Abstract
Background
The authors evaluated the impact of morphological and hemodynamic factors on the rupture of matched-pairs of ruptured-unruptured intracranial aneurysms on one patient’s ipsilateral anterior circulation with 3D reconstruction model and computational fluid dynamic method simulation.
Methods
20 patients with intracranial aneurysms pairs on the same-side of anterior circulation but with different rupture status were retrospectively collected. Each pair was divided into ruptured-unruptured group. Patient-specific models based on their 3D-DSA images were constructed and analyzed. The relative locations, morphologic and hemodynamic factors of these two groups were compared.
Results
There was no significant difference in the relative bleeding location. The morphological factors analysis found that the ruptured aneurysms more often had irregular shape and had significantly higher maximum height and aspect ratio. The hemodynamic factors analysis found lower minimum wall shear stress (WSSmin) and more low-wall shear stress-area (LSA) in the ruptured aneurysms than that of the unruptured ones. The ruptured aneurysms more often had WSSmin on the dome.
Conclusions
Intracranial aneurysms pairs with different rupture status on unilateral side of anterior circulation may be a good disease model to investigate possible characteristics linked to rupture independent of patient characteristics. Irregular shape, larger size, higher aspect ratio, lower WSSmin and more LSA may indicate a higher risk for their rupture.
Keywords: Intracranial aneurysms, Multiple aneurysm, Anterior circulation, Rupture, Computational fluid dynamics
Background
Approximately 15%-35% of aneurysm patients have multiple aneurysms [1-4]. Neurosurgeons face a special problem to treat them because when patient’s general clinical condition can’t undergo a full treatment or aneurysms are presented in locations that cannot be operated through a single treatment, the high-risk or duty aneurysm should be identified and operated first [5,6]. For patients with subarachnoid hemorrhage (SAH), combing clinical, CT and angiographic findings, most bleeding sites can be identified but there are still some difficult cases. The misjudgment is dangerous because the untreated but true ruptured aneurysm may bleed again very soon. As for patients without SAH, considering the high rates of morbidity and mortality in the event of rupture, discriminating the aneurysms in danger of rupture before operation is valuable for preventive treatment too.
Previous studies displayed a number of characteristics that may be related to ruptured aneurysms, such as size, location, irregular shape (i.e. blebs, nipples or multiple lobes), etc. [7-9]. And recently, computational fluid dynamics (CFD) has become a popular tool for studying intracranial aneurysm hemodynamics and discriminating rupture status [10].
In this study, we investigated the rupture-related characteristics on 20 matched-pairs of ruptured-unruptured saccular aneurysms located unilaterally on the anterior circulation in the same patient. For they provide an ideal internal control for such patient-specific variables as genetic, age, sex, blood pressure, individual habits (including smoking, alcoholism), comorbidities present and so forth, and only focus on aneurysms characteristics. We compared their sites, morphology and hemodynamic factors and hope to get some illuminating results on aneurismal rupture. To our knowledge, the matched rupture risk data in one patient including morphological and hemodynamic factors is rarely reported.
Methods
Source of patients
From January 2012 to December 2013, a total of 20 patients with ruptured-unruptured aneurysms pairs in ipsilateral anterior circulation were selected from our institution’s data base (Figure 1).
Figure 1.

Visualization of 20 multiple aneurysms pairs. Double arrows show the ruptured aneurysms while single arrows show the unruptured aneurysms.
The inclusion criteria were: (1) 2 saccular aneurysms with different rupture status in one patient; (2) in ipsilateral anterior circulation. The specific locations include internal carotid artery (ICA, the Bouthillier classification: C4, cavernous; C5, clinoid; C6, ophthalmic; C7, communicating), anterior cerebral artery (ACA), middle cerebral artery (MCA), and anterior communicating artery (ACoA) with inflow only from the ipsilateral ACA (to exclude the contralateral carotid system’s effect); (3) first SAH; (4) complete clinical data and three-dimensional (3D) DSA images were of adequate resolution for CFD analysis; (5) the rupture site was identified by head CT scan imaging or intraoperative findings; (6) the study was consented to by patients or their close relatives. The exclusion criteria were: (1) fusiform or dissecting aneurysms; (2) ACoA aneurysms with good inflow from both sides of ACA; (3) previous SAH; (4) incomplete clinical data or 3D-DSA images were not of adequate resolution for CFD analysis; (5) the rupture site cannot be identified; (6) patients and their relatives did not consent to the study.
All of them were emergency patients for cerebral hemorrhage, underwent DSA at the first time within 2 days before vasospasm happened in our hospital. Written informed consents were obtained from all patients, and the research was permitted by the ethics committee of Beijing Tiantan Hospital. Each pair was divided into two groups, ruptured and unruptured.
Modeling of aneurysms and numerical simulation
Numerical simulation of blood hemodynamics was performed as described previously [11-14]. Briefly, patient-specific 3D-DSA data of all aneurysm pairs, with each case sharing inlet on the same ICA cervical segment, were obtained before treatment. In each case, the last aneurysm along with the brain blood flow was defined as the distal aneurysm, the other aneurysms was defined as the proximal aneurysm. Software package developed in-house was used to create and modify a stereolithographic image that contained the blood vessel luminal surface information. Each model was imported into the ICEM CFD software (ANSYS, Canonsburg, Pennsylvania, USA) to create more than one million finite volume tetrahedral element grids. After meshing, ANSYS CFX 12.1 software was used for simulation of blood hemodynamics. The inflow boundary condition was a pulsatile period velocity profile that was obtained by transcranial Doppler. Two cardiac cycle simulations were performed for numerical stability. The results from the second cardiac cycle were collected as output for the final analyses. The average Reynolds number was within the range of normal blood flow in human cerebral arteries.
Data analysis
Morphologic parameters including the aneurismal size (maximum height), neck width and aspect ratio (dome-to-neck ratio) were measured and calculated from 3D-DSA data. The number of each morphologic types (lateral/bifurcation, regular/irregular) and relative bleeding location (distal/proximal) were recorded. After CFD simulation, these flow pattern characteristics in the aneurysms were analyzed: 1) flow stability, the stable flow pattern persisted during the cardiac cycle, while the unstable flow had flow structure moved or changed during the cardiac cycle [15]; 2) flow complexity, the simple flow pattern had a single vortex structure, while the complex flow contained multiple vortices [15]. The following wall shear stress (WSS) related quantitative parameters were calculated: maximum and minimum WSS (WSSmax, WSSmin), time-averaged WSS (TAWSS), oscillatory shear index (OSI) and low WSS area (LSA). LSA was defined as the proportion of the low WSS area (below 10% of the mean WSS at the anterior genu of the carotid siphon) to the whole area of the aneurysm [16]. All the parameters in the ruptured and unruptured group were calculated and compared.
For qualitative data of relative bleeding location (distal/proximal), morphology type (lateral/bifurcation, regular/irregular), flow stability and complexity, the location of WSSmax (neck/dome) and WSSmin (neck/dome), McNemar’s test was used to compare the differences between the two groups. For quantitative data, one-sample Kolmogorov–Smirnov test was used to test the normal distribution. Then paired-sample t test was used for all the approximately normally distributed parameters with data expressed as mean (SD), and Wilcoxon’s sign rank test was used for non-normally distributed parameters with data expressed as median (quartile). p < 0.05 was regarded as statistically significant. Statistical analysis was performed with an SPSS 15.0 package.
Results
Characteristics of patients and aneurysms
Table 1 shows clinical characteristics of these 20 patients. There were 2 male and 18 females aged between 39 and 83 years (mean 60.6y). For health habits, 4 (20%) were alcohol drinkers, 3 (15%) were cigarette smokers. For comorbidity, 3 (65%) had hypertension (HBP), 6 (30%) had deep vein thrombosis (DVT), 5 (25%) had hyperlipidemia (HPL), 4 (20%) had hyperhomocysteinemia (HHCY), atherosclerosis (AS), 2 (10%) had diabetes mellitus (DM), coronary heart disease (CHD), and 1 (5%) had brain hernia (BH), polycystic kidney disease (PKD), cerebral infarction (CI). 7 (35%) patients had other coexisting aneurysms on the contralateral side of anterior circulation or vertebrobasilar artery. Patients were graded according to the Hunt and Hess scale: grade 1 or 2: 65% and grade ≥3: 35%. For the treatment, 16 (80%) were partial treatments (i.e., some coexisting unruptured aneurysms were left untreated), 12 of them were only rupture-site aneurysm coiling/clipping and 4 of them were only aneurysms on rupture-side clipping; 2 (10%) were two-stage treatments (i.e., complete treatment through 2 operations), one of them was clipping following rupture-site coiling, the other of them was coiling following rupture-side clipping; 2 (10%) chose no operation.
Table 1.
Clinical characteristics of these 20 patients
| No. | Habit, S/D | Comorbidity | Site † | Other site# | Hunt-hess grade | Operation |
|---|---|---|---|---|---|---|
| 1 | HBP, BH | MCA*, C5 | 4 | Bled-site clipping | ||
| 2 | C7*, MCA | 1 | Bled-site coiling | |||
| 3 | HBP, AS, DVT | C7*, C6 | 2 | Bled-site coiling | ||
| 4 | HPL, PKD | C6* , C7 | 2 | Bled-site coiling | ||
| 5 | D | DM, HPL, CI, DVT | C7*, C6 | 2 | Bled-site clipping | |
| 6 | C6*, MCA | 2 | Bled-site coiling | |||
| 7 | D | HBP | ACA*, C5 | 2 | Bled-site coiling | |
| 8 | S | HHCY | C7*, C6 | 2 | Bled-site clipping | |
| 9 | HBP | C7*, MCA | ACA | 2 | Two-stage treatment | |
| 10 | HBP | MCA*, C7 | 3 | Bled-site coiling | ||
| 11 | C7*, C4 | C4 | 1 | No operation | ||
| 12 | S,D | HBP, DVT | ACoA*, C6 | 3 | Bled-site coiling | |
| 13 | S | HBP, HPL,HHCY, AS, DVT, | C7*, MCA | 3 | Bled-site coiling | |
| 14 | HBP, HPL, CHD | C7*, MCA | C7 | 2 | Bled-site coiling | |
| 15 | HBP, DM, AS | C7*, MCA | C7 | 2 | Bled-side clipping | |
| 16 | HBP, HHCY, HD | C7*, C4 | C7, C4 | 3 | No operation | |
| 17 | HBP, HPL | C7*, ACoA | 3 | Bled-side clipping | ||
| 18 | HBP | MCA*, C7 | C7 | 3 | Two-stage treatment | |
| 19 | HBP, DVT | C7*, MCA | 1 | Bled-side clipping | ||
| 20 | D | HHCY, AS, DVT | ACoA*, C7 | BA | 1 | Bled-side clipping |
S/D, Smoker/drinker; HBP, Hypertension; BH, Brain hernia; AS, Atherosclerosis ; DVT, Deep vein thrombosis; HPL, Hyperlipidemia; PKD, Polycystic kidney disease; DM, Diabetes mellitus; CI, Cerebral infarction; HHCY, Hyperhomocysteinemia; CHD, Coronary heart disease;
†the Bouthillier classification of internal carotid artery (ICA) segments: C4, Cavernous; C5, Clinoid; C6, Ophthalmic; C7, Communicating; MCA, Middle cerebral artery; ACA, Anterior cerebral artery; ACoA, Anterior communicating artery;
#coexisting aneurysms on the contralateral side of anterior circulation or vertebrobasilar artery; BA, basilar artery.
*the ruptured group.
The relative rupture location were 11 (55%) distal aneurysms and 9 (45%) proximal aneurysms. Of the ruptured aneurysms 12 were on C7, 3 on MCA, 2 on C6, ACoA and 1 on ACA; of the unruptured aneurysms 7 were on MCA, 4 on C7, C6, 2 on C5, C4, and 1 on ACoA. There was no significant difference in the relative bleeding location (distal/proximal) between the ruptured and unruptured group (Table 2).
Table 2.
Relative locations and morphologic factors
| Variables | Aneurysms | p value ‡ (2-tailed) | ||
|---|---|---|---|---|
| Total (n = 40) | Ruptured group (n = 20) | Unruptured group (n = 20) | ||
| Relative location | ||||
| Distal (%) | 20 (50) | 11 (55) | 9 (45) | 0.824 |
| Proximal (%) | 20 (50) | 9 (45) | 11 (55) | |
| Morphology type | ||||
| Lateral (%) | 15 (37.5) | 6 (30) | 9 (45) | 0.581 |
| Bifurcation (%) | 25 (62.5) | 14 (70) | 11 (55) | |
| Regular | 22 (55) | 5 (25) | 17 (85) | 0.002 |
| Irregular | 18 (45) | 15 (75) | 3 (15) | |
| Maximum height, mm | 3.94 (1.977) | 4.68 (1.605) | 3.20 (2.076) | 0.041 |
| Neck width, mm | 3.58 (1.308) | 3.77 (1.468) | 3.39 (1.131) | 0.351 |
| Surface area, mm2 | 32.20 (38.809) | 42.82 (41.895) | 27.14 (15.980) | 0.062 |
| Volume,mm3 | 20.85 (41.064) | 31.18 (43.944) | 16.08 (14.697) | 0.108 |
| Aspect ratio | 1.11 (0.399) | 1.31 (0.402) | 0.92 (0.290) | 0.004 |
The comparisons of maximum height and aspect ratio between the two groups were of significant differences (p < 0.05). (‡, McNemar’s test, paired-sample t test or Wilcoxon’s sign rank test as appropriate).
Comparison of morphologic factors between ruptured and unruptured group
For aneurysm morphology types, the ruptured aneurysms were more common irregular type, but there were no significant differences in the lateral/bifurcation type between the ruptured and unruptured groups.
As presented in Table 2, the ruptured aneurysms had significantly higher maximum height and aspect ratio than the unruptured aneurysms. But there were no significant differences in the neck width, surface area or volume between the ruptured and unruptured group.
Comparison of hemodynamic factors between ruptured and unruptured group
As presented in Table 3 and Figure 2, the ruptured aneurysms had significantly lower WSSmin and more LSA than the unruptured aneurysms. The ruptured aneurysms more often had WSSmin on the dome. Other hemodynamic factors showed no significant differences between the two groups.
Table 3.
Hemodynamic factors
| Variables | Aneurysms | p value‡ (2-tailed) | ||
|---|---|---|---|---|
| Total (n = 40) | Ruptured group (n = 20) | Unruptured group (n = 20) | ||
| WSSmax, Pa | 53.72 (26.075) | 55.65 (32.700) | 51.78 (17.845) | 0.607 |
| WSSmin, Pa | 0.75 (1.268) | 0.28 (0.289) | 1.22 (1.658) | 0.020 |
| TAWSS, Pa | 13.87 (8.774) | 12.72 (10.234) | 15.02 (7.105) | 0.392 |
| OSI, Pa | 0.0090 (0.00940) | 0.0104 (0.02054) | 0.0061 (0.00871) | 0.156 |
| LSA,% | 0.40 (4.344) | 2.55 (16.536) | 0.12 (1.044) | 0.011 |
| Flow stability | ||||
| Stable (%) | 14 (35) | 6 (30) | 8 (40) | 0.754 |
| Unstable (%) | 26 (65) | 14 (70) | 12 (60) | |
| Flow complexity | ||||
| Simple (%) | 11 (27.5) | 3 (15) | 8 (40) | 0.125 |
| Complex (%) | 29 (72.5) | 17 (85) | 12 (60) | |
| WSSmax location | ||||
| Neck (%) | 33 (82.5) | 17 (85) | 16 (80) | 1.000 |
| Dome (%) | 7 (17.5) | 3 (15) | 4 (20) | |
| WSSmin location | ||||
| Neck (%) | 9 (22.5) | 1 (5) | 8 (40) | 0.039 |
| Dome (%) | 31 (77.5) | 19 (95) | 12 (60) | |
The comparisons of WSSmin, LSA and WSSmin location on the aneurysms between the two groups were of significant differences (p < 0.05). (‡, paired-sample t test, Wilcoxon’s sign rank test or McNemar’s test as appropriate).
Figure 2.
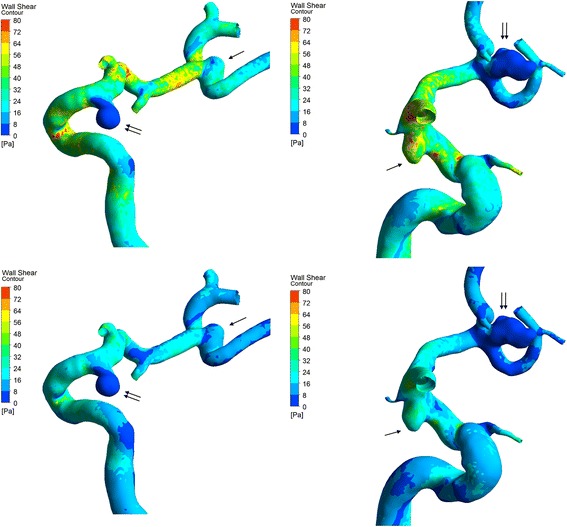
Visualization of the wall shear stress (WSS) of 2 representative aneurysms pairs (case 1, left; case 2, right; upper, at the systolic peak; lower, at the diastolic end). Double arrows show the ruptured aneurysms while single arrows show the unruptured aneurysms. Case 1 has aneurysms on ICA C7 (duty site) and MCA. Case 2 has aneurysms on ICA C7 and ACoA (duty site). The ruptured aneurysms have lower WSS on the dome and more low WSS area.
Discussion
Multiple aneurysms locating unilaterally on the anterior circulation are common and propose special challenge for neurosurgery. K. Mizoi et al. [17] mentioned that 19.4% (72/372) multiple aneurysms located unilaterally in the anterior circulation (not including the ACoA aneurysm) and 42.2% (157/372) multiple aneurysms included at least one on the ACoA (whether they located unilaterally or bilaterally was not concerned). For surgical outcomes, 17 of them got partial treatments only and of which 1 patient died due to postoperative bleeding from the untreated aneurysms [17]. Y. Orz. et al. [18] reported that 66.5% (147/221) multiple aneurysms located unilaterally in the anterior circulation, the total mortality rate after surgery is 15.2% and 15 of them got partial/two-stage treatments [18]. For Rinne et al. [19], the incidence percentage was 16.2% (49/302) and their results suggested that multiple aneurysms signicifantly increase the risk for poor outcome. For Imhof et al. [20], 44.6% (54/121) ruptured aneurysms had the coexisting unruptured aneurysms ipsilateral to the ruptured one. Of all the 124 patients with SAH and multiple aneurysm, 3 passed away immediately after admission and 64 got partial/two-stage treatments [20]. Partial/two-stage treatments are not rare for multiple aneurysms and it put forward a need to identify risky ones among all the coexisting aneurysms. Ruptured-unruptured aneurysm pair on the same patient’s ipsilateral anterior circulation is very interesting. It may be a good disease model not only to investigate characteristics linked to bled aneurysms but also to understand multiple aneurysms better.
As regards to the clinical data, we demonstrate that most cases are female (consistent with previous reports for association between the presence of multiple aneurysms and female sex [1,3,4]) and 65% have hypertension. Other coexisting additional disorders, such as DVT, HPL, AS, etc., can also exist. However, the precise role of these clinical factors in assessing rupture risk needs further study with larger sample size and comparative control group. There is no difference for which relative location (proximal/distal) would bleed first, so Liang-Der Jou et al.’s [21] previous speculation that the proximal aneurysm in serial aneurysms may be subject to a greater rupture risk whose research based on only hemodynamic analysis on 4 multiple aneurysms pairs but without clinical data analysis was not true situation for patients.
The ruptured aneurysms are more common to have irregular shape. Some clinical experience and human studies have reported the same found [7,13,22-24]. The focal bulges on the surface of aneurysm often indicates a weak and thinner wall which indicates a higher risk of aneurysmal rupture [13,24].
The natural history studies on unruptured aneurysms (including single aneurysms and multiple aneurysms together) proposed aneurysms size to be key predictor for rupture, and diameters > 7 mm/10 mm represented much higher bleeding risk [7,25-27]. In our study, the ruptured aneurysms have higher maximum height too. But studies on only multiple aneurysms seem to have different results. In our study the mean maximum height for all these 40 aneurysms is 3.94 ± 1.977 mm, which means that most aneurysms are small aneurysms with diameter <7 mm in size. Similarly, Jagadeesan et al. [28] reported that very small(≤3 mm) and small aneurysm (>3 mm but ≤7 mm) constituted the majority of ruptured aneurysms (24.6% and 50.7%) for multiple aneurysms. Lu et al.’s [2] retrospective study of 294 multiple aneurysms reported that 88.1% of the aneurysms were 5 mm or less. Size may be a good assessment factor for multiple aneurismal rupture, and what is interesting, multiple aneurysms maybe have a smaller size. However, further multicenter studies on larger multiple aneurysms databases are needed to verify the true situation.
The ruptured aneurysms have higher aspect ratio. Consistent with our observation, Sadatomo et al. [29] found that in cases with aspect ratio more than or equal to 1.8, 87% were ruptured aneurysms, whereas in cases with aspect ratio less than 1.8, 92% were unruptured aneurysms. Backes et al. [8] performed conditional univariable logistic regression analysis on 124 patients with 302 multiple aneurysms and found that aspect ratio ≥1.3 was associated with multiple aneurysm rupture independent of aneurysm size and location, and independent of patient characteristics. Ujiie et al. [30] performed an in vivo study and found that aspect ratio determined the intra-aneurysmal flow, a higher aspect ratio (>1.6) indicated a much slower circulation near the dome and flow stagnation was detected in the dome of irregular-shaped aneurysms (dumbbell-shaped and bleb) [30]. The dome often has flow stagnation [31,32], which is related to intra-aneurysmal thrombosis and subsequent inflammatory changes in the aneurysm wall [30]. Higher aspect ratio may suggest a remodeling artery wall and risk for aneurismal rupture.
For hemodynamic factors analysis, we find that the ruptured aneurysms have lower WSSmin and more LSA. These data are consistent with our previous results showing that low WSS might be involved in increasing the risk of rupture [13]. The ruptured aneurysms more often have WSSmin on the dome. Previous studies found that unruptured aneurysms’ thin-walled dome regions co-localized with low WSS and the ruptured aneurysms’ bleeding points were often located at the dome with a low WSS [33]. So we suspect that WSSmin may be associated with flow stagnation at the dome as well as the localized pathologic aneurysm wall degeneration and thinning on the dome. These hemodynamic factors may help us to verify ruptured aneurysms and offer some insight into rupture mechanisms.
The present study had some limitations that need to be addressed. Rigid wall, laminar flow and Newtonian blood were used in our present aneurysm models. The flow conditions were not patient-specific. It is reported that using normal-resolution meshes (1 to 5 million tetrahedral) may underestimate the aneurysm WSS and WSSmax when compared with using high-resolution meshes (300,000 to 1 million elements) [10], while using generalized (typical flow rates in a healthy adult) and patient-specific inflow boundary conditions may results in different WSS magnitudes and hemodynamic characteristics [34]. However, matched pairs design in our study may help control for individual differences affecting the results. Sharing the same inflow boundary conditions on one side of ICA especially help avoid inflow boundary condition confounding. The other limitations were the effects of small sample size and a single center selection bias.
Conclusions
Intracranial aneurysms pairs with different rupture status in one patient’s ipsilateral anterior circulation may be a good disease model to investigate possible features linked to bled aneurysms independent of patient characteristics. The ruptured aneurysms manifested irregular shape, larger size, higher aspect ratio, lower WSSmin and more LSA compared with their unruptured mates. It may promote a better understanding of multiple aneurysms and help neurosurgeons to identify risky sites before operation.
Funding source
This work was supported by the National Natural Science Foundation of China (grant 81301003, 81171079, 81371315 and 81220108007).
Abbreviations
- ACA
Anterior cerebral artery
- ACoA
Anterior communicating artery
- AS
Atherosclerosis
- BH
Brain hernia
- CFD
Computational fluid dynamics
- CHD
Coronary heart disease
- CI
Cerebral infarction
- DM
Diabetes mellitus
- DVT
Deep vein thrombosis
- HBP
Hypertension
- HHCY
Hyperhomocysteinemia
- HPL
Hyperlipidemia
- ICA
Internal carotid artery
- LSA
Low wall shear stress area
- MCA
Middle cerebral artery
- OSI
Oscillatory shear index
- PKD
Polycystic kidney disease
- TAWSS
Time averaged wall shear stress
- WSS
Wall shear stress
- WSSmax
The maximum wall shear stress
- WSSmin
The minimum wall shear stress
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YZ performed the statistical analysis and drafted the manuscript. XY conceived and designed the research, and handled funding and supervision. SW was responsible for aneurysmal model reconstruction and analyzed and interpreted the data. HL was responsible for in-house software exploitation. LJ and YW acquired the data. JL and CL made critical revision to the manuscript for important intellectual content. All authors read and approved the final manuscript.
Contributor Information
Ying Zhang, Email: yingzhang829@163.com.
Xinjian Yang, Email: yang-xj@163.net.
Yang Wang, Email: wangyang7839@163.com.
Jian Liu, Email: 542530730@163.com.
Chuanhui Li, Email: lichuanhui365@163.com.
Linkai Jing, Email: linkai_jing@163.com.
Shengzhang Wang, Email: szwang@fudan.edu.cn.
Haiyun Li, Email: haiyunli@ccmu.edu.cn.
References
- 1.Kaminogo M, Yonekura M, Shibata S. Incidence and outcome of multiple intracranial aneurysms in a defined population. Stroke. 2003;34(1):16–21. doi: 10.1161/01.STR.0000046763.48330.AD. [DOI] [PubMed] [Google Scholar]
- 2.Lu HT, Tan HQ, Gu BX, Wu W, Li MH. Risk factors for multiple intracranial aneurysms rupture: a retrospective study. Clin Neurol Neurosurg. 2013;115(6):690–694. doi: 10.1016/j.clineuro.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Ellamushi HE, Grieve JP, Jager HR, Kitchen ND. Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg. 2001;94(5):728–732. doi: 10.3171/jns.2001.94.5.0728. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi AI, Suarez JI, Parekh PD, Sung G, Geocadin R, Bhardwaj A, Tamargo RJ, Ulatowski JA. Risk factors for multiple intracranial aneurysms. Neurosurgery. 1998;43(1):22–26. doi: 10.1097/00006123-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Crobeddu E, Panciani PP, Garbossa D, Pilloni G, Fornaro R, Ronchetti G, Spena G, Tartara F, Ducati A, Fontanella M: Cerebrovascular diseases in the elderly: the challenge of multiple aneurysms. Int J Neurosci 2014. In press. [DOI] [PubMed]
- 6.Hino A, Fujimoto M, Iwamoto Y, Yamaki T, Katsumori T. False localization of rupture site in patients with multiple cerebral aneurysms and subarachnoid hemorrhage. Neurosurgery. 2000;46(4):825–830. doi: 10.1097/00006123-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 8.Backes D, Vergouwen MD, Velthuis BK, van der Schaaf IC, Bor AS, Algra A, Rinkel GJ. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke. 2014;45(5):1299–1303. doi: 10.1161/STROKEAHA.113.004421. [DOI] [PubMed] [Google Scholar]
- 9.de Rooij NK, Velthuis BK, Algra A, Rinkel GJ. Configuration of the circle of Willis, direction of flow, and shape of the aneurysm as risk factors for rupture of intracranial aneurysms. J Neurol. 2009;256(1):45–50. doi: 10.1007/s00415-009-0028-x. [DOI] [PubMed] [Google Scholar]
- 10.Valen-Sendstad K, Steinman DA. Mind the gap: impact of computational fluid dynamics solution strategy on prediction of intracranial aneurysm hemodynamics and rupture status indicators. AJNR Am J Neuroradiol. 2014;35(3):536–543. doi: 10.3174/ajnr.A3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo B, Yang X, Wang S, Li H, Chen J, Yu H, Zhang Y, Zhang Y, Mu S, Liu Z, Ding G. High shear stress and flow velocity in partially occluded aneurysms prone to recanalization. Stroke. 2011;42(3):745–753. doi: 10.1161/STROKEAHA.110.593517. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Wang S, Chen J, Yu H, Zhang Y, Jiang F, Mu S, Li H, Yang X. Influence of hemodynamics on recanalization of totally occluded intracranial aneurysms: a patient-specific computational fluid dynamic simulation study. J Neurosurg. 2012;117(2):276–283. doi: 10.3171/2012.5.JNS111558. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Mu S, Chen J, Wang S, Li H, Yu H, Jiang F, Yang X. Hemodynamic analysis of intracranial aneurysms with daughter blebs. Eur Neurol. 2011;66(6):359–367. doi: 10.1159/000332814. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Xiang J, Zhang Y, Wang Y, Li H, Meng H, Yang X: Morphologic and hemodynamic analysis of paraclinoid aneurysms: ruptured versus unruptured. J Neurointerventional Surgery 2013. in press. [DOI] [PubMed]
- 15.Cebral JR, Mut F, Weir J, Putman CM. Association of hemodynamic characteristics and cerebral aneurysm rupture. Am J Neuroradiol. 2011;32(2):264–270. doi: 10.3174/ajnr.A2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jou LD, Lee DH, Morsi H, Mawad ME. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. AJNR Am J Neuroradiol. 2008;29(9):1761–1767. doi: 10.3174/ajnr.A1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizoi K, Suzuki J, Yoshimoto T. Surgical treatment of multiple aneurysms. review of experience with 372 cases. Acta Neurochir (Wien) 1989;96(1–2):8–14. doi: 10.1007/BF01403489. [DOI] [PubMed] [Google Scholar]
- 18.Orz Y, Osawa M, Tanaka Y, Kyoshima K, Kobayashi S. Surgical outcome for multiple intracranial aneurysms. Acta Neurochir (Wien) 1996;138(4):411–417. doi: 10.1007/BF01420303. [DOI] [PubMed] [Google Scholar]
- 19.Rinne J, Hernesniemi J, Niskanen M, Vapalahti M. Management outcome for multiple intracranial aneurysms. Neurosurgery. 1995;36(1):31–37. doi: 10.1227/00006123-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Imhof HG, Yonekawa Y. Management of ruptured aneurysms combined with coexisting aneurysms. Acta Neurochir Suppl. 2005;94:93–96. doi: 10.1007/3-211-27911-3_14. [DOI] [PubMed] [Google Scholar]
- 21.Jou L-D, Morsi H, Shaltoni HM, Mawad ME. Hemodynamics of small aneurysm pairs at the internal carotid artery. Med Eng Phys. 2012;34(10):1454–1461. doi: 10.1016/j.medengphy.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Crompton MR. Mechanism of growth and rupture in cerebral berry aneurysms. Br Med J. 1966;1(5496):1138–1142. doi: 10.1136/bmj.1.5496.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan TG, Huston J, 3rd, Brown RD, Jr, Wiebers DO, Piepgras DG. Intracranial saccular aneurysm enlargement determined using serial magnetic resonance angiography. J Neurosurg. 2002;97(5):1023–1028. doi: 10.3171/jns.2002.97.5.1023. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi T, Nishimura S, Kanamori M, Takazawa H, Omodaka S, Sato K, Maeda N, Yokoyama Y, Midorikawa H, Sasaki T, Nishijima M. Distinctive flow pattern of wall shear stress and oscillatory shear index: similarity and dissimilarity in ruptured and unruptured cerebral aneurysm blebs. J Neurosurg. 2012;117(4):774–780. doi: 10.3171/2012.7.JNS111991. [DOI] [PubMed] [Google Scholar]
- 25.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC, International Study of Unruptured Intracranial Aneurysms Investigators Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–110. doi: 10.1016/S0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 26.The International Study of Unruptured Intracranial Aneurysms Investigators: Unruptured intracranial aneurysms — risk of rupture and risks of surgical intervention.New England J Med 1998, 339(24):1725-1733. [DOI] [PubMed]
- 27.Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. 2013;44(9):2414–2421. doi: 10.1161/STROKEAHA.113.001838. [DOI] [PubMed] [Google Scholar]
- 28.Jagadeesan BD, Delgado Almandoz JE, Kadkhodayan Y, Derdeyn CP, Cross DT, 3rd, Chicoine MR, Rich KM, Zipfel GJ, Dacey RG, Moran CJ. Size and anatomic location of ruptured intracranial aneurysms in patients with single and multiple aneurysms: a retrospective study from a single center. J Neurointerventional Surg. 2014;6(3):169–174. doi: 10.1136/neurintsurg-2012-010623. [DOI] [PubMed] [Google Scholar]
- 29.Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K. Morphological differences between ruptured and unruptured cases in middle cerebral artery aneurysms. Neurosurgery. 2008;62(3):602–609. doi: 10.1227/01.NEU.0000311347.35583.0C. [DOI] [PubMed] [Google Scholar]
- 30.Ujiie H, Tachibana H, Hiramatsu O, Hazel AL, Matsumoto T, Ogasawara Y, Nakajima H, Hori T, Takakura K, Kajiya F. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of intracranial aneurysms. Neurosurgery. 1999;45(1):119–129. doi: 10.1097/00006123-199907000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K, Morita A, Kirino T. Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke. 2004;35(11):2500–2505. doi: 10.1161/01.STR.0000144648.89172.0f. [DOI] [PubMed] [Google Scholar]
- 32.Kadasi LM, Dent WC, Malek AM. Colocalization of thin-walled dome regions with low hemodynamic wall shear stress in unruptured cerebral aneurysms. J Neurosurg. 2013;119(1):172–179. doi: 10.3171/2013.2.JNS12968. [DOI] [PubMed] [Google Scholar]
- 33.Omodaka S, Sugiyama S, Inoue T, Funamoto K, Fujimura M, Shimizu H, Hayase T, Takahashi A, Tominaga T. Local hemodynamics at the rupture point of cerebral aneurysms determined by computational fluid dynamics analysis. Cerebrovasc Dis. 2012;34(2):121–129. doi: 10.1159/000339678. [DOI] [PubMed] [Google Scholar]
- 34.Jansen IG, Schneiders JJ, Potters WV, van Ooij P, van den Berg R, van Bavel E, Marquering HA, Majoie CB. Generalized versus patient-specific inflow boundary conditions in computational fluid dynamics simulations of cerebral aneurysmal hemodynamics. AJNR Am J Neuroradiol. 2014;35(8):1543–1548. doi: 10.3174/ajnr.A3901. [DOI] [PMC free article] [PubMed] [Google Scholar]


