Abstract
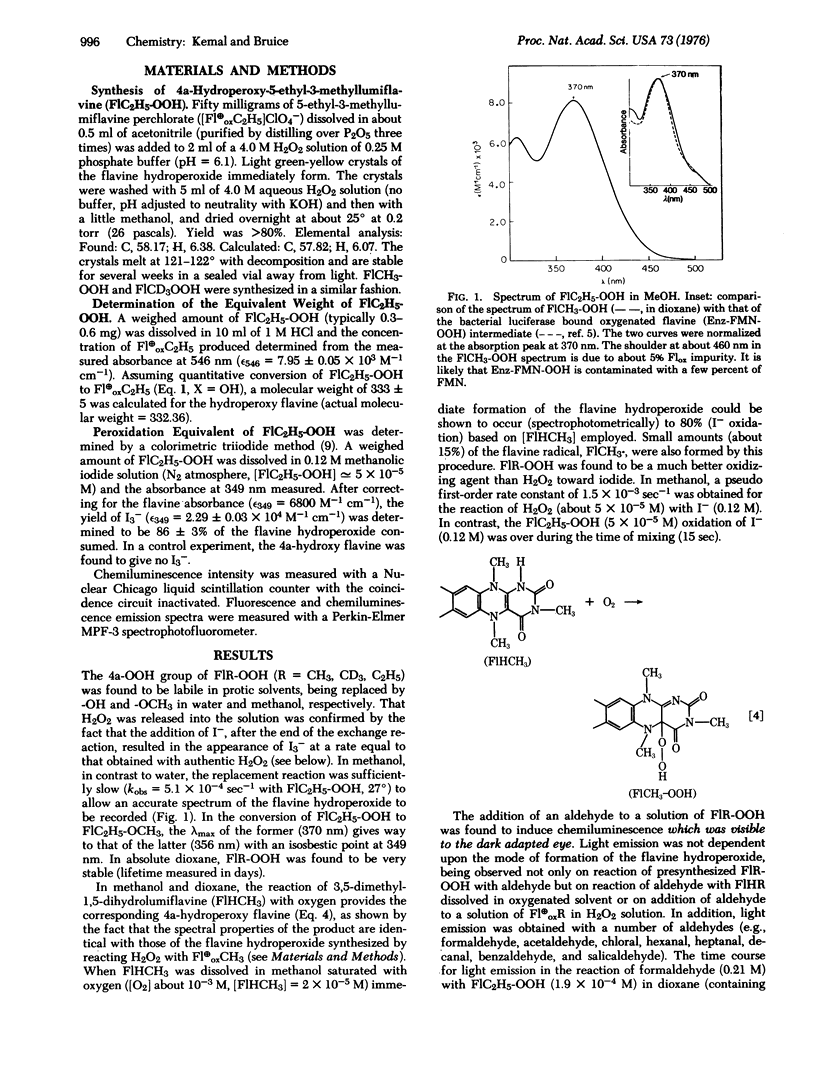
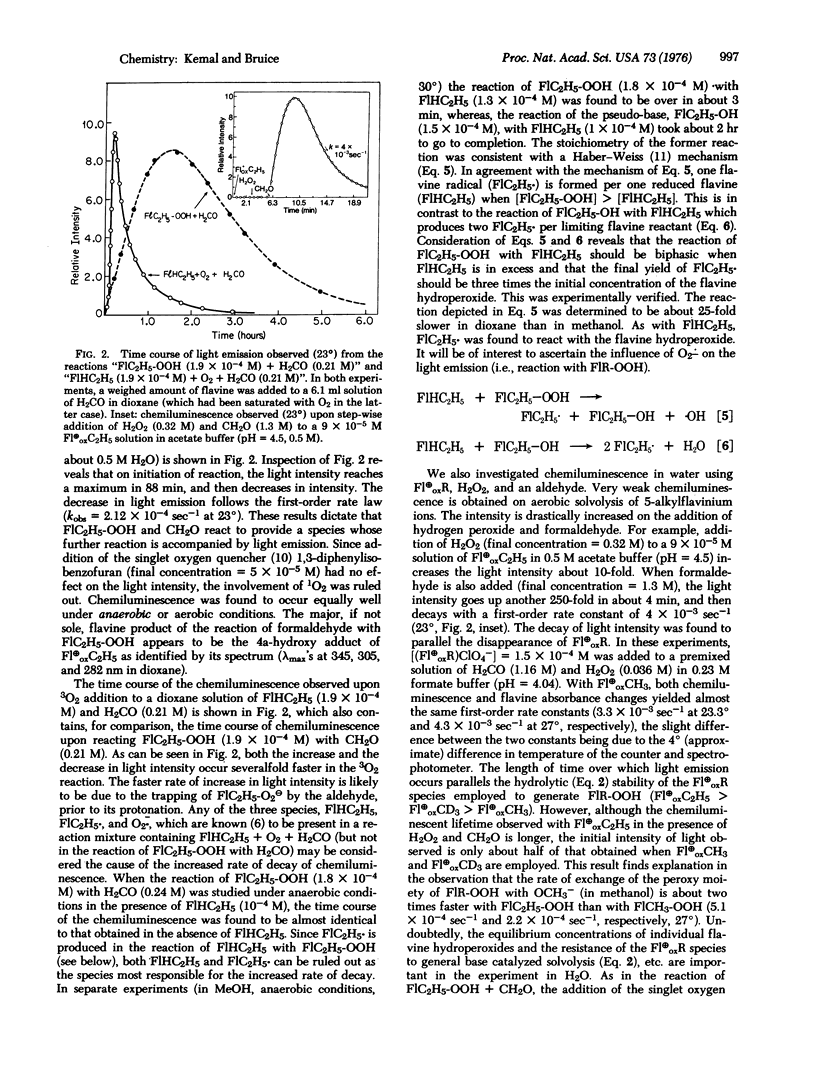
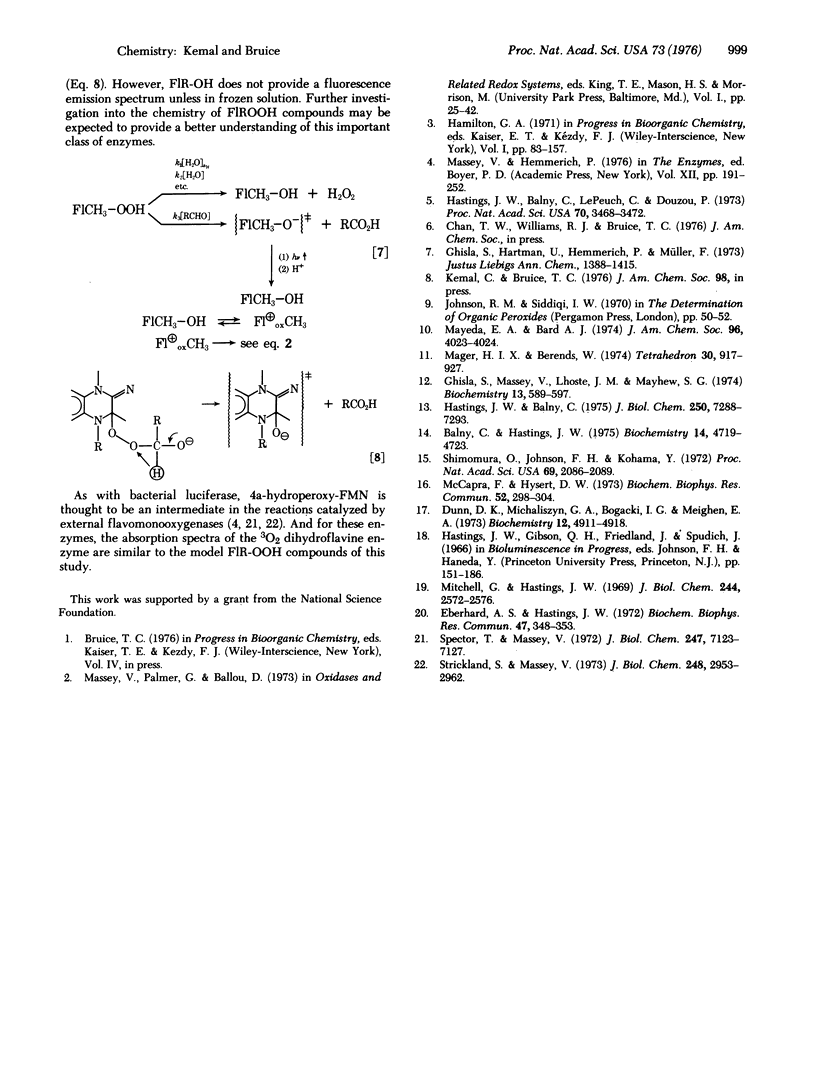
The solution chemistry of N(5)-alkyl flavinium cations and radical species formed by their le- reduction are discussed. Previously unknown, the 4a-flavine hydroperoxides are established to be formed on reaction of N(5)-alkyl flavinium cations with H2O2 or on reaction of N(5)-alkyl-1, 5-dihydroflavines with 3O2. The stability of the 4a-flavine hydroperoxide species is exemplified in the isolation and characterization of 4a-hydroperoxy-N(5)-ethyl-3-methyl-lumiflavine. 4a-Flavine hydroperoxide compounds are shown to be stronger oxidants than H2O2, and to undergo a chemiluminescent reaction in the presence of an aldehyde. Preliminary observations on the chemiluminescent reaction of 4a-flavine hydroperoxides + RCHO are provided, and these are compared to those in the literature dealing with the bioluminescence of bacterial luciferase in the presence of 3O2 and RCHO.
Full text
PDF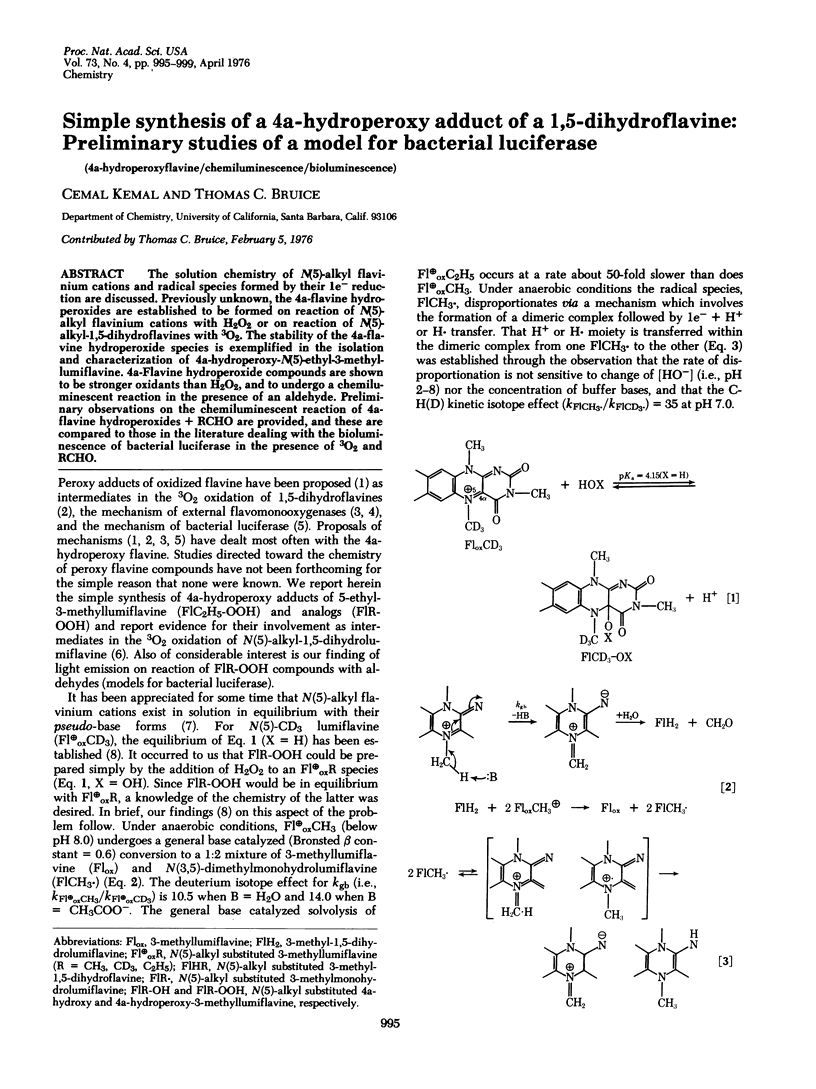

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balny C., Hastings J. W. Fluorescence and bioluminescence of bacterial luciferase intermediates. Biochemistry. 1975 Oct 21;14(21):4719–4723. doi: 10.1021/bi00692a024. [DOI] [PubMed] [Google Scholar]
- Dunn D. K., Michaliszyn G. A., Bogacki I. G., Meighen E. A. Conversion of aldehyde to acid in the bacterial bioluminescent reaction. Biochemistry. 1973 Nov 20;12(24):4911–4918. doi: 10.1021/bi00748a016. [DOI] [PubMed] [Google Scholar]
- Eberhard A., Hastings J. W. A postulated mechanism for the bioluminescent oxidation of reduced flavin mononucleotide. Biochem Biophys Res Commun. 1972 Apr 28;47(2):348–353. doi: 10.1016/0006-291x(72)90719-x. [DOI] [PubMed] [Google Scholar]
- Ghisla S., Massey V., Lhoste J. M., Mayhew S. G. Fluorescence and optical characteristics of reduced flavines and flavoproteins. Biochemistry. 1974 Jan 29;13(3):589–597. doi: 10.1021/bi00700a029. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Balny C., Peuch C. L., Douzou P. Spectral properties of an oxygenated luciferase-flavin intermediate isolated by low-temperature chromatography. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3468–3472. doi: 10.1073/pnas.70.12.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W., Balny C. The oxygenated bacterial luciferase-flavin intermediate. Reaction products via the light and dark pathways. J Biol Chem. 1975 Sep 25;250(18):7288–7293. [PubMed] [Google Scholar]
- Mayeda E. A., Bard A. J. Singlet oxygen. The suppression of its production in dismutation of superoxide ion by superoxide dismutase. J Am Chem Soc. 1974 Jun 12;96(12):4023–4024. doi: 10.1021/ja00819a054. [DOI] [PubMed] [Google Scholar]
- McCapra F., Hysert D. W. Bacterial bioluminescence-identification of fatty acid as product, its quantum yield and a suggested mechanism. Biochem Biophys Res Commun. 1973 May 1;52(1):298–304. doi: 10.1016/0006-291x(73)90987-x. [DOI] [PubMed] [Google Scholar]
- Mitchell G., Hastings J. W. The effect of flavin isomers and analogues upon the color of bacterial bioluminescence. J Biol Chem. 1969 May 25;244(10):2572–2576. [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Kohama Y. Reactions involved in bioluminescence systems of limpet (Latia neritoides) and luminous bacteria. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2086–2089. doi: 10.1073/pnas.69.8.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Massey V. p-Hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Reactivity with oxygen. J Biol Chem. 1972 Nov 25;247(22):7123–7127. [PubMed] [Google Scholar]
- Strickland S., Massey V. The mechanism of action of the flavoprotein melilotate hydroxylase. J Biol Chem. 1973 Apr 25;248(8):2953–2962. [PubMed] [Google Scholar]



