Abstract
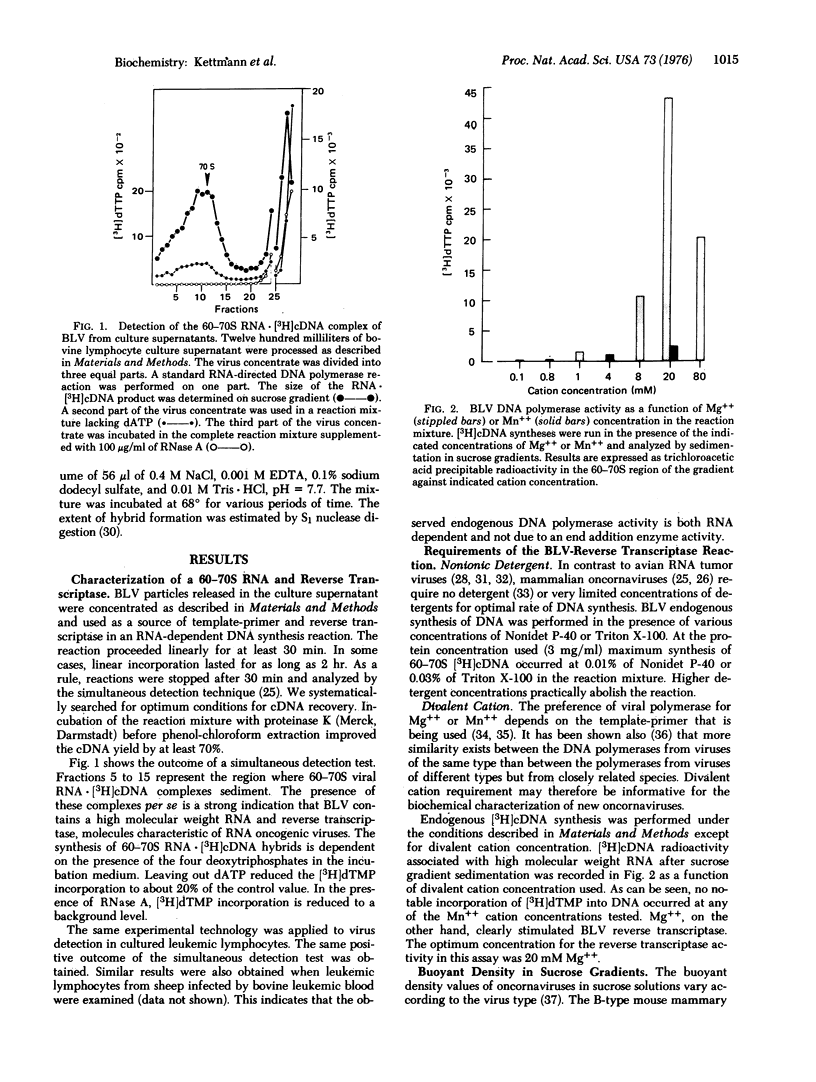
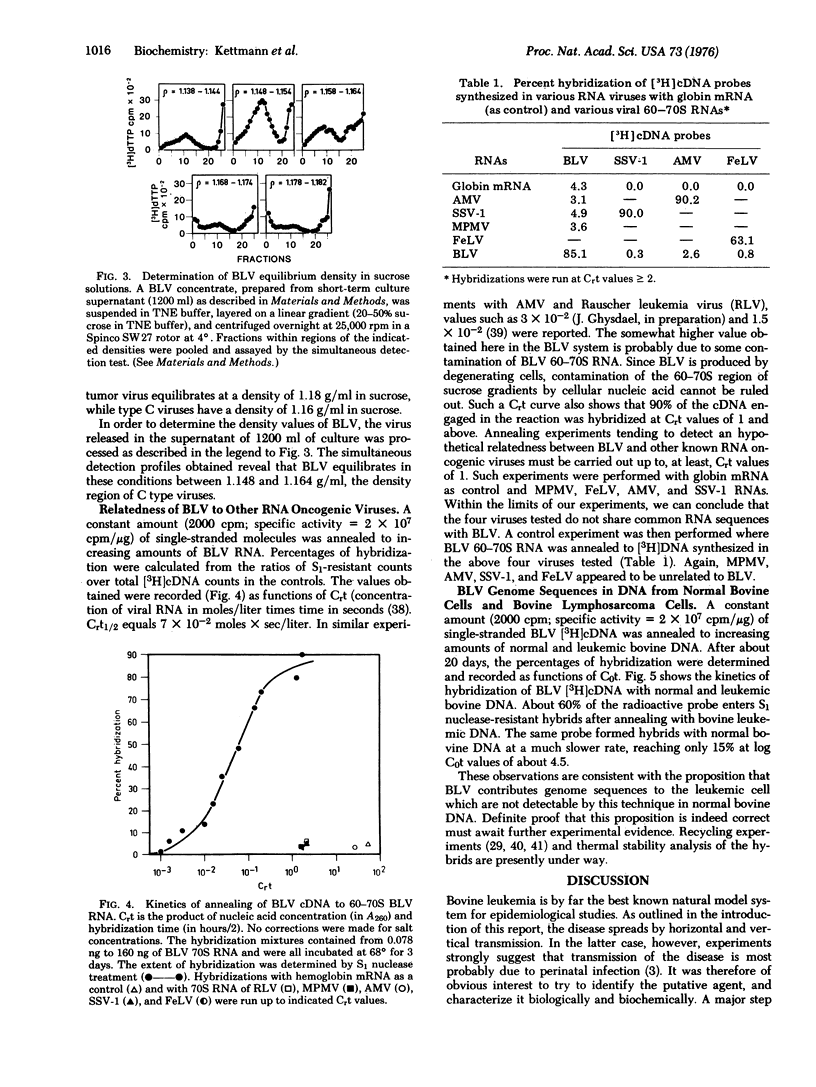
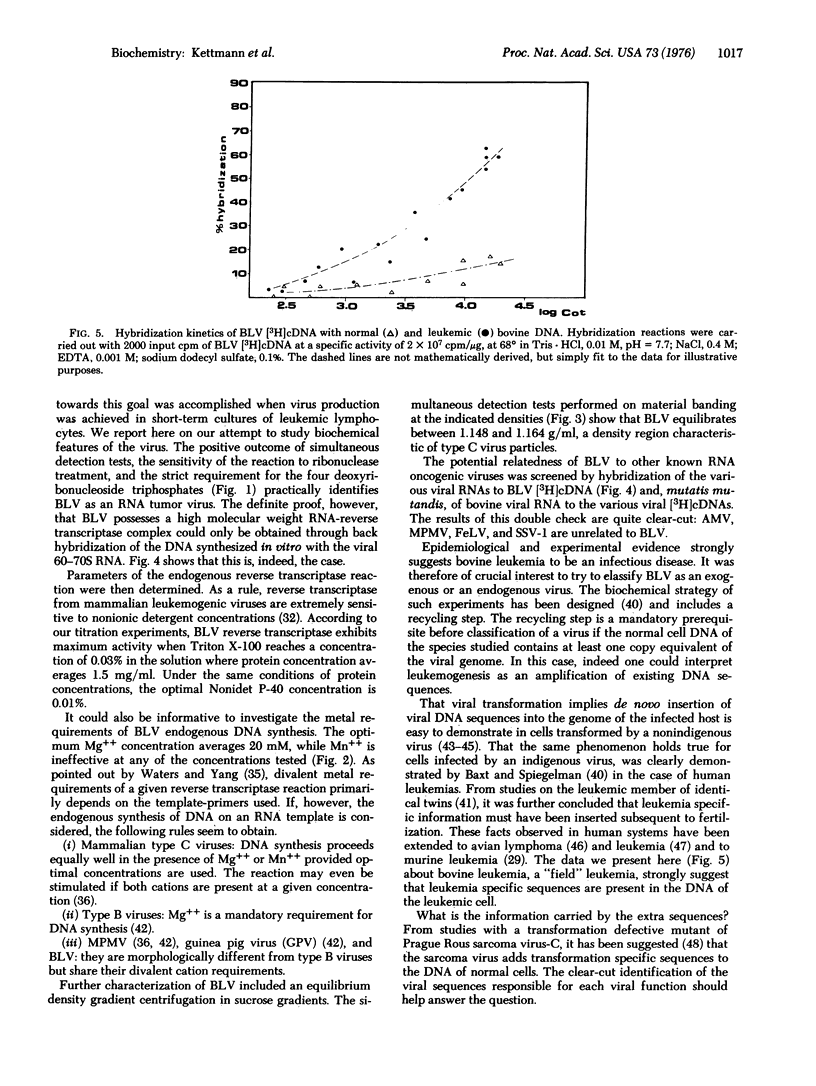
Short-term cultures of bovine leukemic lymphocytes release virus particles with biochemical properties of RNA oncogenic viruses. These particles, tentatively called bovine leukemia virus (BLV), have a high molecular weight RNA-reverse transcriptase complex and a density of 1.155 g/ml in sucrose solutions. Molecular hybridizations between BLV/[3H]cDNA and several viral RNAs show that BLV is not related to Mason-Pfizer monkey virus, simian sarcoma associated virus, feline leukemia virus, or avian myeloblastosis virus. These results were confirmed by hybridization between BLV 70S RNA and [3H]cDNA synthesized in the various viruses tested. The high preference of BLV reverse transciptase for Mg++ as the divalent cation suggests that BLV might be an atypical mammalian leukemogenic "type C" virus. DNA-DNA hybridization studies using BLV [3H]cDNA as a probe strongly suggest that the DNA of bovine leukemic cells contains viral sequences that cannot be detected in normal bovine DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Gallo R. C. Purification, characterization, and comparison of the DNA polymerases from two primate RNA tumor viruses. J Virol. 1973 Sep;12(3):431–439. doi: 10.1128/jvi.12.3.431-439.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt W. G., Spiegelman S. Nuclear DNA sequences present in human leukemic cells and absent in normal leukocytes. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3737–3741. doi: 10.1073/pnas.69.12.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt W., Yates J. W., Wallace H. J., Jr, Holland J. F., Spiegelman S. Leukemia-specific DNA sequences in leukocytes of the leukemic member of identical twins. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2629–2632. doi: 10.1073/pnas.70.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Ruprecht R., Simpson R. W., Spiegelman S. Deoxyribonucleic acid polymerase of Rous sarcoma virus: reaction conditions and analysis of the reaction product nucleic acids. J Virol. 1971 Nov;8(5):730–741. doi: 10.1128/jvi.8.5.730-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- CROSHAW J. E., Jr, ABT D. A., MARSHAK R. R., HARE W. C., SWITZER J., IPSEN J., DUTCHER R. M. PEDIGREE STUDIES IN BOVINE LYMPHOSARCOMA. Ann N Y Acad Sci. 1963 Nov 4;108:1193–1202. doi: 10.1111/j.1749-6632.1963.tb13444.x. [DOI] [PubMed] [Google Scholar]
- Calafat J., Hageman P. C., Ressang A. A. Structure of C-type virus particles in lymphocyte cultures of bovine origin. J Natl Cancer Inst. 1974 Apr;52(4):1251–1257. doi: 10.1093/jnci/52.4.1251. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Avila L., Stock N. D. Serological detection of type C viruses found in bovine cultures. Cancer Res. 1972 Sep;32(9):1864–1870. [PubMed] [Google Scholar]
- Gillespie D., Gallo R. C. RNA processing and RNA tumor virus origin and evolution. Science. 1975 May 23;188(4190):802–811. doi: 10.1126/science.47650. [DOI] [PubMed] [Google Scholar]
- Goodman N. C., Ruprecht R. M., Sweet R. W., Massey R., Deinhardt F., Spiegelman S. Viral-related DNA sequences before and after transformation by RNA tumor viruses. Int J Cancer. 1973 Nov 15;12(3):752–760. doi: 10.1002/ijc.2910120323. [DOI] [PubMed] [Google Scholar]
- Mammerickx M., Dekegel D. Studies on the relationship between persistent lymphocytosis, infection with C-type particles and presence of Trypanosoma theileri, associated with bovine enzootic leukosis. Zentralbl Veterinarmed B. 1975 Jul;22(5):411–419. doi: 10.1111/j.1439-0450.1975.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Schlom J., Dahlberg J., Perk K. Biochemical properties of the bromodeoxyuridine-induced guinea pig virus. J Virol. 1975 Oct;16(4):1039–1050. doi: 10.1128/jvi.16.4.1039-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Miller J. M., Olson C. Precipitating antibody to an internal antigen of the C-type virus associated with bovine lymphosarcoma. J Natl Cancer Inst. 1972 Nov;49(5):1459–1462. [PubMed] [Google Scholar]
- Miller J. M., Van der Maaten M. J. A complement-fixation test for the bovine leukemia (C-type) virus. J Natl Cancer Inst. 1974 Dec;53(6):1699–1702. [PubMed] [Google Scholar]
- Miller L. D., Miller J. M., Olson C. Inoculation of calves with particles resembling C-type virus from cultures of bovine lymphosarcoma. J Natl Cancer Inst. 1972 Feb;48(2):423–428. [PubMed] [Google Scholar]
- Neiman P. E., Purchase H. G., Okazaki W. Chicken leukosis virus genome sequences in DNA from normal chick cells and virus-induced bursal lymphomas. Cell. 1975 Apr;4(4):311–319. doi: 10.1016/0092-8674(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., McMillin C., MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. doi: 10.1128/jvi.13.4.837-846.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C., Hoss H. E., Miller J. M., Baumgartener L. E. Evidence of bovine C-type (leukemia) virus in dairy cattle. J Am Vet Med Assoc. 1973 Aug 15;163(4):355–357. [PubMed] [Google Scholar]
- Olson C., Miller L. D., Miller J. M., Hoss H. E. Transmission of lymphosarcoma from cattle to sheep. J Natl Cancer Inst. 1972 Nov;49(5):1463–1467. [PubMed] [Google Scholar]
- Paulsen J., Rudolph R., Miller J. M. Antibodies to common ovine and bovine C-type virus specific antigen in serum from sheep with spontaneous leukosis and from inoculated animals. Med Microbiol Immunol. 1974;159(2):105–114. doi: 10.1007/BF02123722. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. Separation of B and C type virions by centrifugation in gentle density gradients. J Virol. 1974 May;13(5):1143–1147. doi: 10.1128/jvi.13.5.1143-1147.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Evans R. M., Baluda M. A. Presence in leukemic cells of avian myeloblastosis virus-specific DNA sequences absent in normal chicken cells. J Virol. 1974 Jul;14(1):47–49. doi: 10.1128/jvi.14.1.47-49.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Burny A., Das M. R., Keydar J., Schlom J., Travnicek M., Watson K. Characterization of the products of DNA-directed DNA polymerases in oncogenic RNA viruses. Nature. 1970 Aug 8;227(5258):563–567. doi: 10.1038/227563a0. [DOI] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Sweet R. W., Goodman N. C., Cho J. R., Ruprecht R. M., Redfield R. R., Spiegelman S. The presence of unique DNA sequences after viral induction of leukemia in mice. (RNA tumor virus-nucleic acid hybridization-insertion of viral DNA). Proc Natl Acad Sci U S A. 1974 May;71(5):1705–1709. doi: 10.1073/pnas.71.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Van Der Maaten M. J., Miller J. M., Boothe A. D. Replicating type-C virus particles in monolayer cell cultures of tissues from cattle with lymphosarcoma. J Natl Cancer Inst. 1974 Feb;52(2):491–497. doi: 10.1093/jnci/52.2.491. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Levinson W. E., Bishop J. M. Extent of transcription by the RNA-dependent DNA polymerase of Rous sarcoma virus. Nat New Biol. 1971 Sep 1;233(35):19–21. doi: 10.1038/newbio233019a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Yang W. K. Comparative biochemical properties of RNA-directed DNA polymerases from Rauscher murine leukemia virus and avian myeloblastosis virus. Cancer Res. 1974 Oct;34(10):2585–2593. [PubMed] [Google Scholar]
- Wittmann W., Urbaneck D. Untersuchungen zur Atiologie der Rinderleukose. 8. Ubertragungsversuche mit Blut leukosekranker Rinder auf Schaflämmer (Kurzmitteilung) Arch Exp Veterinarmed. 1969;23(3):709–713. [PubMed] [Google Scholar]



