Abstract
Objective
To determine features of eyelid lesions most predictive of malignancy, and to design a key to assist general practitioners in the triaging of such lesions.
Design
Prospective observational study.
Setting
Department of Ophthalmology at Queen’s University in Kingston, Ont.
Participants
A total of 199 consecutive periocular lesions requiring biopsy or excision were included.
Main outcome measures
First, potential features suggestive of malignancy for eyelid lesions were identified based on a survey sent to Canadian oculoplastic surgeons. The sensitivity, specificity, and odds ratios (ORs) of these features were then determined using 199 consecutive photographed eyelid lesions of patients who presented to the Department of Ophthalmology and underwent biopsy or excision. A triage key was then created based on the features with the highest ORs, and it was pilot-tested by a group of medical students.
Results
Of the 199 lesions included, 161 (80.9%) were benign and 38 (19.1%) were malignant. The 3 features with the highest ORs in predicting malignancy were infiltration (OR = 18.2, P < .01), ulceration (OR = 14.7, P < .01), and loss of eyelashes (OR = 6.0, P < .01). The acronym LUI (loss of eyelashes, ulceration, infiltration) was created to assist in memory recall. After watching a video describing the LUI triage key, the mean total score of a group of medical students for correctly identifying malignant lesions increased from 46% to 70% (P < .001).
Conclusion
Differentiating benign from malignant eyelid lesions can be difficult even for experienced physicians. The LUI triage key provides physicians with an evidence-based, easy-to-remember system for assisting in the triaging of these lesions.
Résumé
Objectif
Déterminer les caractéristiques des lésions palpébrales les plus suggestives de malignité et créer un acronyme susceptible d’aider l’omnipraticien à faire le diagnostic différentiel de ces lésions.
Type d’étude
Étude observationnelle prospective.
Contexte
Le département d’ophtalmologie de l’Université Queen’s à Kingston, Ontario.
Participants
Un total de 199 lésions péri-oculaires nécessitant une biopsie ou une excision ont été incluses.
Principaux paramètres à l’étude
On a d’abord identifié les lésions palpébrales pouvant être suggestives de malignité à l’aide d’une enquête auprès des chirurgiens oculoplasticiens du Canada. On a ensuite établi la sensibilité, la spécificité et les rapports de cotes (RC) de ces caractéristiques à partir de 199 photos de lésions palpébrales de patients consécutifs ayant eu une biopsie ou une excision au département d’ophtalmologie. On a alors créé une triade clé à partir des caractéristiques ayant les RC les plus élevés; cette triade a ensuite fait l’objet d’un test pilote auprès d’un groupe d’étudiants en médecine.
Résultats
Sur les 199 lésions retenues, 161 étaient bénignes (80,9 %) et 38 malignes (19,1 %). Les 3 caractéristiques ayant les plus hauts RC comme prédicteurs de malignité étaient l’infiltration (RC = 18,2, P < ,01), l’ulcération (RC = 14.7, P < ,01) et la perte de cils (RC = 6,0, P < ,01). L’acronyme LUI (Loss of eyelashes, Ulceration, Infiltration) a été créé comme aide-mémoire. Le score total moyen d’un groupe d’étudiants en médecine devant identifier correctement des lésions malignes est passé de 46 % à 70 % (P < ,001) après qu’ils eurent visionné une vidéo décrivant la triade clé LUI.
Conclusion
Différencier les lésions palpébrales malignes des bénignes peut s’avérer difficile, même pour un médecin aguerri. La triade clé LUI procure au médecin un aide-mémoire simple susceptible de l’aider dans le diagnostic différentiel de ces lésions.
The presentation of new or evolving eyelid lesions frequently poses a diagnostic challenge for the examining physician. An array of both benign and malignant lesions can occur in the periocular area, and differentiation between them can often be difficult. It is estimated that malignant lesions make up 10% to 20% of all eyelid lesions,1–5 and that up to 10% of all skin cancers occur on the eyelids.6
Most malignant eyelid lesions are nonmelanoma cancers including (in order of decreasing frequency) basal cell carcinoma, squamous cell carcinoma, sebaceous cell carcinoma, and others. While nonmelanoma cancers tend less toward rapid invasion and metastasis than melanomas do, the proximity of the eyelids to delicate ocular structures can result in substantial morbidity.7 Early diagnosis and treatment is vital, as neglected or incompletely resected tumours can invade adjacent structures and threaten vision and even life. The recurrence rate of periocular nonmelanoma skin cancers is also higher than elsewhere on the body.8
Unlike many skin lesions in other areas of the body, where family physicians might feel comfortable performing biopsies, the intricate anatomy of the eyelid often calls for a referral to an ophthalmologist or plastic surgeon. Differentiating a benign lesion from a malignant one can be challenging owing to the relatively small size, variability in presentation, lack of slit-lamp magnification, and minimal ophthalmologic training in medical school.9 Even ophthalmologists, with exposure to ophthalmic plastic surgery teaching during residency, correctly identify periocular lesions in only 70% of cases.10
In 1985, Friedman et al proposed the ABCD (asymmetry, irregular borders, multiple colours, and diameter ≥ 6 mm) mnemonic for triaging melanoma.11 The ABCD (and expanded ABCDE [asymmetry, irregular borders, multiple colours, diameter ≥ 6 mm, and enlargement]) system is frequently used today and gives health care providers a convenient and easy-to-remember system for assessing risk of malignant melanoma. Currently no such aid exists for eyelid lesions, and there is a scarcity of published guidelines to assist in diagnosing and triaging lesions of the eyelid and periocular area in the primary care literature.
A set of criteria similar to the ABCD system would be of great benefit for eyelid lesions to help differentiate benign from malignant lesions, which warrant more urgent referral. The purpose of our study was to establish a set of criteria in the form of a triage key that helps predict malignant eyelid lesions on clinical examination. In addition, we conducted a pilot study to determine the utility of the triage key for medical students assessing eyelid lesions.
METHODS
Our study took place in the ophthalmic plastic surgery centre in the Department of Ophthalmology at Queen’s University in Kingston, Ont. The first phase involved identifying the clinical features that most reliably differentiate malignant from benign eyelid lesions, as well as creating a triage key of these features to aid in memory recall. The second phase involved testing the triage key in a pilot setting with medical students. Queen’s University research ethics board approval was obtained for the study.
Phase 1: creation of a system for predicting malignancy
An electronic survey was sent to members of the Canadian Society of Oculoplastic Surgery (CSOPS) asking individuals to indicate the top 3 features on clinical examination they believed were most predictive of malignant eyelid lesions. Any feature that was listed more than once by respondents was included for observation.
Concurrently, during an 18-month period all consenting patients referred to the ophthalmic plastic surgery clinic with periocular lesions had a digital photograph taken before proceeding with biopsy or removal. The pathologic diagnosis was determined by a dermatopathologist.
Following completion of our database, 3 ophthalmologists blinded to the diagnosis of the lesions were recruited to identify morphologic features within each photograph. Each observer was first presented with a series of pictures showing an example of all features. A pretest was done to verify that each physician understood the definition and appearance of the features. Following the pretest all 3 physicians independently analyzed the database of lesions to indicate which features were present with each photograph. A feature was considered to be present in a lesion if at least 2 of the 3 physicians indicated its presence.
Statistical analysis using the R statistical environment, version 2.13.2, and the epiR package, version 0.9–45, was used to derive the sensitivity, specificity, and odds ratios (ORs) for the individual features. Confidence intervals were generated using the Fisher exact test. A logistic regression model was used to isolate features or combinations of features that were statistically significant in their association with malignancy. A multivariate model was then used to identify the combination of features that was the most predictive of malignancy. A κ score was generated to determine intraobserver reliability by repeating 4 eyelid lesion photos among the series.
The primary goal of phase 1 of the study was to identify the features associated with the highest ORs and optimum sensitivity and specificity for malignancy among eyelid lesions. To optimize memory recall, we created an acronym with the selected features to use as a triage key.
Phase 2: pilot testing
In phase 2 of our study, the efficacy of our acronym in improving triaging of periocular lesions was tested in a population of medical students. A 5-minute educational video was created on the assessment of periocular lesions and differentiation between benign and malignant processes.12 The triage key created in phase 1 of our study was presented during the video.
Before the start of an ophthalmology lecture given to second-year medical students on an unrelated topic, a set of 12 eyelid lesions was shown on a screen to students. Photographs were randomly selected from a separate database. Participating students were asked to indicate which of the following management categories was most appropriate: urgent referral (1 to 2 months [ie, if a malignant lesion was suspected]), nonurgent referral (4 to 6 months [ie, for indeterminate lesions]), or observation without referral (for clearly benign lesions).
Students were then shown the 5-minute educational video.13 Following the end of the lecture, they were asked to repeat the questionnaire for the same 12 eyelid lesions. For the tests before and after the video, students were compared to the criterion standard of the principal investigator (V.K.). Eight lesions were used to calculate test scores, while 2 were identical and spaced within the sequence to assess intraobserver reliability and 2 were randomly selected but not repeated between the 2 tests to allow for variability between the tests, as the tests were performed within 1 hour of each other. The sequence of lesions was also varied between the tests to allow for variability.
Paired-sample t tests were used to compare the tests done before and after the video in terms of mean total scores, mean percentage of missed urgent referrals, and mean percentage of unnecessary referrals; κ scores were derived to determine if the 2 responses were the same in the repeated photos, regardless of whether the answer was correctly triaged.
RESULTS
Phase 1
An expert panel of 11 members of CSOPS responded to the survey, for a response rate of 34.4% (11 of 32). The survey results reported 5 morphologic features that were believed to be most indicative of malignancy: infiltration, ulceration, loss of eyelashes, telangiectasia, and pigmentation.
Over an 18-month period we photographed 199 lesions that underwent incisional or excisional biopsy. Overall, 161 (80.9%) lesions were benign and 38 (19.1%) were malignant. Of the malignant lesions, there were 34 (89.5%) basal cell carcinomas and 4 (10.5%) squamous cell carcinomas.
Table 1 lists the sensitivity, specificity, and ORs for the 5 features in predicting risk of malignancy. Among the 3 ophthalmologists reviewing photographs, there was complete agreement, with a κ of 1.0. The 3 features with the highest ORs were infiltration (OR = 18.2, P < .01), ulceration (OR = 14.7, P < .01), and loss of eyelashes (OR = 6.0, P < .01). The presence of telangiectasia was associated with a moderately high OR (OR = 4.5, P < .01), but was not included in our scoring system owing to difficulty in identification without slit-lamp biomicroscopy and its poor specificity. Pigmentation was found to have an equivocal OR of 1.0.
Table 1.
Sensitivity, specificity, and ORs (with CIs) for studied features in identifying malignant eyelid lesions
| FEATURE | SENSITIVITY, % (95% CI) | SPECIFICITY, % (95% CI) | OR (95% CI) |
|---|---|---|---|
| Loss of eyelashes | 37 (22 to 54) | 91 (86 to 95) | 6.0 (2.5 to infinity) |
| Ulceration | 50 (33 to 67) | 94 (89 to 97) | 14.7 (6.3 to infinity) |
| Infiltration | 58 (41 to 74) | 93 (88 to 97) | 18.1 (8.0 to infinity) |
| Telangiectasia* | 63 (46 to 78) | 72 (65 to 79) | 4.5 (2.3 to infinity) |
| Pigmentation* | 0 (0 to 13) | 84 (78 to 90) | 1.0 (0.0 to infinity) |
OR—odds ratio.
Not included in LUI (loss of eyelashes, ulceration, and infiltration) triage key owing to poor OR or poor specificity.
Based on the 3 features with the highest ORs for predicting malignancy, we created the triage key LUI (loss of eyelashes, ulceration, and infiltration). A description and example of each of these features is shown in Figure 1. Table 2 describes the sensitivity and specificity of these features in isolation or combination in identifying malignant lesions within our database. If all 3 features are present, the sensitivity, specificity, and positive predictive value of malignancy are 21%, 99%, and 80%, respectively.
Figure 1.
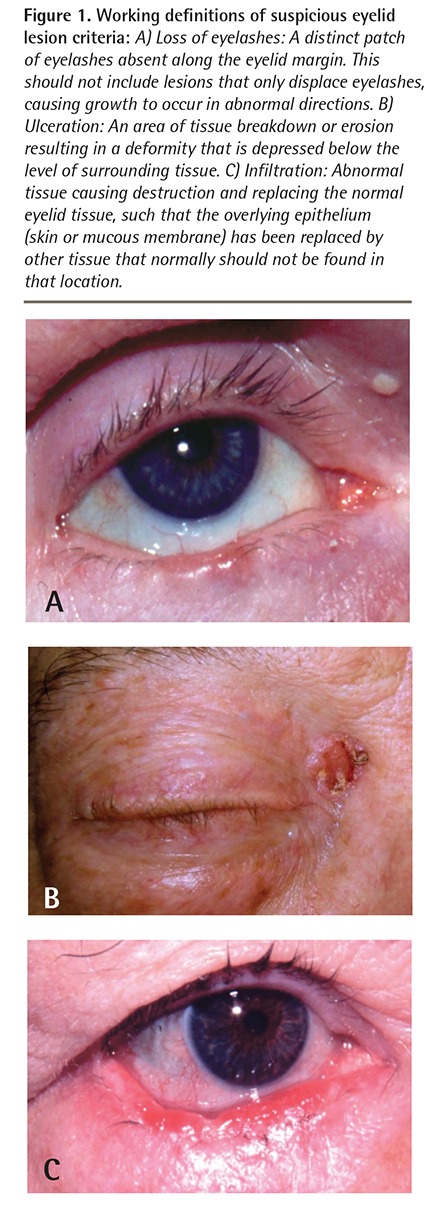
Working definitions of suspicious eyelid lesion criteria: A) Loss of eyelashes: A distinct patch of eyelashes absent along the eyelid margin. This should not include lesions that only displace eyelashes, causing growth to occur in abnormal directions. B) Ulceration: An area of tissue breakdown or erosion resulting in a deformity that is depressed below the level of surrounding tissue. C) Infiltration: Abnormal tissue causing destruction and replacing the normal eyelid tissue, such that the overlying epithelium (skin or mucous membrane) has been replaced by other tissue that normally should not be found in that location.
Table 2.
Sensitivity and specificity of LUI (loss of eyelashes, ulceration, and infiltration) triage key features alone or in combination in identifying malignant eyelid lesions
| CRITERIA | SENSITIVITY, % | SPECIFICITY, % | POSITIVE LIKELIHOOD RATIO | NEGATIVE LIKELIHOOD RATIO |
|---|---|---|---|---|
| Loss of eyelashes | 37 | 91 | 4.1 | 0.69 |
| Ulceration | 50 | 94 | 8.3 | 0.53 |
| Infiltration of tissues | 58 | 93 | 8.3 | 0.45 |
| ≥ 1 criteria | 76 | 85 | 5.1 | 0.28 |
| ≥ 2 criteria | 47 | 94 | 7.8 | 0.56 |
| All 3 criteria | 21 | 99 | 21.0 | 0.80 |
Phase 2
A total of 84 medical students participated in phase 2 of the study. Before watching the educational video describing the LUI features, the mean total score for correctly identifying malignant lesions was 46% (mean [SD] 3.7 [1.4] out of 8). This improved to 70% (mean [SD] 5.6 [1.3] out of 8, P < .001) after watching the video. The number of unnecessary referrals decreased from 71% in the pretest to 50% in the post-test (P = .004). The number of missed urgent referrals for malignant-appearing lesions decreased from 8% to 3% (P < .001). Intraobserver reliability in providing the same response for identical pictures improved from a pretest κ score of 0.23 to a posttest κ score of 0.47 after the educational video.
DISCUSSION
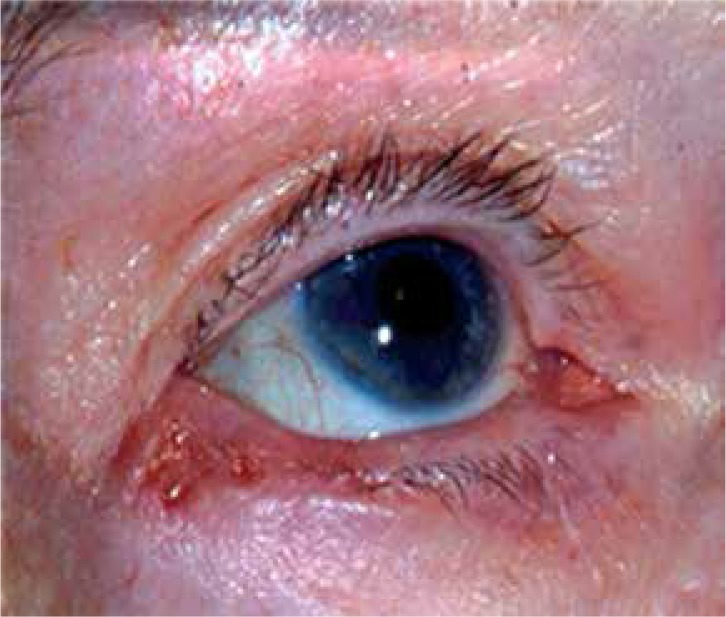
Through a survey of ophthalmic plastic surgeons and a clinical series of periocular lesions with histopathologic confirmation of diagnosis, the LUI triage key was created to assist health care professionals in establishing the likelihood of periorbital skin malignancy. The LUI triage key contains the 3 features most predictive of malignant lesions in our clinical series. Any feature alone substantially increases the likelihood of a malignant lesion being present, and while malignant lesions often do not display all 3 features, the presence of all LUI features in a single lesion is highly suggestive of malignancy. An example of a lesion with 2 LUI features is shown in Figure 2. The presence of all 3 features is associated with a positive likelihood ratio of malignancy of 21.0 (Table 2).
Figure 2.
Use of the LUI (loss of eyelashes, ulceration, and infiltration) triage key to warrant urgent referral: The lesion displays both loss of eyelashes and ulceration, and would warrant urgent referral to an ophthalmologist or oculoplastic surgeon.
The goal of creating a triage key acronym such as LUI is to provide primary care physicians who do not have access to slit-lamp biomicroscopy with an organized, evidence-based method for assessing periocular lesions similar to the ABCD criteria used for pigmented cutaneous lesions. Studies have demonstrated the utility of mnemonics such as acronyms in memory recall.14 The ABCD mnemonic still forms an integral part of medical school curricula today; of interest, the diagnostic accuracy of LUI features is comparable to that of ABCD (Table 3).15
Table 3.
Sensitivity and specificity of the ABCDE (asymmetry, irregular borders, multiple colours, diameter ≥ 6 mm, and enlargement) signs in diagnosing melanoma
| CRITERIA | SENSITIVITY, % | SPECIFICITY, % |
|---|---|---|
| Asymmetry | 57 | 72 |
| Irregular borders | 57 | 71 |
| Multiple colours | 65 | 59 |
| Diameter ≥ 6 mm | 90 | 63 |
| Horizontal enlargement | 84 | 90 |
| ≥ 1 criteria | 97 | 36 |
| ≥ 2 criteria | 89 | 65 |
| ≥ 3 criteria | 66 | 80 |
| ≥ 4 criteria | 54 | 94 |
| All 5 criteria | 43 | 100 |
Adapted from Thomas et al.15
In addition to creating the LUI triage key, we also conducted a pilot study in which a short video introduced the concept to second-year medical students. Previous studies have shown the value of multimedia learning tools over traditional learning providing knowledge acquisition to medical students in a time-efficient manner.12 Completion of a short questionnaire before and after the video resulted in a significantly lower number of unnecessary referrals and lower number of missed malignant lesions, suggesting that students were able to quickly apply the LUI system despite limited clinical experience.
It should be noted that while the LUI triage key is of assistance in predicting malignancy of eyelid lesions, all patients presenting with a periocular lesion should have a complete workup including history taking and examination. Additional questions to ask about the patient’s medical history and other relevant findings on examination are listed in Box 1. More important, suspicion of malignancy (eg, based on LUI features) should be clearly communicated to the consultant physician.
Box 1.
Clinical workup of periocular lesions
Relevant questions during history taking
Patient demographic characteristics—age and race or ethnicity of patient should be considered
Timing of lesion onset—a long-standing, stable lesion is less concerning than a newly noticed lesion
Growth rate—most periocular malignancies grow over a period of months
History of previous excisions—might provide clues to underlying pathologic diagnosis
Presence of skin malignancies elsewhere on body— patients with a history of skin cancer are at greater risk of future sun-related malignancies
Presence of systemic conditions predisposing to malignant processes (eg, basal cell nevus syndrome, Torre syndrome)
Relevant examination findings
Location of lesion (eg, upper vs lower eyelid, eyelid margin involvement)
Size of lesion—measure in millimetres or by percentage of eyelid involved
Morphologic features—especially presence of LUI (loss of eyelashes, ulceration, infiltration) features
Limitations
First, we did not test an exhaustive list of potential features in identifying malignancy, but rather limited it to features that were ranked the highest by members of the CSOPS. Second, features were identified from 2-dimensional photographs without any background information regarding the lesion. In a clinical setting, history taking is possible and examination allows 3- dimensional viewing, palpation, and other techniques such as transillumination to be performed, all of which would aid in clinical decision making. Third, the malignant lesions in this study included only basal cell carcinomas and squamous cell carcinomas, and did not include samples of rarer, more invasive lesions such as malignant melanoma. Finally, it is possible that elements of the video, such as displaying examples of obviously benign versus obviously malignant lesions, might have contributed to identification improvement independent of the LUI acronym.
Conclusion
We have created an evidence-based triage key (LUI) and associated video to assist health care providers in assessing the likelihood of malignancy in periocular lesions and making more appropriate referral decisions. Our LUI system has been shown to be valid and reliable in a clinical setting. At our centre, it has been incorporated into ophthalmology teaching sessions at both the undergraduate medical student and the family medicine resident level. We believe this represents a pragmatic method of evaluating such lesions that is suitable for teaching on a widespread level.
EDITOR’S KEY POINTS
Differentiating benign from malignant eyelid lesions can be difficult, even for experienced physicians. Early detection of malignant lesions is critical to preventing substantial morbidity and mortality.
The 3 features most predictive of malignancy for eyelid lesions are loss of eyelashes, ulceration, and infiltration of tissues, presented as a triage key known by the acronym LUI.
Incorporating the LUI (loss of eyelashes, ulceration, and infiltration) triage key into medical education has the potential to improve detection of malignant eyelid lesions and improve patient outcomes.
POINTS DE REPÈRE DU RÉDACTEUR
Il peut être difficile, même pour un médecin expérimenté, de distinguer les lésions palpébrales malignes de celles qui ne le sont pas. Une détection précoce des lésions malignes est cruciale pour prévenir des conséquences importantes de morbidité et de mortalité.
Les trois caractéristiques des paupières les plus suggestives de malignité sont la perte de cils, l’ulcération et l’infiltration des tissus, une triade qu’on propose de représenter par l’acronyme anglais LUI (Loss of eyelashes, Ulceration, Infiltration).
L’introduction de cette triade clé dans la formation médicale pourrait améliorer la détection des lésions palpébrales malignes et ainsi réduire les conséquences pour le patient.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Cook BE, Jr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106(4):746–50. doi: 10.1016/S0161-6420(99)90161-6. [DOI] [PubMed] [Google Scholar]
- 2.Lin HY, Cheng CY, Hsu WM, Kao WH, Chou P. Incidence of eyelid cancers in Taiwan: a 21-year review. Ophthalmology. 2006;113(11):2101–7. doi: 10.1016/j.ophtha.2006.06.001. Epub 2006 Sep 7. [DOI] [PubMed] [Google Scholar]
- 3.Pornpanich K, Chindasub P. Eyelid tumors in Siriraj Hospital from 2000–2004. J Med Assoc Thai. 2005;88(Suppl 9):S11–4. [PubMed] [Google Scholar]
- 4.Tesluk GC. Eyelid lesions: incidence and comparison of benign and malignant lesions. Ann Ophthalmol. 1985;17(11):704–7. [PubMed] [Google Scholar]
- 5.Xu XL, Li B, Sun XL, Li LQ, Ren RJ, Gao F, et al. Eyelid neoplasms in the Beijing Tongren Eye Centre between 1997 and 2006. Ophthalmic Surg Lasers Imaging. 2008;39(5):367–72. doi: 10.3928/15428877-20080901-18. [DOI] [PubMed] [Google Scholar]
- 6.Cook BE, Jr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108(11):2088–98. doi: 10.1016/s0161-6420(01)00796-5. [DOI] [PubMed] [Google Scholar]
- 7.Deprez M, Uffer S. Clinicopathological features of eyelid skin tumors. A retrospective study of 5504 cases and review of literature. Am J Dermatopathol. 2009;31(3):256–62. doi: 10.1097/DAD.0b013e3181961861. [DOI] [PubMed] [Google Scholar]
- 8.Wang JK, Liao SL, Jou JR, Lai PC, Kao SC, Hou PK, et al. Malignant eyelid tumours in Taiwan. Eye (Lond) 2003;17(2):216–20. doi: 10.1038/sj.eye.6700231. [DOI] [PubMed] [Google Scholar]
- 9.Noble J, Somal K, Gill HS, Lam WC. An analysis of undergraduate ophthalmology training in Canada. Can J Ophthalmol. 2009;44(5):513–8. doi: 10.3129/i09-127. [DOI] [PubMed] [Google Scholar]
- 10.Hillson TR, Harvey JT, Hurwitz JJ, Liu E, Oestreicher JH, Pashby RC. Sensitivity and specificity of the diagnosis of periocular lesions by oculoplastic surgeons. Can J Ophthalmol. 1998;33(7):377–83. [PubMed] [Google Scholar]
- 11.Friedman RJ, Rigel DS, Kopf AW. Early detection of malignant melanoma: the role of physician examination and self-examination of the skin. CA Cancer J Clin. 1985;35(3):130–51. doi: 10.3322/canjclin.35.3.130. [DOI] [PubMed] [Google Scholar]
- 12.Steedman M, Abouammoh M, Sharma S. Multimedia learning tools for teaching undergraduate ophthalmology: results of a randomized clinical study. Can J Ophthalmol. 2012;47(1):66–71. doi: 10.1016/j.jcjo.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Kratky V. In the clinic—Dr. Vladimir Kratky, MD on eyelid lumps and bumps. 2007. Insidermedicine.comAvailable from: https://insidermedicine.com/archives/In_the_Clinic_-_Dr_Vladimir_Kratky_MD_on_Eyelid_Lumps_and_Bumps_1794.aspx. Accessed 2014 Dec 15.
- 14.Beitz JM. Unleashing the power of memory. The mighty mnemonic. Nurse Educ. 1997;22(2):25–9. doi: 10.1097/00006223-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197(1):11–7. doi: 10.1159/000017969. [DOI] [PubMed] [Google Scholar]