic assessment programs. Toronto, ON: Cancer Care Ontario; 2011. Available from: www.cancercare.on.ca/cms/one.aspx?objectId=8133&contextId=1377. Accessed 2012 Feb 23. [Google Scholar]
- 15.Manpower and Standards of Care in Radiation Oncology Committee. Definition of RT waiting. Ottawa, ON: Canadian Association of Radiation Oncology; 2000. Available from: www.caro-acro.ca/Assets/Publications/Manpower+and+Standards+of+Care+in+Radiation+Oncology+Committee+-+Definition+of+RT+Waiting+-+September+2000.pdf?method=1. Accessed 2012 Feb 23. [Google Scholar]
- 16.Hamilton W, Sharp DJ, Peters TJ, Round AP. Clinical features of prostate cancer before diagnosis: a population-based, case-control study. Br J Gen Pract. 2006;56(531):756–62. [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan MR, O’Daly BJ, O’Brien MF, Gardner S, Lennon G, Mulvin DW, et al. The value of appropriate assessment prior to specialist referral in men with prostatic symptoms. Ir J Med Sci. 2009;178(3):281–5. doi: 10.1007/s11845-009-0337-1. [DOI] [PubMed] [Google Scholar]
- 18.Gjengstø P, Eide J, Frugard J, Bakke A, Hoisaeter PA. The potentially curable prostate cancer patient and the pathways leading to diagnosis and treatment. Scand J Urol Nephrol. 2004;38(1):15–8. doi: 10.1080/00365590310019990. [DOI] [PubMed] [Google Scholar]
- 19.Hawary AM, Warburton HE, Brough RJ, Collins GN, Brown SC, O’Reilly PH, et al. The ‘2-week wait’ rule for referrals for suspected urological cancers— urgent need for refinement of criteria. Ann R Coll Surg Engl. 2008;90(6):517–22. doi: 10.1308/003588408X301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell CS, Fielding AM, Rosser K, Ames AC, Vaughton KC. Prostate specific antigen—a screening test for prostatic cancer? Br J Urol. 1989;64(5):504–6. doi: 10.1111/j.1464-410x.1989.tb05287.x. [DOI] [PubMed] [Google Scholar]
- 21.D’Amico AV, Cote K, Loffredo M, Renshaw AA, Schultz D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20(23):4567–73. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 23.Borre M. Screening by lower urinary tract symptoms vs asymptomatic prostate-specific antigen levels leading to radical prostatectomy in Danish men: tumour characteristics and treatment outcome. BJU Int. 2009;104(2):205–8. doi: 10.1111/j.1464-410X.2008.08306.x. [DOI] [PubMed] [Google Scholar]
- 24.Nickel JC, Mendez-Probst CE, Whelan TF, Paterson RF, Razvi H. 2010 update: guidelines for the management of benign prostatic hyperplasia. Can Urol Assoc J. 2010;4(5):310–6. doi: 10.5489/cuaj.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183(3):963–8. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 26.Nam RK, Kattan MW, Chin JL, Trachtenberg J, Singal R, Rendon R, et al. Prospective multi-institutional study evaluating the performance of prostate cancer risk calculators. J Clin Oncol. 2011;29(22):2959–64. doi: 10.1200/JCO.2010.32.6371. [DOI] [PubMed] [Google Scholar]
- 27.Nam RK, Toi A, Klotz LH, Trachtenberg J, Jewett MA, Appu S, et al. Assessing individual risk for prostate cancer. J Clin Oncol. 2007;25(24):3582–8. doi: 10.1200/JCO.2007.10.6450. [DOI] [PubMed] [Google Scholar]