Abstract
Background & Objectives
Little is known about actual dietary patterns and their associations with clinical outcomes in hemodialysis patients. We identified dietary patterns in hemodialysis patients in Japan and examined associations between dietary patterns and clinical outcomes.
Design, setting, participants, measurements
We used data from 3,080 general-population participants in the Hisayama study (year 2007), and data from 1,355 hemodialysis patients in the Japan Dialysis Outcomes and Practice Patterns Study (JDOPPS: years 2005–2007). Food intake was measured using a brief self-administered diet-history questionnaire (BDHQ). To identify food groups with the Hisayama population data, we used principal components analysis with Promax rotation. We adjusted the resulting food groups for total daily energy intake, and then we used those adjusted food-group scores to identify dietary patterns in the JDOPPS patients by cluster analysis (Ward’s method). We then used Cox regression to examine the association between dietary patterns and a composite of adverse clinical outcomes: hospitalization due to cardiovascular disease or death due to any cause.
Results
We identified three food groups: meat, fish, and vegetables. Using those groups we then identified three dietary patterns: well-balanced, unbalanced, and other. After adjusting for potential confounders, we found an association between an unbalanced diet and important clinical events (hazard ratio 1.90, 95% C.I. 1.19–3.04).
Conclusions
Hemodialysis patients whose diet was unbalanced were more likely to have adverse clinical outcomes. Thus hemodialysis patients might benefit not only from portion control, but also from a diet that is well-balanced diet with regard to the food groups identified here as meat, fish, and vegetables.
Introduction
Dietary management is important to improve outcomes in hemodialysis patients. Clinical guidelines provide a recommended intake of micronutrients[1] to prevent hyperphosphatemia, hyperkalemia, hypertension, and water retention. Reduced intakes of protein, raw vegetables, and salt are recommended.[2–8] Excessive dietary restriction may of course result in malnutrition, but details of dietary patterns that might improve outcomes in hemodialysis patients are largely unknown.
Some previous research on nutritional epidemiology in kidney disease has focused on the absolute amounts of foods and micronutrients[7,9]. We focused instead on dietary patterns, which were identified by their balance (or unbalance) among food groups. Given that the prognosis of hemodialysis patients is better in Japan than in the US and Europe, we expected that an understanding of the relationship between dietary pattern and prognosis in hemodialysis patients in Japan would also provide useful information for hemodialysis care in other countries.
Here we report the results of a cohort study using data from hemodialysis patients participating in the Japan Dialysis Outcomes and Practice Patterns Study (JDOPPS) [10,11]. Our goals were to identify dietary patterns in those patients and to investigate relationships between dietary patterns and important clinical outcomes.
Methods
Ethics
The ethics committees of Kyushu University (Fukuoka, Japan) and Kyoto University (Kyoto, Japan) approved this study. Written informed consent was obtained from participants in the Hisayama study[12,13] and in the JDOPPS. The data were analyzed anonymously.
Participants and setting
The participants were selected from among Japanese volunteers participating in the Hisayama study[12,13] and Japanese hemodialysis patients participating in the JDOPPS.
The Hisayama study is a population-based study that has been conducted since 1961 in Hisayama-cho in the Kyushu region of Japan. Subjects are volunteers of various ages, and are considered to represent the age distribution of the population of Japan.[14,15] In the present study, we analyzed data from 3,080 people enrolled in the Hisayama study in 2007.
The JDOPPS is part of the International Dialysis Outcomes and Practice Patterns Study, an international longitudinal study of hemodialysis patients. Patients in the JDOPPS were selected randomly from among representative dialysis facilities in Japan, and they appear to represent all hemodialysis patients in Japan. The design of the DOPPS is detailed elsewhere.[16] After we excluded data from hemodialysis patients whose dietary intake was not measured and those with a daily energy intake of less than 500 kcal or more than 4,000 kcal, data from 1,355 hemodialysis patients who participated in the third phase of the JDOPPS between 2005 and 2007 were available for analysis.
The predictors
The methods regarding the predictors had four steps: (1) collection of data on food consumption, (2) identification of food groups, (3) computation of food-group scores, and (4) identification of dietary patterns. Those four steps are described in sequence below. We note that this method for identifying dietary patterns is based on foods and food groups, not on micronutrients, and that methods such as the one we used in this study are common in nutritional epidemiology.[17–20]
(1) Collection of data on food consumption (Hisayama study): Data on foods consumed were obtained using a brief self-administered diet-history questionnaire (the BDHQ).[21–23] The BDHQ is a 4-page structured questionnaire that contains questions about 58 foods and beverages, and allows the total energy intake and the intake of micronutrients to be estimated. Reports of previous studies indicate that food intake estimated using the BDHQ is consistent with intake measured using semi-weighted 16-day dietary records.[21,24] Food intake was measured with the BDHQ in the Hisayama study in 2007 and in the JDOPPS during the second year of JDOPPS enrollment, between 2006 and 2007.
(2) Identification of food groups (Hisayama study): To identify food groups, we conducted a principal components analysis (PCA). We used PCA with Promax rotation to reduce the results regarding the many foods listed in the BDHQ to a smaller set of food groups. That is, we used PCA to identify groups of foods that were eaten with approximately equal frequencies by the same people. We did those analyses with data from 3,080 participants in the Hisayama study. Here it is important to remember one similarity between PCA and other multivariate analyses: When the values of an independent variable are nearly the same among almost all participants, then that independent variable contributes little or no information to the results, and such variables should be deleted from the analyses. Therefore, in PCA it is common practice to delete items that vary by only small amounts between individuals [25], so for the PCA in this study we used 20 foods from the 58 in the BDHQ.
(3) Computation of food-group scores (Hisayama study and JDOPPS): After identifying food groups, we standardized the frequency of consumption of each food by using the mean and standard deviation in the Hisayama data. Then we used those standardized frequencies to compute food-group scores for each JDOPPS patient, and we used the residual method [26] to adjust those food-group scores for the total daily energy intake
(4) Identification of dietary patterns (JDOPPS): To identify dietary patterns in the JDOPPS patients, we used Ward’s method of cluster analysis[27] on the energy-adjusted food-group scores. Thus, the patterns we identified were based on the relative amounts of foods from each food group that the JDOPPS patients actually ate.
The outcome
This study had one outcome, which was a composite of important adverse clinical events: hospitalization due to cardiovascular disease or death due to any cause. Cardiovascular disease included coronary heart disease, arrhythmia, congestive heart failure, cardiac valvular disease, cardiac myopathy, and pericarditis. The date and cause of hospitalization was ascertained approximately every 4 months in the JDOPPS.
Analyses (associations between dietary patterns and the outcome)
Cox regression analysis was used to investigate relationships between dietary patterns and the composite outcome. Those relationships were expressed as hazard ratios. The time between the second year of food-intake measurement using the BDHQ and the composite outcome was studied first. Two models were used. In Model 1, the covariates considered in estimating the hazard ratio were age, sex, and hemodialysis duration. In Model 2, the covariates were body mass index, serum albumin, total daily energy intake, and comorbid conditions (coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, and diabetes).
In a sensitivity analysis, we adjusted for hemoglobin level, the dose of erythropoietin-stimulating agent (ESA), and Kt/V, in addition to the covariates included in Model 2. In another sensitivity analysis, we adjusted for smoking habit in addition to the covariates included in Model 2.
All analyses were done with SAS 9.2 (SAS Institute, Cary, NC) and STATA 13.1 software (STATA, College Station, TX).
Results
Population characteristics
Table 1 shows the characteristics of participants in the Hisayama study and in the JDOPPS. We included 3,080 participants from the Hisayama study. The mean of their ages was 62.7 years, and 10.6% of them had diabetes. We also included 1,355 hemodialysis patients from the JDOPPS. The mean of their ages was 61.4 years, and 32.1% of them had diabetes. The mean duration of their dialysis was 7.6 years. The proportions of comorbidities, including diabetes and cardiovascular disease, were higher in the JDOPPS group than in the Hisayama group.
Table 1. Demographic and clinical characteristics of the participants in the Hisayama study and in the JDOPPS.
| Hisayama(n = 3,080) | JDOPPS(n = 1,355) | |
|---|---|---|
| Mean (SD) age, years | 62.7 (12.0) | 61.4 (11.9) |
| Male (%) | 43.6 | 61.4 |
| Mean (SD) dialysis duration, years | NA | 7.6 (7.2) |
| Mean (SD) BMI | 23.1 (3.5) | 21.1 (3.2) |
| Comorbid conditions (%) | ||
| Diabetes | 10.6 | 32.1 |
| Coronary heart disease | 6.0 | 41.3 |
| Cerebrovascular disease | 3.7 | 11.5 |
| Other cardiovascular disease | 8.2 | 30.6 |
| Peripheral vascular disease | 0.2 | 15.9 |
| Cancer | 7.5 | 9.4 |
| Mean (SD) albumin, g/dL | 4.2 (0.3) | 3.8 (0.4) |
JDOPPS: Japan Dialysis Outcomes and Practice Patterns Study, NA: not applicable, BMI: body mass index.
Food groups (general–population results)
In the first PCA, “natto (fermented soybean)” had a moderate loading on 2 components. We therefore deleted “natto” and ran the PCA again. The first three components had eigenvalues greater than 1: 5.69, 1.53, and 1.35, which accounted for 28.4%, 7.8%, and 6.74% of the variance, respectively. As shown in Table 2, three food groups were identified. The first group included carrot & pumpkin, root vegetables, cabbage (cooked), mushrooms, seaweed, lettuce & cabbage (raw), potatoes, tofu (bean curd) & fried tofu, turnip (radish), and tomato. This we call the vegetables group. The second group included squid & octopus & shrimp & shellfish, dried fish, fatty fish, lean fish, and small fish with bones. This we call the fish group. The third group included ham, pork & beef, chicken, and eggs. This we call the meat group.
Table 2. Coefficients after Promax rotation (Principal Components Analysis, Hisayama data).
| Component | ||||
|---|---|---|---|---|
| Food Item | 1 | 2 | 3 | |
| Carrot/pumpkin | 0.798 | −0.079 | 0.001 | |
| Root vegetables | 0.754 | −0.055 | −0.022 | |
| Green leafy vegetables | 0.705 | −0.092 | 0.067 | |
| Cabbage (cooked) | 0.686 | −0.146 | 0.148 | |
| Mushrooms | 0.636 | 0.058 | 0.013 | |
| Seaweed | 0.591 | 0.135 | −0.127 | |
| Lettuce/cabbage (raw) | 0.543 | −0.122 | 0.260 | |
| Potatoes | 0.530 | 0.168 | −0.057 | |
| Tofu (bean curd)/fried tofu | 0.504 | 0.154 | −0.026 | |
| Turnip (radish) | 0.497 | 0.189 | −0.106 | |
| Tomato | 0.426 | 0.104 | −0.003 | |
| Dried fish | −0.081 | 0.693 | 0.087 | |
| Fatty fish | 0.027 | 0.630 | 0.116 | |
| Lean fish | 0.103 | 0.594 | −0.034 | |
| Small fish with bones | 0.160 | 0.591 | −0.179 | |
| Squid, octopus, shrimp, shellfish | −0.095 | 0.536 | 0.248 | |
| Ham | −0.135 | 0.042 | 0.718 | |
| Pork/beef | 0.054 | 0.019 | 0.715 | |
| Chicken | 0.057 | 0.121 | 0.564 | |
| Eggs |
|
0.116 |
−0.012 |
0.474 |
| Coefficents of correlation |
1 | 1.000 | 0.430 | 0.267 |
| 2 | 1.000 | 0.242 | ||
| 3 |
|
|
1.000 |
|
| Deleted food | ||||
| Natto | ||||
Data on 20 foods were analyzed with principal components analysis (Promax rotation). The first three components had eigenvalues greater than 1: 5.69, 1.53, and 1.35, which accounted for 28.4%, 7.8%, and 6.74% of the variance.
Dietary patterns in hemodialysis patients
Cluster analysis of the adjusted food-group scores revealed three clusters, which we call (1) “well-balanced diet”, (2) “unbalanced diet,” and (3) “other diet” (Table 3). Patients in the first of those three clusters, i.e. those whose diet was well-balanced, were those who ate approximately equal amounts of food from the meat, fish, and vegetable groups. Almost half of the JDOPPS patients had a well-balanced diet (49.2%). Patients in the second of the three clusters, i.e. those whose diet was unbalanced, were those who ate a much larger amount from the vegetable group than from the meat group and the fish group. They amounted to 14% of the JDOPPS patients.
Table 3. Adjusted food-group scores for each cluster (JDOPPS data).
| Food-group score | ||||
|---|---|---|---|---|
| Cluster | n | Vegetables | Fish | Meat |
| Well-balanced | 666 49.2% | 0.297 (0.460) | 0.216 (0.936) | 0.319 (0.874) |
| Unbalanced | 189 14.0% | 1.522 (0.454) | 0.528 (0.809) | 0.315 (0.838) |
| Other | 500 36.9% | −0.971 (0.643) | −0.488 (0.945) | −0.544 (0.980) |
Each food-group score was adjusted for total daily energy intake by the residual method [20]. Values in parentheses are standard deviations.
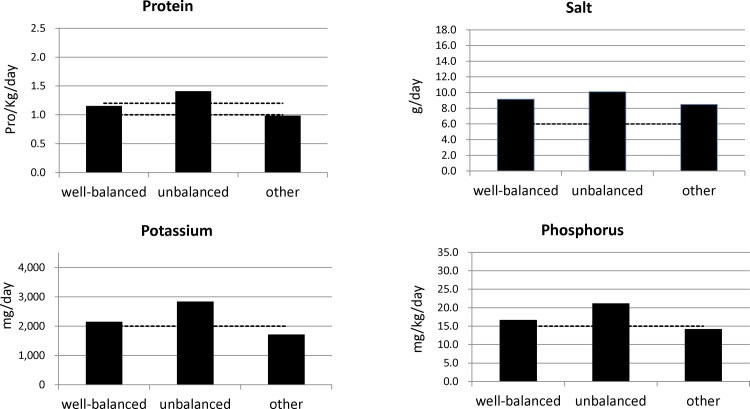
Fig. 1 shows the amounts of micronutrients for each cluster of JDOPPS patients. According to clinical guidelines, protein intake was within the prescribed range among those who ate a well-balanced diet, too high among those who ate an unbalanced diet, and too low among the others. [1] The mean salt intake was more than 6 g/day in all groups, and was highest among those who ate an unbalanced diet. Potassium intake was within the prescribed range among those who ate a well-balanced diet, too high among those who ate an unbalanced diet, and too low among the others. Phosphorus intake was similar to potassium intake.
Figure 1. Micronutrient intake stratified by dietary pattern.
Estimated micronutrient intake stratified by dietary pattern. Dotted lines show dietary standards according to Japan’s clinical guidelines (Dietary recommendations for chronic kidney disease, 2007, Japanese Society of Nephrology).
Patient characteristics by dietary pattern
Table 4 shows characteristics of the JDOPPS patients, stratified by the three dietary patterns. Patients who ate an unbalanced diet were older than those who ate a well-balanced diet, and fewer of them were male. Total daily energy intake, protein intake, salt intake, and potassium intake were highest among those whose diet was unbalanced.
Table 4. JDOPPS patient characteristics at baseline, by dietary pattern (n = 1,355).
| Well-balanced (49.2%) | Unbalanced (14.0%) | Other (36.9%) | |
|---|---|---|---|
| Mean (SD) age, years | 62.3 (11.8) | 64.2 (11.9) | 59.2 (11.5) |
| Male (%) | 57.2 | 40.7 | 74.8 |
| Mean (SD) dialysis duration, years | 7.7 (7.4) | 7.2 (7.2) | 7.6 (7.0) |
| Mean (SD) BMI | 21.2 (3.3) | 20.2 (3.0) | 21.5 (3.1) |
| Comorbid conditions (%) | |||
| Diabetes | 31.7 | 32.8 | 32.2 |
| Coronary Heart Disease | 44.3 | 41.8 | 37.2 |
| Cerebrovascular Disease | 12.8 | 12.7 | 9.4 |
| Other Cardiovascular Disease | 28.5 | 36.0 | 31.2 |
| Peripheral Vascular Disease | 15.6 | 22.2 | 14.0 |
| Cancer | 8.7 | 9.6 | 10.3 |
| Mean (SD) serum albumin, g/dL | 3.8 (0.4) | 3.8 (0.5) | 3.9 (0.4) |
| Mean (SD) phosphorus, mg/dL | 5.6 (1.3) | 5.3 (1.4) | 5.6 (1.4) |
| Mean (SD) serum potassium, mEq/L | 5.1 (0.8) | 5.1 (0.8) | 5.0 (0.8) |
| Mean (SD) energy intake, cal/Kg/day | 1592 (563) | 1707 (538) | 1640 (656) |
| Mean (SD) protein intake, g/Kg/day | 1.16 (0.51) | 1.41 (0.59) | 0.99 (0.49) |
| Mean (SD) salt intake, g/day | 9.16 (3.32) | 10.10 (3.21) | 8.48 (3.60) |
| Mean (SD) potassium intake, g/day | 2.15 (0.86) | 2.84 (1.01) | 1.72 (0.84) |
| Mean (SD) phosphorus intake, mg/day | 883 (370) | 1018 (376) | 793 (395) |
The “well-balanced diet” was characterized by approximately equal intake of the three food groups (fish, meat, and vegetables). The “unbalanced diet” was characterized by relatively large vegetable intake compared with meat and fish intake, and the “other diet” refers to other intake patterns.
Association between dietary pattern and clinical outcomes in hemodialysis patients
Table 5 shows associations between dietary patterns and the composite outcome. In Model 1, which included adjustments for age, gender, and dialysis duration, the unbalanced diet was associated with a higher event rate than the well-balanced diet (adjusted hazard ratio [HR] 1.81, 95% CI 1.15–2.85). A similar association was seen in Model 2 (adjusted HR 1.90, 95% CI 1.19–3.04), that is, after adjustment for serum albumin, BMI, and total daily energy intake, in addition to the covariates included in Model 1.
Table 5. Dietary patterns and the composite outcome (JDOPPS data).
| Dietary patterns | Composite outcome rate (/100 person-years) | Model 1 | Model 2 |
|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||
| Well-balanced | 7.4 | Reference | |
| Unbalanced | 10.3 | 1.81 (1.15–2.85) | 1.90 (1.19–3.04) |
| Other | 6.1 | 1.23 (0.82–1.83) | 1.21 (0.81–1.82) |
The composite outcome included hospitalization due to cardiovascular disease, and death due to any cause. Model 1: Adjusted for age, gender, and dialysis duration. Model 2: Adjusted for age, gender, dialysis duration, serum albumin, BMI, total daily energy intake, and comorbid conditions (diabetes, coronary heart disease, cerebrovascular disease, other cardiovascular disease, and peripheral vascular disease).
In the sensitivity analysis adjusted for the covariates included in Model 2 and also adjusted for hemoglobin level, ESA dose, and single-pool Kt/V, we also found a similar association between unbalanced diet and the composite outcome (adjusted HR 1.89, 95% CI 1.11–3.23). In the other sensitivity analysis, adjusted for the covariates included in Model 2 and also for smoking habit, we again found a similar association between unbalanced diet and adverse clinical events (adjusted HR 1.85, 95% CI 1.16–2.97).
Discussion
Using PCA with data from a representative sample of the general population of Japan, we identified three food groups: meat, fish, and vegetables. We then found that hemodialysis patients could be said to have diets that were “well-balanced” or “unbalanced” with regard to those three food groups. (As noted previously, to identify dietary patterns based on foods or on food groups, as we did in this study, is common in nutritional epidemiology.[17–19]) The hemodialysis patients whose diet was unbalanced were more likely to have important clinical events. These findings suggest that limiting food portions, which is often recommended for hemodialysis patients to prevent severe adverse clinical outcome, is not enough. In addition to portion control, a diet that is balanced among meat, fish, and vegetables might help to prevent adverse outcomes.
Nutritional epidemiologic research in hemodialysis patients has largely focused on relationships between individual food items, micronutrients, and outcomes. For example, relationships between fish consumption, phosphate consumption, and outcomes in these patients have been reported.[7,28] However, hemodialysis patients do not eat only one specific food item, but rather various foods, and therefore dietary patterns should be determined on the basis of the combinations of foods that people actually eat. We began with PCA, from which we identified three groups of foods that are in fact eaten by people in Japan: meat, fish, and vegetables. We then used cluster analysis, from which we identified hemodialysis patients’ actual patterns of food consumption with reference to those groups. Those patterns (well-balanced, unbalanced, and other) were associated with important clinical outcomes.
In hemodialysis patients, adequate protein intake (1.0 to 1.2 g/kg per day), such as can be obtained from the meat and fish groups we identified, is recommended to counteract loss of protein via the dialysate.[29] Sufficient protein intake is critical to preventing malnutrition, but excessive protein intake may lead to hyperphosphatemia, which may in turn lead to cardiovascular events. Hemodialysis patients should also avoid excessive vegetable intake to prevent hyperkalemia, which, like hyperphosphatemia, is associated with cardiovascular events. It is therefore physiologically plausible that a diet well-balanced among food groups would be associated with good clinical outcomes, as was found in this study.
The present study had a number of strengths. First, the Hisayama study and the JDOPPS used representative samples of the general population of Japan and of hemodialysis patients in Japan, respectively. Therefore the findings should be generalizable to all hemodialysis patients in Japan. To the extent that differences in dietary patterns between hemodialysis patients in Japan and those in other countries can result in differences in clinical outcomes, the present findings might be used for nutritional research and possibly also for dietary recommendations to improve the prognosis of patients in, for example, the US and Europe. Second, the use of the BDHQ enabled us to measure food intake in clinical settings.[21–24] Third, results of the sensitivity analyses indicated that the association of dietary pattern with the composite outcome was robust with respect to hemoglobin level, ESA dose, Kt/V, and smoking habit.
One possible limitation of this study is that food intake was self-reported. Actual food intake might have differed from that estimated from the food-frequency questionnaire.[30] In particular, social-desirability bias might have caused hemodialysis patients, who were aware of their dietary proscriptions, to report inaccurately-low levels of food intake, and the estimated intake of micronutrients might therefore have been incorrect.
In summary, eating a diet that was not balanced among meat, fish, and vegetables was associated with important adverse clinical events, which suggests that hemodialysis patients should not only limit their food intake but should also strive for a proper balance among those three food groups.
Acknowledgments
We thank the residents of Hisayama for participating in the survey and the staff of the Division of Health and Welfare of Hisayama for cooperating in this study. We also thank the patients in the JDOPPS for participating in the survey, and the staff of the dialysis facilities participating in the JDOPPS. We thank Joseph Green for advice and suggestions.
Funding Statement
This study is supported by scientific research grants from Kyowa Hakko Kirin (Japan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kopple JD (2001) National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis 37: S66–70. [DOI] [PubMed] [Google Scholar]
- 2. Sanghavi S, Whiting S, Uribarri J (2013) Potassium balance in dialysis patients. Semin Dial 26: 597–603. 10.1111/sdi.12123 [DOI] [PubMed] [Google Scholar]
- 3. Sherman RA, Mehta O (2009) Dietary phosphorus restriction in dialysis patients: potential impact of processed meat, poultry, and fish products as protein sources. Am J Kidney Dis 54: 18–23. 10.1053/j.ajkd.2009.01.269 [DOI] [PubMed] [Google Scholar]
- 4. Mailloux LU (2000) The overlooked role of salt restriction in dialysis patients. Semin Dial 13: 150–151. [DOI] [PubMed] [Google Scholar]
- 5. Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, et al. (2009) Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 373: 1009–1015. 10.1016/S0140-6736(09)60212-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, et al. (2011) Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305: 1119–1127. 10.1001/jama.2011.308 [DOI] [PubMed] [Google Scholar]
- 7. Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, et al. (2010) Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol 5: 683–692. 10.2215/CJN.08601209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, et al. (2009) Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119: 671–679. 10.1161/CIRCULATIONAHA.108.807362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, et al. (2012) High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306. 10.1038/ki.2011.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Port FK, Eknoyan G (2004) The Dialysis Outcomes and Practice Patterns Study (DOPPS) and the Kidney Disease Outcomes Quality Initiative (K/DOQI): a cooperative initiative to improve outcomes for hemodialysis patients worldwide. Am J Kidney Dis 44: 1–6. [DOI] [PubMed] [Google Scholar]
- 11. Kuriyama S (2008) Characteristics of the clinical practice patterns of hemodialysis in Japan in consideration of DOPPS and the NKF/DOQI guidelines. Clin Exp Nephrol 12: 165–170. 10.1007/s10157-007-0020-7 [DOI] [PubMed] [Google Scholar]
- 12. Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Arima H, et al. (2003) Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke 34: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 13. Fukuhara M, Arima H, Ninomiya T, Hata J, Yonemoto K, et al. (2012) Impact of lower range of prehypertension on cardiovascular events in a general population: the Hisayama Study. J Hypertens 30: 893–900. 10.1097/HJH.0b013e328351d380 [DOI] [PubMed] [Google Scholar]
- 14. Katsuki S (1966) Epidemiological and clinicopathological study on cerebrovascular disease in Japan. Prog Brain Res 21: 64–89. [DOI] [PubMed] [Google Scholar]
- 15. Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, et al. (2013) Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961–2009). Circulation 128: 1198–1205. 10.1161/CIRCULATIONAHA.113.002424 [DOI] [PubMed] [Google Scholar]
- 16. Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, et al. (2004) The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 44: 7–15. [DOI] [PubMed] [Google Scholar]
- 17. Reedy J, Wirfalt E, Flood A, Mitrou PN, Krebs-Smith SM, et al. (2010) Comparing 3 dietary pattern methods—cluster analysis, factor analysis, and index analysis—With colorectal cancer risk: The NIH-AARP Diet and Health Study. Am J Epidemiol 171: 479–487. 10.1093/aje/kwp393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okubo H, Sasaki S, Horiguchi H, Oguma E, Miyamoto K, et al. (2006) Dietary patterns associated with bone mineral density in premenopausal Japanese farmwomen. Am J Clin Nutr 83: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 19. Wirfalt E, Midthune D, Reedy J, Mitrou P, Flood A, et al. (2009) Associations between food patterns defined by cluster analysis and colorectal cancer incidence in the NIH-AARP diet and health study. Eur J Clin Nutr 63: 707–717. 10.1038/ejcn.2008.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flood A, Rastogi T, Wirfalt E, Mitrou PN, Reedy J, et al. (2008) Dietary patterns as identified by factor analysis and colorectal cancer among middle-aged Americans. Am J Clin Nutr 88: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, et al. (2012) Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol 22: 151–159. 10.2188/jea.JE20110075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sasaki S, Yanagibori R, Amano K (1998) Self-administered diet history questionnaire developed for health education: a relative validation of the test-version by comparison with 3-day diet record in women. J Epidemiol 8: 203–215. [DOI] [PubMed] [Google Scholar]
- 23. Sasaki S, Ushio F, Amano K, Morihara M, Todoriki O, et al. (2000) Serum biomarker-based validation of a self-administered diet history questionnaire for Japanese subjects. J Nutr Sci Vitaminol (Tokyo) 46: 285–296. [DOI] [PubMed] [Google Scholar]
- 24. Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, et al. (2011) Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr 14: 1200–1211. 10.1017/S1368980011000504 [DOI] [PubMed] [Google Scholar]
- 25. Cattell RB (1965) Factor Analyis: An Introduction to Essentials. Ii. The Role of Factor Analysis in Research. Biometrics 21: 405–435. [PubMed] [Google Scholar]
- 26. Willett W, Stampfer MJ (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124: 17–27. [DOI] [PubMed] [Google Scholar]
- 27. Everitt BLS, Lesse M (2001) Cluster analysis. 4th ed. London: Arnold. [Google Scholar]
- 28. Kutner NG, Clow PW, Zhang R, Aviles X (2002) Association of fish intake and survival in a cohort of incident dialysis patients. Am J Kidney Dis 39: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 29. Combe C, McCullough KP, Asano Y, Ginsberg N, Maroni BJ, et al. (2004) Kidney Disease Outcomes Quality Initiative (K/DOQI) and the Dialysis Outcomes and Practice Patterns Study (DOPPS): nutrition guidelines, indicators, and practices. Am J Kidney Dis 44: 39–46. [DOI] [PubMed] [Google Scholar]
- 30. Mahabir S, Baer DJ, Giffen C, Subar A, Campbell W, et al. (2006) Calorie intake misreporting by diet record and food frequency questionnaire compared to doubly labeled water among postmenopausal women. Eur J Clin Nutr 60: 561–565. [DOI] [PubMed] [Google Scholar]