Abstract
The potato cyst nematode, Globodera rostochiensis, is an important pest of potato. Like other pathogens, plant parasitic nematodes are presumed to employ effector proteins, secreted into the apoplast as well as the host cytoplasm, to alter plant cellular functions and successfully infect their hosts. We have generated a library of ORFs encoding putative G. rostochiensis putative apoplastic effectors in vectors for expression in planta. These clones were assessed for morphological and developmental effects on plants as well as their ability to induce or suppress plant defenses. Several CLAVATA3/ESR-like proteins induced developmental phenotypes, whereas predicted cell wall-modifying proteins induced necrosis and chlorosis, consistent with roles in cell fate alteration and tissue invasion, respectively. When directed to the apoplast with a signal peptide, two effectors, an ubiquitin extension protein (GrUBCEP12) and an expansin-like protein (GrEXPB2), suppressed defense responses including NB-LRR signaling induced in the cytoplasm. GrEXPB2 also elicited defense response in species- and sequence-specific manner. Our results are consistent with the scenario whereby potato cyst nematodes secrete effectors that modulate host cell fate and metabolism as well as modifying host cell walls. Furthermore, we show a novel role for an apoplastic expansin-like protein in suppressing intra-cellular defense responses.
Introduction
Plants are hosts for microbial and viral pathogens, as well as for multicellular parasites such as insects, parasitic plants and nematodes. Despite the many biological differences between microbial and multicellular pathogens, many of the principles governing the molecular interactions between these pathogens and their hosts are thought to be similar. Plants detect microbes through the recognition of pathogen-associated molecular patterns (PAMPs), by membrane spanning proteins known as pattern-recognition receptors (PRRs). These PRRs induce PAMP-triggered immunity (PTI) by triggering signaling cascades that initiate mitogen-activated protein kinase (MAPK) cascades, production of reactive oxygen species (ROS), production of antimicrobial compounds, expression of pathogenesis related (PR) genes and localized deposition of callose [1].
To overcome PTI, host-adapted pathogens employ secreted proteins known as effectors to promote infection. Many effectors are delivered to the host cytoplasm and a number of these have been shown to interfere with intracellular signaling pathways induced by PTI mechanisms [2–4]. Many pathogens also deliver effector proteins to the plant apoplast, some of which have also been show to promote pathogenesis, either by masking PAMP recognition or by directly inhibiting host apoplastic defense proteins [5–10].
Effector proteins can also induce effector-triggered immunity (ETI) by virtue of their being recognized by the nucleotide-binding and leucine-rich repeat (NB-LRR) proteins encoded by disease resistance (R) genes [11]. NB-LRR proteins recognize effectors delivered to the host cytoplasm and induce a much stronger response than PTI, often associated with a type of cell death known as the hypersensitive response (HR). Apoplastic effectors can also induce ETI by being recognized by receptor-like proteins (RLPs) present in the plant plasma membrane [12,13].
The potato cyst nematode (PCN), Globodera rostochiensis, is an obligate biotroph and parasitizes crops such as potato, tomato and eggplant. After hatching from eggs in the soil, cyst nematodes penetrate the roots as infective juveniles and move intracellularly into the root cortex. Phytopathogenic nematodes secrete effector proteins through a hollow stylet either into the cytoplasm of the host cell or into the apoplast [14]. Like microbial effectors, several nematode effectors have been shown to inhibit plant defense responses and to be recognized by NB-LRR and RLP proteins [13,15,16]. Unlike most plant pathogens however, nematodes also induce dramatic changes in cell identity and metabolism. Cyst nematodes induce the development of a specialized feeding structure called a syncytium, a large, multinucleate, and metabolically active cell that provides nutrients to the developing nematode. The syncytium expands as a result of directed local cell wall break down of the initial syncytial and neighboring cells and subsequent fusion of the protoplast [17]. These alterations are presumed to be mediated, in large part, by secreted nematode effector proteins [14].
Most secreted effector-like proteins produced by cyst nematodes are believed to be produced in the pharyngeal gland cells (two subventral and one dorsal), and are thought to be delivered to the host through the stylet [18]. Subventral glands are highly active during the penetration and migratory stage of parasitism and tend to produce effector proteins presumed to function in the apoplast, such as cell wall-modifying proteins [14]. In the sedentary stage, the subventral glands shrink in size while the dorsal gland enlarges and becomes active during syncytium formation and maintenance [19]. Effectors produced in the latter gland are thought to be delivered to the host cytoplasm [14].
Several approaches have been used to identify effector proteins from nematodes, including cDNA-AFLP, microarrays, EST mining, proteomics and candidate gene approaches [14]. With other pathogens, local and systemic expression of effectors in planta via viral vectors has been shown to be an effective method to identify effectors that cause dramatic phenotypes in plants that may be indicative of their importance in pathogenesis. For example, the crinkler (CRN) family of proteins in Phytophthora infestans were defined by such a strategy and have since been shown to form a major class of effectors in all oomycetes [20].
We have identified at least fourteen putative G. rostochiensis apoplastic effector proteins from public databases. When transiently expressed by agroexpression and/or a potato virus X (PVX) expression vector in different solanaceous plants, approximately half of the effectors caused phenotypes, including the induction of cell death, chlorosis and dwarfing as well as developmental phenotypes. In addition, the ubiquitin extension protein GrUBCEP12, as well as an expansin-like protein GrEXPB2, also showed the potential to suppress defense-related cell death in Nicotiana benthamiana and/or N. tabacum. These extracellular effectors were able to inhibit the resistance mediated by the signaling induced by the N and Rx NB-LRR proteins in N. benthamiana. The GrEXPB2 protein also elicited defense responses in different plant species. Our results demonstrate that apoplastic effector proteins can affect intra-cellular signaling pathways and suggest novel functions for expansin-like proteins in plant-nematode interactions.
Material and Methods
Bacterial strains, plants growth conditions
Expression vectors based on PVX were delivered using Agrobacterium tumefaciens strain GV3101 by infiltration or toothpick inoculation [21]. All other binary vectors were delivered by Agro-infiltration using strain C58C1 as previously described [22]. All plants were grown at 22°C, 50% humidity in controlled growth chamber condition with 14h/10h light/dark cycle.
Construction of Gateway compatible plasmids
The gateway cassette with terminal attR recombination sites and ccdB selection gene was amplified by PCR from the vector pGBKCg with a forward primer including restriction sites XbaI and ClaI and a reverse primer containing an Xho1 site (S3 Table). The resulting PCR product was ligated into the SmaI site of pEAQ_35SE (Brosseau and Moffett unpublished) a derivative of pEAQSelectK [23] to make pEAQ_35SE-Gw (pEAQ35S). The gateway cassette was subsequently excised from pEAQ35S with XhoI and ClaI and cloned into the ClaI and SalI sites of pGR106 and pGR103 [21] to make pGR106-Gw (PVX) and pGR103-Gw (PVX-HB) respectively.
Identification and amplification of candidate secreted effector protein
Candidate secreted effector proteins (CSEPs) were identified by searching all Globodera rostochiensis predicted ORFs on NCBI and EST databases (http://www.nematodes.org/downloads/databases/NEMBASE4/GRC_nuc.fsa). The resulting collection of ORFs was investigated for the presence of N-terminal signal peptides in the predicted proteins using SignalP version 3.0 [24]. All proteins with a predicted signal peptide (SP) identified by hidden markov models (HMM) algorithm of SignalP version 3.0 http://www.cbs.dtu.dk/services/SignalP/) [24] were retained as potential candidate effectors. Proteins not predicted to be secreted by SignalP but referenced as secreted in the literature were also kept as CSEPs. To verify further, a pipeline of bioinformatics tools and software; TargetP, TMHMM and ProtComp as described previously [25] were used for the prediction of CSEPs.
Cloning of effectors and expression in planta
Pre-parasitic second-stage juveniles (pre-J2s) of G. rostochiensis or infected potato roots containing different nematode parasitic stages from Québec populations [26] were used for RNA isolation using Trizol as previously described [27]. mRNA was converted to cDNA by RT-PCR using an oligo dT primer and the superscript CellsDirect cDNA synthesis system (Invitrogen life technology). Selected genes were amplified with specific primers (S3 Table) using high fidelity KOD hot start DNA polymerase (EMD Millipore). Sense primers were designed to amplify the effectors including, at their 5′ end, the attB1 sequence (GGGGACAAGTTTGTACAAAAAAGCAGGCTTC) followed by a Kozak consensus sequence and a start codon (AGAACCATG). Reverse primers contained the attB2 sequence (GGGGACCACTTTGTACAAGAAAGCTGGGTC) followed by the gene-specific sequence including the native stop codon. Effector sequences differing from previously published sequences are listed in S2 Table and have been deposited in Genbank (accessions KF963513-KF963529) and are shown in S5 Fig.
PCR products were cloned into pDONR207 or pDONR221 using BP clonase and recombined into the gateway compatible binary vector pEAQ35S, PVX and PVX-HB by LR clonase reaction (Invitrogen) following the manufacturer’s instructions.
Four to six week-old N. tabacum, N. benthamiana, potato and tomato were used for agroinfiltration and agroinfection as previously described [21,28].
Cell death and disease resistance suppression assays
For cell death suppression assays, Agrobacterium strains carrying the CSEP either in the pEAQ35S or PVX constructs were diluted in 10 mM MgCl2 such that all effectors were infiltrated at a final OD600 of 0.2 and the cell death inducers and P38, the viral suppressor of RNA silencing of Turnip Crinkle Virus (TCV) [29], at a final OD600 of 0.1. A control with the cell death inducer and empty vector was always infiltrated on the opposite side of the leaf. All experiments were repeated at least three times. Cell death symptoms were scored 3–5 DPI and pictures were taken 5 DPI. Cell death suppression was assessed visually on a scale of 0 to 2. A complete absence of cell death was given a score of 2 while partial suppression was given a score of 1 and no suppression was attributed a score of zero. The overall suppression activity for suppressors was calculated from 12–15 infiltration sites. The scores from all infiltrated sites for a particular effector were added together and divided by the theoretical maximum score for that effector (i.e. the number of assays times two) to obtain a percentage cell death score.
PVX resistance assays induced by Rx or by N were performed as previously described [30,31].
Gene expression analysis by quantitative real-time RT-PCR (qRT-PCR) assays
RNA extractions and qRT-PCR assays (iQ SYBR Green Supermix; Bio-Rad Laboratories) were performed using primers listed in S3 Table as previously described [32,33]. mRNA samples for each developmental stage were prepared from two independent experiments and used for cDNA synthesis. All qPCR assays consisted of three technical replicates for each cDNA sample. The G. rostochiensis β-actin gene (Gract-1) (EF437156) was used as an endogenous reference for data analysis using the 2−ΔΔCt method [34]. For each developmental stage, 2−ΔΔCt represented the amount of the target gene expression that was normalized to Gract-1 and relative to a calibrator that had the lowest expression in the cyst or other life stage.
Results
Identification of candidate secreted effectors proteins (CSEPs) in G. rostochiensis
A common property of eukaryotic pathogen effectors is that they are secreted proteins, containing an N-terminal signal peptide (SP), regardless of whether their eventual location of action is in the cytoplasm or the apoplast [3]. From more than 373 G. rostochiensis sequences obtained from NCBI and the nematode EST database, NEMBASE4, we identified thirty-seven proteins having a putative SP. Of the proteins identified, many were known to be probable intracellular effectors (e.g. SPRYSEC proteins) and these will be described elsewhere. Several candidate ORFs are predicted to encode enzymes with pectate lyase, endoglucanase, glutathione peroxidase and metalloproteinase activities based on homology and previous reports [35,36]. Due to their predicted functions, these proteins are very likely to function in the apoplast (Table 1). In agreement with this, homologues of many of these proteins (S1 Table), have been reported either as being expressed in the esophageal glands and/or being secreted into the apoplast [13, 37,38]. Apoplastic effectors are often cysteine rich and we found that ten out of twenty-three of these proteins have four or more Cys residues in the mature protein (S1 Table). We also included in this study a number of effectors of unknown function whose location of action could not be predicted (Table 1).
Table 1. Description of cloned apoplastic secreted effector proteins.
| Effector | Predicted protein | Predicted localization | Predicted or known function | Phenotype observed in planta |
|---|---|---|---|---|
| GrCLE1 | CLE-like peptide | Apoplastic | Mimicking plant CLE peptides, involved in syncytium formation | downward curving leaves and chlorosis in N. benthamiana ¶ |
| GrCLE-4A | CLE-like peptide | Apoplastic | Mimicking plant CLE peptides, involved in syncytium formation | No visible phenotype |
| GrCLE-4D | CLE-like peptide | Apoplastic | Mimicking plant CLE peptides, involved in syncytium formation | downward curving leaves and chlorosis in N. benthamiana ¶ |
| GrCLE-4B | CLE-like peptide | Apoplastic | Mimicking plant CLEs peptides, involved in syncytium formation | downward curving leaves and chlorosis in N. benthamiana ¶ |
| GrCLE-4C | CLE-like peptide | Apoplastic | Mimicking plant CLE peptides, involved in syncytium formation | downward curving leaves and chlorosis in N. benthamiana ¶ |
| GrEXPB1 | Expansin-like protein | Apoplastic | Cell wall extension | No visible phenotype |
| GrEXPB2 | Expansin-like protein | Apoplastic | Cell wall extension | Chlorosis and dwarfing in N. benthamiana ¶; necrosis in tomato and potato; and suppression of host defense |
| GrVAP1 | Venom allergen protein | Apoplastic | Defense, potential avirulence protein | No visible phenotype |
| GrENG1 | Endoglucanase | Apoplastic | Cell wall modification | No visible phenotype |
| GrENG2 | Endoglucanase | Apoplastic | Cell wall modification | No visible phenotype |
| GrENG3 | Endoglucanase | Apoplastic | Cell wall modification | No visible phenotype |
| GrPEL1 | Pectate lyase | Apoplastic | Plant cell wall degradation | Severe malformation in the leaves and death at the end in N. benthamiana ¶; dwarfing and necrosis in tomato |
| GrPEL2 | Pectate lyase | Apoplastic | Plant cell wall degradation | Severe malformation in the leaves and death in N. benthamiana ¶; dwarfing and necrosis in tomato |
| GrMTP | Metalloprotease | Apoplastic | Protein degradation | Curling leaves and necrotic collapse of the leaves¶ |
| GrGPX | detoxification of ROS | Unknown | Detoxification of ROS and plant defense suppression | No visible phenotype |
| GrTPX | detoxification of ROS | Unknown | Detoxification of ROS and plant defense suppression | No visible phenotype |
| GrAMS1 | Sensory protein | Unknown | Sensory protein, help in location of the host | No visible phenotype |
| Gr SXP1 | Unknown | Unknown | Unknown | No visible phenotype |
| Gr4D06 | Unknown | Unknown | Unknown | No visible phenotype |
| GrE9 | Unknown | Unknown | Unknown | No visible phenotype |
| GrA42 | Unknown | Unknown | Unknown | No visible phenotype |
| GrUBCEP12 | Ubiquitin extension-like peptide | Nuclear and cytoplasmic, apoplastic | Feeding cell formation and plant defense suppression | shrunken downward curling leaves and succumbing to a necrotic collapse¶ and Suppression of host defense* § |
| GrSKP-1 | Ubiquitin ligase component | Cytoplasmic, apoplastic | Involved in signal transduction, protein degradation | Bushy plant with smaller leaves in N. benthamiana ¶ |
*Transient expression in N. benthamiana
§Transient expression in N. tabacum
¶Systemic expression in N. benthamiana via PVX
We conducted WUBLASTP searches against the genome sequence of G. pallida [39], a close relative of G. rostochiensis, and the genus Heterodera that includes cyst nematodes of soybean, sugar beet and cereals. As expected, all of the candidate effectors have a homolog in G. pallida with protein identities of 37–97% (S1 Table). Several effectors also have a homolog in the genus Heterodera with protein identities of 31–85% (S1 Table). We also conducted BLASTX in GenBank excluding Globodera and Heterodera species. Eleven of the CSEPs also showed homology (with protein identity of ≥ 40%) to predicted effectors from plant parasitic nematodes outside the Globodera and Heterodera genera (S1 Table).
One of the identified CSEP-encoding genes, GrExp1 appears to be the result of alternative splicing of ExpB1. Compared to GrEXPB1, GrEXP1 is missing a continuous stretch of ten amino acids in the C-terminal region and we were unable to amplify this isoform from cDNA of preparasitic second-stage juveniles (pre-J2s). Three other candidate effectors encoding genes, GrCLE-4B1, GrCLE-4A3 and GrENG-4 (S1 Table), appear to be alleles or copies of previously described genes GrCLE4B, GrCLE4D and GrENG3, respectively as they show only point mutation differences with published sequences.
Candidate ORFs were amplified by RT-PCR (S2 Table), followed by cloning and sequencing (see Materials and Methods for details). In our library of twenty-three CSEP clones, fourteen showed sequence differences with sequences reported in NCBI (S2 Table, S5 Fig.). Nine of these genes showed nonsynonymous amino acid variation, while four genes have synonymous mutations.
Transcriptional profiles of several G. rostochiensis putative apoplastic CSEP-encoding genes
We used quantitative real-time RT-PCR (qRT-PCR) to determine the expression profile of four of the CSEP-encoding genes through the five nematode developmental stages: egg, pre-J2 and parasitic second-, third- and fourth-stage juveniles (par-J2, J3 and J4) (S1 Fig.). GrPEL1 and GrENG1 showed similar expression patterns, having consistent expression from egg to early parasitic stages. Expression of GrExpB2 was highly upregulated in the pre-J2 stage, but significantly decreased in parasitic stages. In contrast to GrExpB2 expression, GrSkp1 showed dramatic and continuous upregulation in both early and late nematode parasitic stages.
Construction of a G. rostochiensis CSEP library
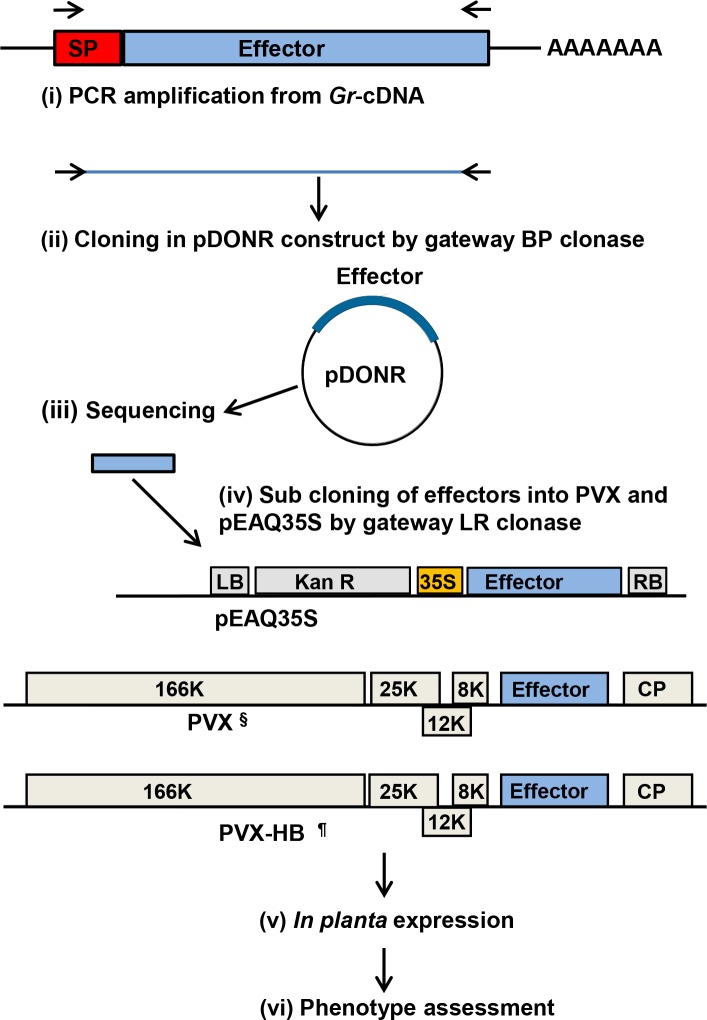
G. rostochiensis is an obligate biotroph for which no transformation system is available and loss- or gain-of-function mutant analysis is not possible in this organism at a high throughput level. To gain insight into the biological function of the GrCSEPs we generated effector clone libraries in constructs for transient and systemic expression in planta (S2 Table). Effector ORFs were cloned in three different constructs: pEAQ35S; pGR106-Gw (herein referred to as PVX), and pGR103-Gw (expressing the Rx-breaking coat protein from PVX strain HB; herein referred to as PVX-HB) (Fig. 1). The latter was used for the expression of effectors in potato cultivars expressing the Rx gene [40]. CSEPs were transiently and systemically expressed in N. benthamiana, N. tabacum, tomato and potato both by agroinfiltration and agroinfection from binary vector pEAQ35S as well as from PVX vectors (Fig. 1). Phenotypes were assessed either for the induction of visual changes in plant health, morphology or architecture in systemically infected plants or in the induction of visible changes in agroinfiltrated leaf patches (Table 1).
Figure 1. Cloning and expression strategy for putative G. rostochiensis effector proteins.
Cloning and functional analysis of candidate secreted effector proteins in planta included; (i) PCR amplification of effector encoding genes from G. rostochiensis cDNA with their cognate SP (red); (ii) Cloning into the donor vector pDONR207 or pDONR221; (iii) Sequencing of multiple clones for sequence confirmation; (iv) Transfer of cDNAs from donor vector to gateway compatible pEAQ35S, PVX and PVX-HB, where § is Gateway compatible pGR106 and ¶ is Gateway compatible pGR103; (v) In planta expression of effectors by agroinfiltration and agroinfection; (vi) Identification of phenotype induced in different solanaceous plants and assessment for cell death suppression.
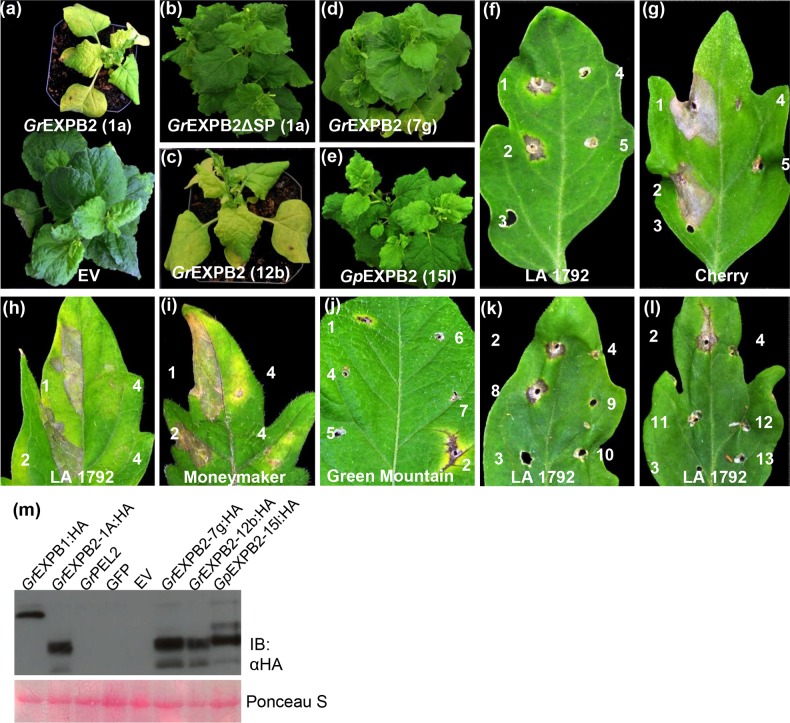
GrEXPB2 induces chlorosis in N. benthamiana and cell death in tomato and potato
Expansins are cell wall-loosening proteins that are involved in growth as well as in cell wall disassembly [41]. In nematodes, expansin-like proteins are thought to be secreted into the apoplast during invasion of the roots [42,43]. We identified two effectors, GrEXPB1 and GrEXPB2, predicted to encode expansin-like proteins, although GrEXPB2 lacks a carbohydrate binding domain (CBD II) commonly found in expansin-like proteins (S2 Fig.). GrEXPB2 induced dramatic symptoms when expressed systemically via PVX in N. benthamiana, including severe chlorosis and dwarfing (Fig. 2A). Consistent with its predicted apoplastic localization, GrEXPB2-induced symptoms were completely abrogated when the SP was deleted (PVX-GrEXPB2ΔSP; Fig. 2B), although we cannot rule out the possibility that deletion of this N-terminal region might affect protein stability or function. GrEXPB2 has been reported to present a significant variability between individuals and between populations, although the most common sequence variant is the GrEXPB2 “type” sequence reported here [44]. We expressed HA epitope-tagged versions of GrEXPB2 “type” (12b), a variant GrEXPB2 (7b) from an Ro5 population, an EXPB2 homolog from G. pallida (15l) [44] (S3 Fig.), as well as GrEXPB1. Only 12b induced symptoms when expressed from PVX in N. benthamiana, despite all four versions being expressed, as confirmed by western blotting (Fig. 2M). However, PVX-GrEXPB2 did not induce any symptoms in N. tabacum, either by agroinfiltration or agroinfection, aside from the typical mosaic symptoms indicative of PVX infection (data not shown).
Figure 2. GrEXPB2 induces necrosis in tomato and potato and chlorosis and dwarfing in N. benthamiana.
N. benthamiana infected with (a) PVX-GrEXPB2 clone 1A (top) or empty PVX vector (EV) (bottom). (b) PVX-GrEXPB2ΔSP clone 1A, (c) PVX-GrEXPB2:HIS-HA clone 12b, (d) PVX-GrEXPB2:HIS-HA clone 7g, (e) PVX-GpEXPB2:HIS-HA clone 15l. Plants were photographed at 20 DPI. Tomato cultivars (f, k, l) LA 1792 and (g) Cherry, were tooth pick inoculated with PVX derivatives or tooth pick only. The numbers correspond to 1, GrEXPB2 clone 1A; 2, GrEXPB2:HIS-HA clone 1A; 3, tooth pick only; 4, GrEXPB2ΔSP clone 1A; 5, EXPB1:HIS-HA; 6, GFP; 7, GrPEL1; 8, GrEXPB2:HIS-HA clone 12b; 9, GpEXPB2:HIS-HA clone 15l; 10, GrEXPB2:HIS-HA clone 22j; 11, GrEXPB2:HIS-HA clone 5a; 12, GrEXPB2:HIS-HA clone 6b; and 13, GrEXPB2:HIS-HA clone 7g. Leaves were photographed at 10 DPI. Tomato cultivars (h) LA 1792 and (i) Moneymaker were agroinfiltrated with pEAQ35S constructs expressing 1, GrEXPB2 clone 1A; 2, AtRx and 4, Empty vector. Leaves were photographed at 6 DPI. Potato cultivar (j) Green Mountain was tooth pick inoculated with PVX constructs as in (f, g, k, l). Leaves were photographed at 15 DPI. (m) HA-tagged versions of EXPB2 variants, as indicated, were expressed in N. benthamiana leaves from the PVX vector and total protein extracts were prepared from infiltrated patches 4 DPI, followed by Anti HA immune blotting. Ponceau S staining (lower panel) was used to show equal loading.
We next expressed GrEXPB2 in eight tomato cultivars (LA1972, Bush Beefsteak, Yellow Pear-shaped, Cherry, Rose de Berne, Starfire, Earliana and Moneymaker) and four potato cultivars (Katahdin, Green Mountain, Hilite Russet, and Miranda) with PVX-GrEXPB2. Potato cultivars Atlantic and Divina, which express the Rx gene, were inoculated with PVX-HB-GrEXPB2. After agroinfection with PVX-GrEXPB2, we observed a strong necrotic response around the inoculated site 7–10 days post inoculation (DPI) in all tomato and potato cultivars listed above. As in N. benthamiana the symptoms in tomato appeared only when the SP was included indicating that the phenotype is induced by GrEXPB2 in the apoplast (Fig. 2F and 2G). To demonstrate that the necrotic phenotype is not affected by PVX we transiently expressed GrEXPB2 from the binary construct, pEAQ35S in leaves of two tomato cultivars (LA1972 and Moneymaker). Cell death was induced in tomato by pEAQ35S-GrEXPB2 at 3–4 DPI, similar to the response induced by expression of an autoactive mutant Rx (AtRx) protein [45] included as a control, while no symptoms were observed with the empty vector, GrEXPB2ΔSP or several other effectors (Fig. 2H and 2I). PVX-GrEXPB2 also induced strong necrotic symptoms at 10–15 DPI in agroinfected leaves in all potato cultivars tested (Fig. 2J), whereas no symptoms were observed in control sites inoculated with PVX-GFP, PVX-GrPEL1 or PVX-GrEXPB2ΔSP (Fig. 2J). Similar to the results obtained in N. benthamiana, only GrEXPB2 clone 12b induced symptoms in tomato (Fig. 2K and 2L).
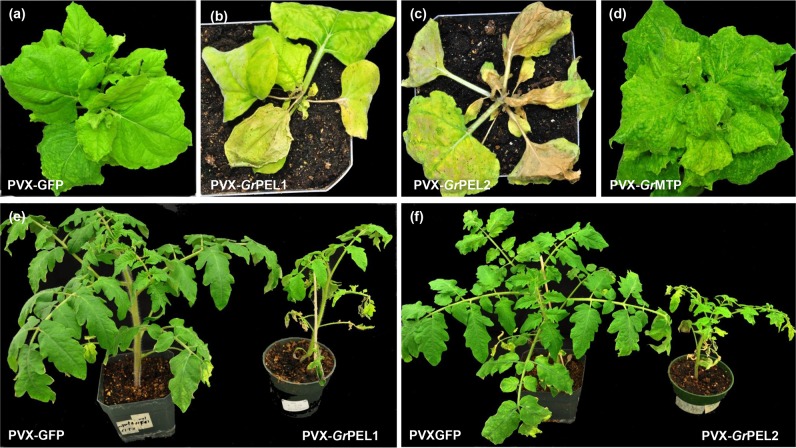
Expression of GrPEL1, GrPEL2 and GrMTP induces systemic chlorosis and necrosis
Systemic expression of GrPEL1, GrPEL2 and GrMTP in N. benthamiana resulted in severe malformations within the infiltrated leaves at six DPI. At fourteen DPI, PVX-GrPEL1 induced severe curling, chlorosis and wrinkling in the upper leaves, eventually killing the plant at 21 DPI (Fig. 3B). PVX-GrPEL2 did not induce severe chlorotic symptoms in the infiltrated leaves, but as it moved systemically it killed the midrib of the leaves, with necrosis spreading with virus movement, eventually killing the upper part of the plant as well as the inoculated leaves (Fig. 3C). GrMTP induced malformation in the systemic leaves, with the leaves eventually becoming extremely discolored and cup shaped (Fig. 3D).
Figure 3. GrPEL1, GrPEL2 and GrMTP induce chlorosis and necrosis in N. benthamiana and tomato.
N. benthamiana plants were infected by agroinfiltration with (a) PVX-GFP, (b) PVX-GrPEL1, (c) PVX-GrPEL2, (d) PVX-GrMTP. Plants were photographed at 21 DPI. Tomato plants, cultivar Starfire, were inoculated with (e) PVX-GrPEL1 and (f) PVX-GrPEL2 or with PVX-GFP (e, f, right hand side). Plants were photographed at 28 DPI.
In tomato GrPEL1 and GrPEL2 induced systemic leaf crinkling and necrotic flecks, eventually killing some of the branches and resulting in the infected plants being significantly dwarfed (Fig. 3E and 3F).
Expression of GrCLE peptides produces dramatic developmental phenotypes
Five CLAVATA3/ESR-like (CLE) effectors (GrCLE1, GrCLE4D, GrCLE4B, GrCLE4C and GrCLE4A) were expressed from PVX both with and without SP in N. benthamiana, N. tabacum, tomato and potato. GrCLE1 induced downward curving leaves and chlorosis in the systemic leaves of infected N. benthamiana plants compared to the empty vector inoculated plant (Fig. 4A and 4B). A similar phenotype was observed both for the full-length GrCLE1 construct as well as the GrCLE1ΔSP construct lacking SP (Fig. 4B and 4C). GrCLE4B, GrCLE4BΔSP and GrCLE4D induced severed chlorosis and downward curling in systemic leaves (Fig. 4D-F) with the leaves becoming very narrow and yellow. These plants also showed a significant increase in axillary shoots. The curved leaf phenotype was less pronounced in GrCLE4C and no phenotype was observed with GrCLE4A in N. benthamiana (data not shown). No unusual symptoms were observed with any of GrCLE constructs in the other plant species tested.
Figure 4. CLE peptides induce dramatic phenotypes in N. benthamiana.

N. benthamiana plants were infected by agroinfiltration with (a) empty PVX vector (EV), (b) PVX-GrCLE1, (c) PVX-GrCLE1ΔSP, (d) PVX-GrCLE4B, (e) PVX-GrCLE4BΔSP and (f) PVX-GrCLE4D. Photographs were taken at 21 DPI.
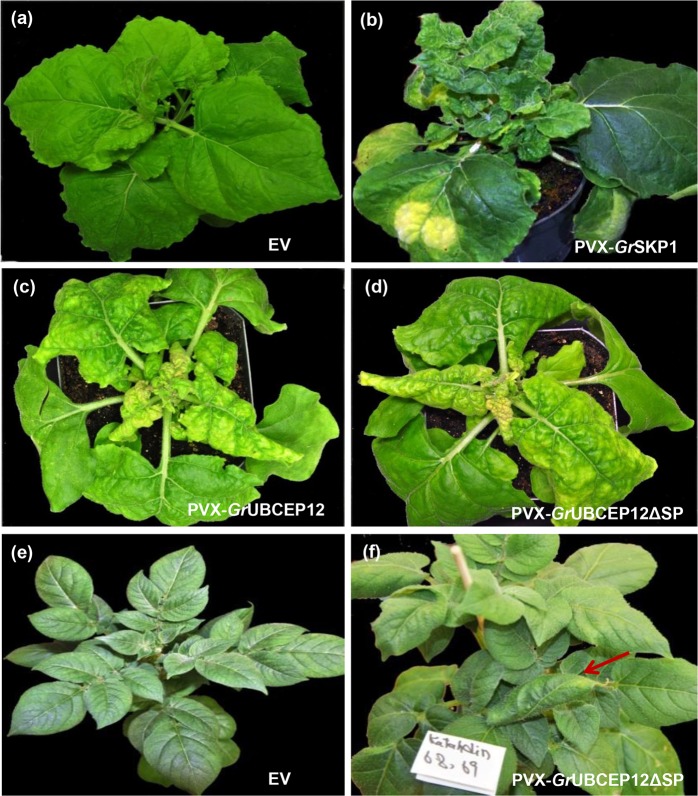
GrUBCEP12 and GrSKP1 affect plant morphology and may function in the apoplast
The effectors GrUBCEP12 and GrSKP1 show striking homology to plant proteins involved in protein degradation. GrSKP1 shows 75% identity to the Arabidopsis SKP1-related protein ASK10 (AT3G21860) over a 160 aa stretch whereas GrUBCEP12 encodes a monoubiquitin domain and a small C-terminal carboxyl extension protein [32]. Although, intuitively, these proteins would be expected to function in the plant cytoplasm, we found that expressing the full-length proteins (including the SP) induced dramatic phenotypes. Expression of GrSKP1 from PVX in N. benthamiana produced very bushy plants with an increased number of smaller leaves in the upper part of the plant. These leaves also presented a bubble-like phenotype on their upper surface compared to the empty vector inoculated plant (Fig. 5A and 5B). Expression of GrUBCEP12 from PVX induced one of the most pronounced phenotypes in N. benthamiana and in potato. In N. benthamiana the phenotype first became visible 7–10 DPI, inducing shrunken downward curling leaves and eventually leading to the leaves turning brown and succumbing to a necrotic collapse after 16 DPI (Fig. 5D and 5D). A similar phenotype was observed with both full length GrUBCEP12 and a version lacking the SP (GrUBCEP12ΔSP) (Fig. 5C and 5D). PVX-GrUBCEP12 was the only construct to induce a morphological phenotype in potato (cultivar Katahdin), where it induced leaf-curling symptoms (Fig. 5E and 5F).
Figure 5. GrUBCEP12 and GrSKP1 alter plant morphology.
N. benthamiana plants were infected by agroinfiltration with (a) empty PVX vector (EV), (b) PVX-GrSKP1, (c) PVX-GrUBCEP12, or (d) PVX-GrUBCEP12ΔSP. Photographs were taken at 16 DPI. Systemic expression in potato of (e) PVX and (f) PVX-GrUBCEP12ΔSP in potato cultivar Katahdin. Photographs were taken at 30 DPI.
Suppression of immunity-associated cell death by G. rostochiensis effectors
We screened the CSEP constructs for their ability to suppress cell death. In the first screen, we transiently expressed effectors from PVX in the leaves of N. benthamiana and N. tabacum by agroinfiltration together with three cell death inducers, including the P. infestans elicitor PiNPP [46,47] as well as an autoactive mutant (D460V) of the NB-LRR Rx (AtRx) [28,45] and the combination of the NB-LRR Bs2 with its cognate effector AvrBs2 [48] (see Material and Methods). Assessment of cell death suppression activities is listed in Table 2 and representative assays are shown in Fig. 6. The cell death percentage scores were calculated from 12–15 infiltration sites based on three to four independent experiments. Two putative apoplastic effectors, GrEXPB2 and GrUBCEP12, inhibited the cell death induced by PiNPP, AtRX and AvrBs2/Bs2 in N. benthamiana and N. tabacum leaves (Table 2).
Table 2. Suppression of cell death by G. rostochiensis apoplastic effectors.
| Effectora | Suppression of cell death in N. benthamianab | Suppression of cell death in N. tabacumb | ||||
|---|---|---|---|---|---|---|
| AtRx | PiNPP | Bs2/AvrBs2 | AtRx | PiNPP | Bs2/AvrBs2 | |
| GrCLE1 | − | − | − | − | − | − |
| GrCLE1ΔSP | − | − | − | − | − | − |
| GrCLE-4A | − | − | − | − | − | − |
| GrCLE-4D | − | − | − | − | − | − |
| GrCLE-4DΔSP | − | − | − | − | − | − |
| GrCLE-4B1 | − | − | − | − | − | − |
| GrCLE-4BΔSP | − | − | − | − | − | − |
| GrCLE-4C | − | − | − | − | − | − |
| GrCLE-4CΔSP | − | − | − | − | − | − |
| GrEXPB1 | − | − | − | − | − | − |
| GrEXPB1ΔSP | − | − | − | − | − | − |
| GrEXPB2 | + | +++ | +++ | ++ | + | ++ |
| GrEXPB2ΔSP | − | + | − | − | − | − |
| GrVAP1 | − | − | − | − | − | − |
| GrAMS1 | − | − | − | − | − | − |
| GrAMS1ΔSP | − | − | − | − | − | − |
| GrSXP1 | − | − | − | − | − | − |
| GrSXP1ΔSP | − | − | − | − | − | − |
| GrA42 | − | − | − | − | − | − |
| GrUBCEP12 | + | + | + | ++ | Nt | + |
| GrUBCEP12ΔSP | ++ | + | ++ | + | Nt | ++ |
| GrSKP1 | − | − | − | − | − | − |
| Gr4D06 | − | − | − | − | − | − |
| Gr4D06-ΔSP | − | − | − | − | − | − |
| GrTPX | − | − | − | − | − | − |
| GrENG1 | − | − | − | − | − | − |
| GrENG2 | − | − | − | − | − | − |
| GrENG3 | − | − | − | − | − | − |
| GrGPX | − | − | − | − | − | − |
| GrPEL1 | − | − | − | − | − | − |
| GrPEL2 | ||||||
| GrMTP | − | − | − | − | − | − |
| GrGPX2 | − | − | − | − | − | − |
| GrCM-1-A | − | − | − | − | − | − |
| P. infestans Avr3a ΔSP | Nt | ++ | Nt | Nt | + | Nt |
| P. sojae Avr3b ΔSP | + | Nt | Nt | + | + | + |
| pGR106-empty | − | − | − | − | − | − |
aAll effectors were expressed from PVX based constructs.
b(+++) Cell death suppression in at least 75% of infiltrated patches; (++) Cell death suppression in at least 50% of infiltrated patches; (+) Cell death suppression in at least 25% of infiltrated patches; (−) No suppression or less than 25%; (Nt) Not tested.
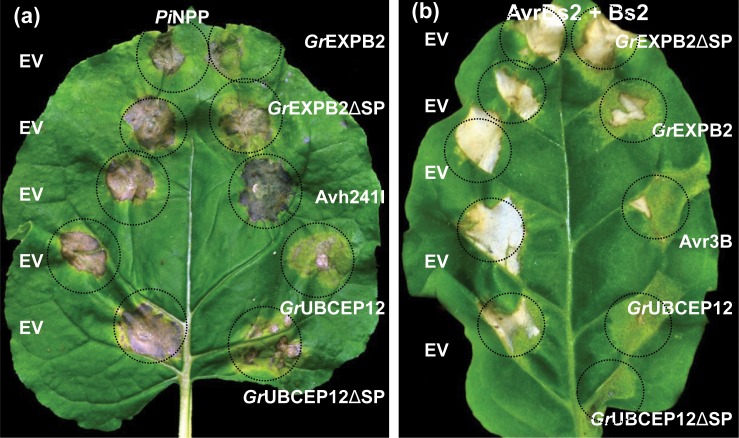
Figure 6. Suppression of cell death induced by NB-LRR proteins by apolastic effectors in N. benthamiana and N. tabacum.
(a) N. benthamiana leaves were co-infiltrated with Agrobacterium carrying binary vectors expressing PiNPP and P38 together with either empty vector (EV, left hand side) or the indicated effectors expressed from pEAQ35S (right hand side). (b) N. tabacum leaves were co-infiltrated with Agrobacterium containing binary vectors expressing AvrBS2, Bs2 and P38 together with either empty vector (EV, left hand side) or the indicated effectors expressed from pEAQ35S (right hand side). Cell death symptoms were assessed (Table 2) at 3–5 DPI and photographs were taken at 5 DPI.
The same effectors were also expressed in N. benthamiana and N. tabacum from pEAQ35S together with binary vectors expressing PiNPP and AvrBs2/Bs2. In N. benthamiana and N. tabacum the cell death induced by PiNPP and AvrBs2/Bs2 was suppressed by both GrEXPB2 and GrUBCEP12 (Fig. 6A and 6B).
Suppression of disease resistance mediated by the NB-LRR proteins Rx and N
Cell death is not required for preventing pathogen proliferation in plants in many cases, suggesting that additional mechanisms contribute to immunity. We investigated whether the putative apoplastic effectors that abrogate defense-related cell death could also suppress disease resistance mediated by the Rx and N proteins, which do not require the induction of cell death to confer resistance to viruses [30, 49]. In this assay, PVX expressing GFP (PVX-GFP) was agroexpressed in N. benthamiana leaves with the Rx gene along with either empty vector or the putative apoplastic effectors identified in the cell death suppression assays above. Virus accumulation was detected in N. benthamiana leaves by visualizing GFP by UV illumination and by immune-blotting at 4 DPI. Little or no GFP was observed in the leaf patches co-infiltrated with PVX-GFP, Rx and empty vector whereas both GrEXPB2 and GrUBCEP12 allowed significant accumulation of GFP in the infiltrated areas as assessed visually and by anti-GFP immune-blotting (Fig. 7). We also used an assay based on the N gene, which confers resistance to Tobacco mosaic virus (TMV) through the recognition of the P50 fragment of the viral replicase [30]. We have previously shown that co-expression of N and P50 in N. benthamiana leaves can inhibit the accumulation of an unrelated virus (PVX-GFP) in the absence of cell death [30]. Here, we co-expressed N and P50 plus PVX-GFP together with the two putative apoplastic defense-suppressing effectors and monitored the accumulation of PVX-GFP visually and by immune-blotting. The polerovirus P0 protein was included as a positive control in this assay as it has been shown to inhibit anti-viral defense responses, but not cell death [30]. As expected, P0 and GrEXPB2 and GrUBCEP12 inhibited the ability of N to suppress GFP accumulation in this assay (Fig. 8).
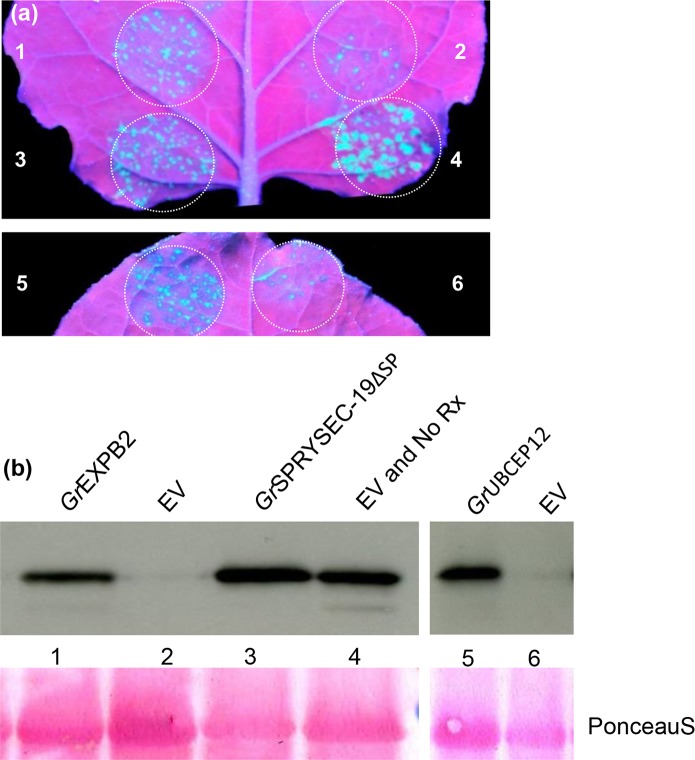
Figure 7. GrEXPB2 and GrUBCEP12 suppress Rx-mediated resistance to PVX.
N. benthamiana leaves were co-infiltrated with Agrobacterium carrying binary vectors expressing PVX-GFP and 35S-Rx together with pEAQ35S vectors expressing 1, GrEXPB2; 2, empty vector; 3, GrSPRYSEC-19ΔSP; 4, Rx replaced with empty vector; 5, GrUBCEP12; 6, empty vector. (a) GFP expression was visualized and photographed under UV illumination at 4 DPI. (b) Anti-GFP immune blotting was performed on total protein samples taken at 4 DPI from N. benthamiana patches expressing the same construct combinations as described above. Ponceau S staining (lower panel) was used to show equal loading.
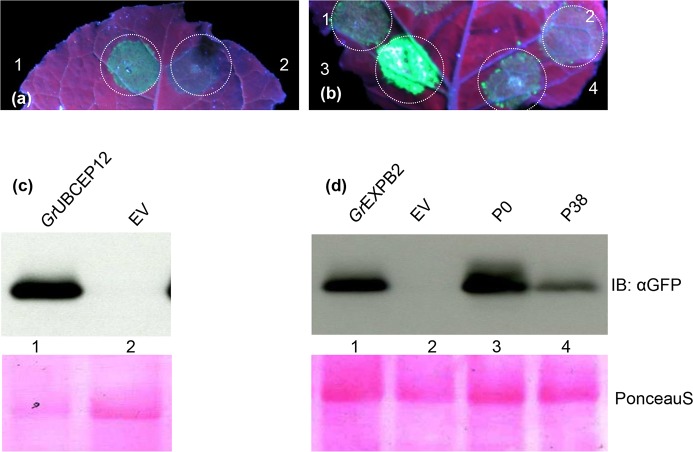
Figure 8. GrEXPB2 and GrUBCEP12 proteins suppress virus resistance mediated by the N gene.
N. benthamiana leaves were co-infiltrated with Agrobacterium carrying binary vectors expressing PVX-GFP, N and P50 together with pEAQ35S expressing (a) 1, GrUBCEP12; 2, empty vector; (b) 1, GrEXPB2; 2, empty vector; 3, P0; 4, P38. GFP expression was visualized under UV illumination at 4 DPI. (c-d) Anti GFP immune blotting was performed on total protein samples taken at 4 DPI from N. benthamiana leaf patches co-expressing the combinations of constructs described in a and b. The number on the blot corresponds to the number on the leaf above each blot. Ponceau S staining (lower panel) was used to show equal loading.
Discussion
We have identified, cloned and partially characterized twenty-three putative CSEPs from G. rostochiensis. Given their activity when expressed with their SP, together with previous reports, at least fourteen of these are likely to function in the apoplast (Table 1). Surprisingly the GrSKP1 and GrUBCEP12 proteins, predicted to function in the cytoplasm, also induced effects when expressed with their SP, suggesting that these proteins may also function outside the cell. An additional seven proteins, which did not induce any effect in planta, and whose predicted functions do not suggest a likely site of action, remain to be characterized. While the phenotypes were observed in above ground tissues, G. rostochiensis is able to infect leaves [50] and as such it is reasonable to assume that its effectors will function similarly in these tissues, particularly for recognition by, and inhibition of, the immune system which likely functions similarly in all tissues.
Phytopathogenic nematodes encode at least three classes of effectors based broadly on their biological activities: effectors that degrade or modify host cell walls; effectors involved in reprogramming cellular identity and metabolism; and effectors involved in suppressing host defenses. Members of the first class, including several predicted cell wall degrading enzymes such as endoglucanases (GrENG1, GrENG2, GrENG3), pectate lyases (GrPEL1, GrPEL2) as well as expansin-like (GrEXPB1, GrEXPB2) proteins and a metalloproteinase (GrMTP), would be expected to be apoplastic. Representative genes from this group (GrPEL1, GrENG1 and GrEXPB2) showed relatively high expression in pre-J2s and during early stages of infection (S1 Fig.). The most severe phenotypes observed were those produced by over expression of GrPEL1 and GrPEL2 in N. benthamiana and tomato (Fig. 3). Despite showing only 28% identity and being predicted to target different pectic polysaccharides [51], both proteins induced severe necrosis. The phenotype that we observed with GrPEL1 and GrPEL2 expression (Fig. 3) is consistent with a role for pectate lyases in tissue maceration associated with numerous pathogens encoding pectate lyase activity [52]. In nematode infection, this phenotype would be expected to be highly localized, but would be beneficial to the nematode during its migration towards the target host cell.
The GrCLE1 peptide has been shown to change root growth and morphology in Arabidopsis as well as in host plants potato and tomato [53] and GrCLE4 peptides can rescue the Arabidopsis clv3 mutant [34]. The morphological phenotypes induced by PVX-GrCLE constructs (Fig. 4) are consistent with these nematode-secreted effector peptides mimicking endogenous plant CLE peptides and in reprogramming plant cell fate during syncytium formation and maintenance [14]. The fact that GrCLE1 and GrCLE4B can induce similar phenotypes with and without a SP is consistent with a report showing that mature propeptides of soybean cyst nematode CLE proteins are delivered first to the syncytium cytoplasm and then translocated to the apoplast by an unknown pathway [54,55]. Other candidate effectors that might be involved in reprogramming host cell identity and metabolism include GrSKP1 and GrUBCEP12. These two proteins induce the most striking morphological phenotypes when expressed from PVX (Fig. 5) and, given their strong similarity to proteins involved in protein turnover, it is plausible that they may alter multiple host pathways. Interestingly, GrUBCEP12 induced a strong phenotype when expressed from PVX either with or without a signal peptide (Fig. 5C, 5D and 5F). GrUBCEP12 has been shown to be processed into two functional units inside the cell, a free ubiquitin moiety and a 12-amino acid peptide from its carboxyl terminus [32]. It remains to be seen which of these moieties contribute to the observed phenotypes and whether they possess properties that allow them to exit (or enter) the cell by non-canonical mechanisms, as with the CLE peptides [54,55].
Although many cytoplasmic-delivered effectors have been shown to suppress the signaling pathways that lead to PTI [56], we have demonstrated that defense responses initiated from both the apoplast (PiNPP) and the cytoplasm (Rx, N) can be blocked by at least one apoplastic effector. Although the localizations of GrUBCEP12 and GrSKP1 remain to be definitively established, GrEXPB2 appears to function in the apoplast given the fact that it does not function without its SP, consistent with its predicted function in binding cell wall-associated carbohydrates.
Expression of GrEXPB2 is highly upregulated in the pre-J2 stage and diminishes quickly upon plant infection (S1 Fig.). GrEXPB2 protein was also identified at high levels with several cell wall modifying enzymes and VAP1 in stylet secretions from pre-J2s indicating that these proteins are secreted into the apoplast to facilitate nematode invasion and migration, possibly through a cell wall-loosening activity [13]. The G. rostochiensis stylet can deliver apoplastic effectors, such as VAP1, ENG1 and EXPB2, in the preparasitic stage [13]. However cytoplasmic effectors can presumably only be delivered directly to the one cell the stylet eventually pierces. Thus it would make sense for the nematode to secrete apoplastic effectors that could suppress plant defenses during tissue invasion. GrEXPB2 could be involved in suppression of early PTI and/or ETI during this stage. Other pathogens have been reported to suppress defenses from the apoplast. Pep1, an apoplastic effector from Ustilago maydis, suppresses cell death in maize by directly binding to cell wall-associated/apoplastic peroxidase [57]. Likewise, an apoplastic effector, calreticulin (Mi-CRT) from the root knot nematode Meloidogyne incognita, increases plant susceptibility to M. incognita and to an oomycete [58]. In addition, the extracellular growth-promoting peptide hormone phytosulfokine inhibits PTI in Arabidopsis [59]. These examples indicate that it is possible to suppress intracellular host defense signalling from the apoplast. However, this is, to our knowledge, the first example of an apoplastic effector that is able to inhibit NB-LRR responses.
Many effectors elicit defense responses, possibly because, in tampering with the host defense response, they inadvertently set off the response they were meant to defuse [60,61]. As such, one could predict that those effectors that suppress defense response might be the most likely to be recognized by the plant innate immune system. Indeed, the apoplastic GrVAP1 protein interferes with protease-based defenses and in doing so elicits a resistance response from the Cf-2 protein [13]. Likewise, we suggest that GrEXPB2 may also have the dual properties of suppressing and eliciting plant defenses.
GrEXPB1 and GrEXPB2 belong to an expansin-like family of proteins present in cyst, root-knot and migratory nematodes [42,43,62–65]. Proteins with expansin domains have also been reported in plant-parasitic bacteria and fungi [66,67]. In the saprophytic fungus Trichoderma reesei an expansin-like protein, the swollenin protein functions as a PAMP in Trichoderma-plant interaction by inducing local defense genes [67]. Homogenates from G. rostochiensis juveniles as well as extracts from leaves expressing GrEXPB1 have been shown to induce plant cell wall extension [43]. Nonetheless, in contrast to GrEXPB2 (type variant), GrEXPB1 does not induce any phenotype in our in planta assays, nor does the GrEXPB2 variant 7g or GpEXPB2 (Fig. 2). As such, we suggest that the necrotic phenotypes induced by GrEXPB2 are likely due to recognition by components of the plant innate immune system present in tomato and potato. This in turn would explain the fact that recombinant PVX expressing “avirulent” GrEXPB2 is unable to infect tomato systemically (data not shown). Likewise, the fact that GrEXPB2 appears to be recognized in potato and tomato, but not tobacco, plus the fact that the GrEXPB2 (7g) and GpEXPB2 are not recognized in tomato (Fig. 2) suggest specific recognition by the host immune system. We have modelled the 3D structure of GrEXPB2 using the crystal structure of the EXPB1 protein (Zea m 1) [68] in the Swiss-Model Workspace [69,70] and found that all three amino acids that are altered in the two variant proteins were positioned on the outer surface of the protein (S4 Fig.). This suggests that these differences could affect interactions with other proteins, potentially including PRRs. As such, the chlorotic phenotype induced in N. benthamiana by PVX-EXPB2 may be due to a weak recognition of GrEXPB2, whereas recognition would appear to be absent in tobacco. Defense induction by GrEXPB2 is presumably mediated through transmembrane RLK or RLP proteins in tomato and potato. Why recognition of GrEXPB2 does not confer resistance to G. rostochiensis in these plants is unclear. However, it may also be due to additional effectors that cooperate in suppressing defense responses as has been proposed for the interaction between Gp-RBP-1 and Gpa2 [16]. The nature of the interplay between GrEXPB2’s role in suppressing and inducing plant defenses is a subject for future studies. Nonetheless, we have identified an important player in plant-nematode interactions, which may play a role in the often multi-genic mechanisms of defense against cyst nematodes.
Supporting Information
The relative expression of four CSEP-encoding genes was determined using quantitative RT-PCR in five G. rostochiensis life stages: cyst, pre-parasitic second-stage juvenile (pre-J2) and parasitic second-, third- and fourth-stage juveniles (par-J2, J3 and J4). Values are means ± SE of two biological replicates, normalized to the G. rostochiensis β-actin gene and relative to expression in the egg stage.
(XLSX)
CLUSTAL W2.1 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) was used to align the amino acid sequences of GrEXPB1 and GrEXPB2. The signal peptide (SP; yellow highlighting) and Rare lipoprotein A (RlpA)-like double-psi beta-barrel (DPBB_1; grey highlighting) domains are conserved between the two proteins while carbohydrate binding domain (CDB_II; green highlighting) is absent from GrEXPB2. Non identical residues or residues present only in one protein are represented by red coloring.
(PDF)
(A) Nucleotide sequence alignment of three G. rostochiensis, EXPB2 clones; GrExpB2 clone 1A, GrExpB2 12b and GrExpB2 7g and G. pallida ExpB2, GpExpB2 clone 15l. Individual gDNA clones were obtained by PCR from gDNA isolated from cysts. Clones GrExpB2 7g and GpExpB2 15l were PCR amplified from gDNA and GrExpB2 clone 1A, GrExpB2 12b were amplified from cDNA. The four introns are absent in GpExpB2 clone 15l. Mismatched nucleotides are highlighted in red and identical nucleotides in yellow, the 3’ UTR is highlighted in green.
(B) Amino acid sequence alignment of GrEXPB2 clone 1A, GrEXPB2 12b, GrEXPB2 7g and GpEXPB2 clone 15l. The single mismatched amino acid between the two GrEXPB2 clones is highlighted in pink and the two amino acids that differ from GpEXPB2 are highlighted in red, all matched amino acids are highlighted in yellow.
(PDF)
ORFs from EXPB2 clones 12b (GenBank acc. no. GQ152150), 7g (GenBank acc. no. GQ152166) and 15l (GenBank acc. no. CAC84564.1) were used for 3D modelling with the Swiss-Model Workspace. Shown is the front and back view of the 3D structure model of GrEXPB2 type protein (12b) as modelled on the crystal structure of the Zea mays protein EXPB1 (PDB ID: 2hczX). Residues variable between the type protein and variant clones are marked in colour: clone 7g (red) A65V; clone 15l (cyan) C79Y and L119M (green).
(PDF)
Multiple sequence alignment with hierarchical clustering (http://multalin.toulouse.inra.fr/multalin/multalin.html) was used for sequence alignment. Matched nucleotides are shown in red while the nucleotide that differ are shown either by blue or black and Genbank accession numbers are shown in parentheses. (A) Alignment of CLE-4A (top) with Reference CLE-4A. (B) Alignment of CLE-4B1 (top) with Reference CLE-4B1. (C) Alignment of ENG-1 (top) with Reference ENG-1. (D) Alignment of ENG-2 (bottom) with Reference ENG-2 (top). (E) Alignment of ENG-3 (top) with Reference ENG-3 (bottom). (F) Alignment of VAP1 (top) with Reference VAP1 (bottom). (G) Alignment of PEL1 (bottom) with Reference PEL1 (top). (H) Alignment of PEL2 (bottom) with Reference PEL2 (top). (I) Alignment of MTP (top) with Reference MTP (bottom). (J) Alignment of GPX2 (bottom) with Reference GPX (top). (K) Alignment of AMS1 (top) with Reference AMS1 (bottom). (L) Alignment of GPX1 (top) with Reference GPX1 (bottom). (M) Alignment of SKP1 (top) with Reference SKP1 (bottom). (N) Alignment of TPX (top) with Reference TPX (bottom).
(PPTX)
(XLS)
(XLS)
(DOCX)
Acknowledgments
We are grateful to Geert Smant for sharing SPRYSEC sequences, to Sophien Kamoun and Yuanchao Wang for control effector and Inf1 expression constructs, to Mohamed El Oirdi for technical assistance, to Chantal Brosseau for the pEAQ expression vector, to Benjamin Mimee, Annie Christine Boucher, Marco Duceppe and Jean Carpentier for assistance in preparing nematode cDNA and to Maxime Gosselin-Théberge and Taharima Habib for their assistance in infiltration experiments.
Data Availability
New effector sequences have been deposited in Genbank (http://www.ncbi.nlm.nih.gov/genbank) under accession numbers KF963513-KF963529.
Funding Statement
This work was supported by funds from the Agriculture and Agri-food Canada Growing Canadian Agri-Innovations Program to G.B. and P.M., a discovery grant from the National Sciences and Engineering Research Council (NSERC) to P.M., and funds from the US Department of Agriculture, Agricultural Research Service to X.W. S.A. was supported by the visiting fellowships in government laboratories program (NSERC) and by a postdoctoral fellowship from the Fonds de Recherche Québecois Nature et Technologie (FRQNT), and this work benefited from links established under CropSustaIn FP7 Project.
References
- 1. Macho AP, Zipfel C (2014) Plant PRRs and the activation of innate immune signaling. Mol Cell 54: 263–272. 10.1016/j.molcel.2014.03.028 [DOI] [PubMed] [Google Scholar]
- 2. Dodds PN, Rathjen JP (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nature Rev Genet 11: 539–548 10.1038/nrg2812 [DOI] [PubMed] [Google Scholar]
- 3. Win J, Chaparro-Garcia A, Belhaj K, Saunders D, Yoshida K, et al. (2012) Effector biology of plant-associated organisms: concepts and perspectives, Cold Spring Harb Symp Quant Biol. 77:235–47 10.1101/sqb.2012.77.015933 [DOI] [PubMed] [Google Scholar]
- 4. Ali S, Bakkeren G (2011) Fungal and oomycete effectors—strategies to subdue a host. Can J Plant Pathol 33: 425–446. [Google Scholar]
- 5. van den Burg HA, Harrison SJ, Joosten MH, Vervoort J, de Wit PJ (2006) Cladosporium fulvum Avr4 protects fungal cell walls against hydrolysis by plant chitinases accumulating during infection. Mol Plant Microbe Interact 19: 1420–1430. 10.1094/MPMI-19-1420 [DOI] [PubMed] [Google Scholar]
- 6. van Esse HP, Bolton MD, Stergiopoulos I, de Wit PJ, Thomma BP (2007) The chitin-binding Cladosporium fulvum effector protein Avr4 is a virulence factor. Mol Plant Microbe Interact 20: 1092–1101. 10.1094/MPMI-20-9-1092 [DOI] [PubMed] [Google Scholar]
- 7. Rooney HC, Van’t Klooster JW, van der Hoorn RA, Joosten MH, Jones JD, et al. (2005) Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance. Science 308: 1783–1786. 10.1126/science.1111404 [DOI] [PubMed] [Google Scholar]
- 8. Tian M, Win J, Song J, van der Hoorn R, van der Knaap E, et al. (2007) A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease. Plant Physiol 143: 364–377. 10.1104/pp.106.090050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shabab M, Shindo T, Gu C, Kaschani F, Pansuriya T, et al. (2008) Fungal effector protein AVR2 targets diversifying defense-related cys proteases of tomato. Plant Cell 20: 1169–1183. 10.1105/tpc.107.056325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Esse HP, Van’t Klooster JW, Bolton MD, Yadeta KA, van Baarlen P, et al. (2008) The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 20: 1948–1963. 10.1105/tpc.108.059394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814. 10.1016/j.cell.2006.02.008 [DOI] [PubMed] [Google Scholar]
- 12. Song J, Win J, Tian M, Schornack S, Kaschani F, et al. (2009) Apoplastic effectors secreted by two unrelated eukaryotic plant pathogens target the tomato defense protease Rcr3. Proc Nat Acad Sci USA 106: 1654–1659. 10.1073/pnas.0809201106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lozano-Torres JL, Wilbers RHP, Gawronski P, Boshoven JC, Finkers-Tomczak A, et al. (2012) Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc Nat Acad Sci USA 109: 10119–10124. 10.1073/pnas.1202867109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, et al. (2013) Nematode effector proteins: an emerging paradigm of parasitism. New Phytol. 199(4):879–94. 10.1111/nph.12323 [DOI] [PubMed] [Google Scholar]
- 15. Postma WJ, Slootweg EJ, Rehman S, Finkers-Tomczak A, Tytgat TOG, et al. (2012) The effector SPRYSEC-19 of Globodera rostochiensis suppresses CC-NB-LRR-mediated disease resistance in plants. Plant Physiol 160: 944–954. 10.1104/pp.112.200188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacco MA, Koropacka K, Grenier E, Jaubert MJ, Blanchard A, et al. (2009) The cyst nematode SPRYSEC protein RBP-1 elicits Gpa2- and RanGAP2-dependent plant cell death. PLoS Pathog 5 (8): e1000564 10.1371/journal.ppat.1000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohlmann H, Sobczak M (2014) The plant cell wall in the feeding sites of cyst nematodes. Front Plant Sci 5: 89 10.3389/fpls.2014.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davis EL, Hussey RS, Mitchum MG, Baum TJ (2008) Parasitism proteins in nematode-plant interactions. Curr Opin Plant Biol 11: 360–366. 10.1016/j.pbi.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 19. Davis EL, Hussey RS, Baum TJ, Bakker J, Schots A, et al. (2000) Nematode Parasitism Genes. Ann Rev Phytopathol 38: 365–396. [DOI] [PubMed] [Google Scholar]
- 20. Stam R, Jupe J, Howden AJM, Morris JA, Boevink PC, et al. (2013) Identification and characterisation CRN effectors in Phytophthora capsici shows modularity and functional diversity. PLoS One 8: e59517 10.1371/journal.pone.0059517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takken FL, Luderer R, Gabriels SH, Westerink N, Lu R, et al. (2000) A functional cloning strategy, based on a binary PVX-expression vector, to isolate HR-inducing cDNAs of plant pathogens. Plant J 24: 275–283. 10.1046/j.1365-313x.2000.00866.x [DOI] [PubMed] [Google Scholar]
- 22. Moffett P (2011) Fragment complementation and co-immunoprecipitation assays for understanding R protein structure and function. Methods Mol Biol 712: 9–20. 10.1007/978-1-61737-998-7_2 [DOI] [PubMed] [Google Scholar]
- 23. Sainsbury F, Thuenemann EC, Lomonossoff GP (2009) pEAQ: versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotech J 7: 682–693. 10.1111/j.1467-7652.2009.00434.x [DOI] [PubMed] [Google Scholar]
- 24. Dyrløv Bendtsen J, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795. 10.1016/j.jmb.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 25. Laurie JD, Ali S, Linning R, Mannhaupt G, Wong P, et al. (2012) Genome comparison of barley and maize smut fungi reveals targeted loss of RNA silencing components and species-specific presence of transposable elements. Plant Cell 24 1733–1745. 10.1105/tpc.112.097261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boucher AC, Mimee B, Montarry J, Bardou-Valette S, Belair G, et al. (2013) Genetic diversity of the golden potato cyst nematode Globodera rostochiensis and determination of the origin of populations in Quebec, Canada. Mol Phylogenet Evol 69: 75–82. 10.1016/j.ympev.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 27. Carpentier J, Esquibet M, Fouville D, Manzanares-Dauleux MJ, Kerlan M-C, et al. (2012) The evolution of the Gp-Rbp-1 gene in Globodera pallida includes multiple selective replacements. Mol Plant Pathol 13: 546–555. 10.1111/j.1364-3703.2011.00769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rairdan GJ, Moffett P (2006) Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation. Plant Cell 18: 2082–2093. 10.1105/tpc.106.042747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qu F, Ren T, Morris TJ (2003) The coat protein of Turnip crinkle virus suppresses posttranscriptional gene silencing at an early initiation step. J Virol 77: 511–522. 10.1128/JVI.77.1.511-522.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhattacharjee S, Zamora A, Azhar MT, Sacco MA, Lambert LH, et al. (2009) Virus resistance induced by NB-LRR proteins involves Argonaute4-dependent translational control. Plant J 58: 940–951. 10.1111/j.1365-313X.2009.03832.x [DOI] [PubMed] [Google Scholar]
- 31. Rairdan GJ, Collier SM, Sacco MA, Baldwin TT, Boettrich T, et al. (2008) The coiled-coil and nucleotide binding domains of the potato Rx disease resistance protein function in pathogen recognition and signaling. Plant Cell 20: 739–751. 10.1105/tpc.107.056036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chronis D, Chen S, Lu S, Hewezi T, Carpenter SCD, et al. (2013) A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J 74(2):185–96. 10.1111/tpj.12125 [DOI] [PubMed] [Google Scholar]
- 33. Lu S-W, Tian D, Borchardt-Wier HB, Wang X (2008) Alternative splicing: A novel mechanism of regulation identified in the chorismate mutase gene of the potato cyst nematode Globodera rostochiensis . Mol Biochem Parasitol 162: 1–15. 10.1016/j.molbiopara.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 34. Lu SW, Chen S, Wang J, Yu H, Chronis D, et al. (2009) Structural and functional diversity of CLAVATA3/ESR (CLE)-like genes from the potato cyst nematode Globodera rostochiensis . Mol Plant Microbe Interact 22: 1128–1142. 10.1094/MPMI-22-9-1128 [DOI] [PubMed] [Google Scholar]
- 35. Haegeman A, Mantelin S, Jones JT, Gheysen G (2012) Functional roles of effectors of plant-parasitic nematodes. Gene 492: 19–31. 10.1016/j.gene.2011.10.040 [DOI] [PubMed] [Google Scholar]
- 36. Quentin M, Abad P, Favery B (2013) Plant parasitic nematode effectors target host defense and nuclear functions to establish feeding cells. Front Plant Sci 4 53 10.3389/fpls.2013.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Replogle A, Hussey R, Baum T, Wang X, et al. (2011) Identification of potential host plant mimics of CLAVATA3/ESR (CLE)-like peptides from the plant-parasitic nematode Heterodera schachtii . Mol Plant Pathol 12: 177–186. 10.1111/j.1364-3703.2010.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang X, Allen R, Ding X, Goellner M, Maier T, et al. (2001) Signal peptide-selection of cDNA cloned directly from the esophageal gland cells of the soybean cyst nematode Heterodera glycines . Mol Plant Microbe Interact 14: 536–544. 10.1094/MPMI.2001.14.4.536 [DOI] [PubMed] [Google Scholar]
- 39. Cotton JA, Lilley CJ, Jones LM, Kikuchi T, Reid AJ, et al. (2014) The genome and life-stage specific transcriptomes of Globodera pallida elucidate key aspects of plant parasitism by a cyst nematode. Genome Biol 15: R43 10.1186/gb-2014-15-3-r43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bendahmane A, Kohm BA, Dedi C, Baulcombe DC (1995) The coat protein of potato virus X is a strain-specific elicitor of Rx1-mediated virus resistance in potato. Plant J 8: 933–941. 10.1046/j.1365-313X.1995.8060933.x [DOI] [PubMed] [Google Scholar]
- 41. Sampedro J, Cosgrove DJ (2005) The expansin superfamily. Genome Biol 6: 242 10.1186/gb-2005-6-12-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tomalova I, Iachia C, Mulet K, Castagnone-Sereno P (2012) The map-1 gene family in root-knot nematodes, Meloidogyne spp.: a set of taxonomically restricted genes specific to clonal species. PLoS One 7: e38656 10.1371/journal.pone.0038656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qin L, Kudla U, Roze EH, Goverse A, Popeijus H, et al. (2004) Plant degradation: a nematode expansin acting on plants. Nature 427: 30 10.1038/427030a [DOI] [PubMed] [Google Scholar]
- 44. Gerič Stare B, Lamovšek J, Širca S, Urek G (2012) Assessment of sequence variability in putative parasitism factor, expansin (EXPB2) from diverse populations of potato cyst nematode Globodera rostochiensis. Physiol Mol Plant Pathol 79: 49–54. [Google Scholar]
- 45. Bendahmane A, Farnham G, Moffett P, Baulcombe DC (2002) Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J 32: 195–204. 10.1046/j.1365-313X.2002.01413.x [DOI] [PubMed] [Google Scholar]
- 46. Kanneganti T-D, Huitema E, Cakir Kamoun S (2006) Synergistic interactions of the plant cell death pathways induced by Phytophthora infestans Nep1-like protein PiNPP1.1 and INF1 elicitin. Mol Plant Microbe Interact 19: 854–863. 10.1094/MPMI-19-0854 [DOI] [PubMed] [Google Scholar]
- 47. Qutob D, Tedman-Jones J, Gijzen M (2006) Effector-triggered immunity by the plant pathogen Phytophthora. Trends in Microbiol 14: 470–473. 10.1016/j.tim.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 48. Tai TH, Dahlbeck D, Clark ET, Gajiwala P, Pasion R, et al. (1999) Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc Natl Acad Sci USA 96: 14153–14158. 10.1073/pnas.96.24.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bendahmane A, Kanyuka K, Baulcombe DC (1999) The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11: 781–792. 10.2307/3870814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poch HL, Lopez RH, Kanyuka K (2006) Functionality of resistance gene Hero, which controls plant root-infecting potato cyst nematodes, in leaves of tomato. Plant Cell Environ 29: 1372–1378. 10.1111/j.1365-3040.2006.01517.x [DOI] [PubMed] [Google Scholar]
- 51. Kudla U, Milac A-L, Qin L, Overmars H, Roze E, et al. (2007) Structural and functional characterization of a novel, host penetration-related pectate lyase from the potato cyst nematode Globodera rostochiensis. Mol Plant Pathol 8: 293–305. 10.1111/j.1364-3703.2007.00394.x [DOI] [PubMed] [Google Scholar]
- 52. Kelemu S, Collmer A (1993) Erwinia chrysanthemi EC16 produces a second set of plant-inducible pectate lyase isozymes. Appl and Environ Microbiol 59: 1756–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guo Y, Ni J, Denver R, Wang X, Clark SE (2011) Mechanisms of molecular mimicry of plant CLE peptide ligands by the parasitic nematode Globodera rostochiensis . Plant Physiol 157: 476–484. 10.1104/pp.111.180554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang J, Lee C, Replogle A, Joshi S, Korkin D, et al. (2010) Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector proteins. New Phytol 187: 1003–1017. 10.1111/j.1469-8137.2010.03300.x [DOI] [PubMed] [Google Scholar]
- 55. Wang J, Joshi S, Korkin D, Mitchum MG (2010) Variable domain I of nematode CLEs directs post-translational targeting of CLE peptides to the extracellular space. Plant Signal Behav 5: 1633–1635. 10.4161/psb.5.12.13774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science 341: 746–751. 10.1126/science.1236011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hemetsberger C, Herrberger C, Zechmann B, Hillmer M, Doehlemann G (2012) The Ustilago maydis effector Pep1 suppresses plant immunity by inhibition of host peroxidase activity. PLoS Pathog 8: e1002684 10.1371/journal.ppat.1002684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jaouannet M, Magliano M, Arguel M, Gourgues M, Evangelisti E, et al. (2013) The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant Microbe Interact 26: 97–105. 10.1094/MPMI-05-12-0130-R [DOI] [PubMed] [Google Scholar]
- 59. Igarashi D, Tsuda K, Katagiri F (2012) The peptide growth factor, phytosulfokine, attenuates pattern-triggered immunity. Plant J 71: 194–204. 10.1111/j.1365-313X.2012.04950.x [DOI] [PubMed] [Google Scholar]
- 60. van der Hoorn RA, Kamoun S (2008) From Guard to Decoy: a new model for perception of plant pathogen effectors. Plant Cell 20: 2009–2017. 10.1105/tpc.108.060194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Collier SM, Moffett P (2009) NB-LRRs work a “bait and switch” on pathogens. Trends Plant Sci 14 (10): 521–529. 10.1016/j.tplants.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 62. Peng H, Gao BL, Kong LA, Yu Q, Huang WK, et al. (2013) Exploring the host parasitism of the migratory plant-parasitic nematode Ditylenchus destuctor by expressed sequence tags analysis. PLoS One 8: e69579 10.1371/journal.pone.0069579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abad P, Gouzy J, Aury J-M, Castagnone-Sereno P, Danchin EGJ, et al. (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26: 909–915. 10.1038/nbt.1482 [DOI] [PubMed] [Google Scholar]
- 64. Kikuchi T, Cotton JA, Dalzell JJ, Hasegawa K, Kanzaki N, et al. (2011) Genomic insights into the origin of parasitism in the emerging plant pathogen bursaphelenchus xylophilus . PLoS Pathogens 7 10.1371/journal.ppat.1002219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haegeman A, Jacob J, Vanholme B, Kyndt T, Mitreva M, et al. (2009) Expressed sequence tags of the peanut pod nematode Ditylenchus africanus: The first transcriptome analysis of an anguinid nematode. Mol Biochem Parasitol 167: 32–40. 10.1016/j.molbiopara.2009.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laine MJ, Haapalainen M, Wahlroos T, Kankare K, Nissinen R, et al. (2000) The cellulase encoded by the native plasmid of Clavibacter michiganensis ssp. sepedonicus plays a role in virulence and contains an expansin-like domain. Physiol Mol Plant Pathol 57: 221–233. [Google Scholar]
- 67. Brotman Y, Briff E, Viterbo A, Chet I (2008) Role of swollenin, an expansin-like protein from Trichoderma, in plant root colonization. Plant Physiol 147 779–789. 10.1104/pp.108.116293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yennawar NH, Li L-C, Dudzinski DM, Tabuchi A, Cosgrove DJ (2006) Crystal structure and activities of EXPB1 (Zea m 1), a β-expansin and group-1 pollen allergen from maize. Proc Nat Acad Sci USA 103: 14664–14671. 10.1073/pnas.0605979103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22: 195–201. 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
- 70. Gerič Stare B, S. Š, Urek G (2013) Model of 3D structure of putative parasitism factor, expansin (EXPB2) from golden potato cyst nematode Globodera rostochiensis Acta Biolog Slove 56: 75–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The relative expression of four CSEP-encoding genes was determined using quantitative RT-PCR in five G. rostochiensis life stages: cyst, pre-parasitic second-stage juvenile (pre-J2) and parasitic second-, third- and fourth-stage juveniles (par-J2, J3 and J4). Values are means ± SE of two biological replicates, normalized to the G. rostochiensis β-actin gene and relative to expression in the egg stage.
(XLSX)
CLUSTAL W2.1 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) was used to align the amino acid sequences of GrEXPB1 and GrEXPB2. The signal peptide (SP; yellow highlighting) and Rare lipoprotein A (RlpA)-like double-psi beta-barrel (DPBB_1; grey highlighting) domains are conserved between the two proteins while carbohydrate binding domain (CDB_II; green highlighting) is absent from GrEXPB2. Non identical residues or residues present only in one protein are represented by red coloring.
(PDF)
(A) Nucleotide sequence alignment of three G. rostochiensis, EXPB2 clones; GrExpB2 clone 1A, GrExpB2 12b and GrExpB2 7g and G. pallida ExpB2, GpExpB2 clone 15l. Individual gDNA clones were obtained by PCR from gDNA isolated from cysts. Clones GrExpB2 7g and GpExpB2 15l were PCR amplified from gDNA and GrExpB2 clone 1A, GrExpB2 12b were amplified from cDNA. The four introns are absent in GpExpB2 clone 15l. Mismatched nucleotides are highlighted in red and identical nucleotides in yellow, the 3’ UTR is highlighted in green.
(B) Amino acid sequence alignment of GrEXPB2 clone 1A, GrEXPB2 12b, GrEXPB2 7g and GpEXPB2 clone 15l. The single mismatched amino acid between the two GrEXPB2 clones is highlighted in pink and the two amino acids that differ from GpEXPB2 are highlighted in red, all matched amino acids are highlighted in yellow.
(PDF)
ORFs from EXPB2 clones 12b (GenBank acc. no. GQ152150), 7g (GenBank acc. no. GQ152166) and 15l (GenBank acc. no. CAC84564.1) were used for 3D modelling with the Swiss-Model Workspace. Shown is the front and back view of the 3D structure model of GrEXPB2 type protein (12b) as modelled on the crystal structure of the Zea mays protein EXPB1 (PDB ID: 2hczX). Residues variable between the type protein and variant clones are marked in colour: clone 7g (red) A65V; clone 15l (cyan) C79Y and L119M (green).
(PDF)
Multiple sequence alignment with hierarchical clustering (http://multalin.toulouse.inra.fr/multalin/multalin.html) was used for sequence alignment. Matched nucleotides are shown in red while the nucleotide that differ are shown either by blue or black and Genbank accession numbers are shown in parentheses. (A) Alignment of CLE-4A (top) with Reference CLE-4A. (B) Alignment of CLE-4B1 (top) with Reference CLE-4B1. (C) Alignment of ENG-1 (top) with Reference ENG-1. (D) Alignment of ENG-2 (bottom) with Reference ENG-2 (top). (E) Alignment of ENG-3 (top) with Reference ENG-3 (bottom). (F) Alignment of VAP1 (top) with Reference VAP1 (bottom). (G) Alignment of PEL1 (bottom) with Reference PEL1 (top). (H) Alignment of PEL2 (bottom) with Reference PEL2 (top). (I) Alignment of MTP (top) with Reference MTP (bottom). (J) Alignment of GPX2 (bottom) with Reference GPX (top). (K) Alignment of AMS1 (top) with Reference AMS1 (bottom). (L) Alignment of GPX1 (top) with Reference GPX1 (bottom). (M) Alignment of SKP1 (top) with Reference SKP1 (bottom). (N) Alignment of TPX (top) with Reference TPX (bottom).
(PPTX)
(XLS)
(XLS)
(DOCX)
Data Availability Statement
New effector sequences have been deposited in Genbank (http://www.ncbi.nlm.nih.gov/genbank) under accession numbers KF963513-KF963529.