Abstract
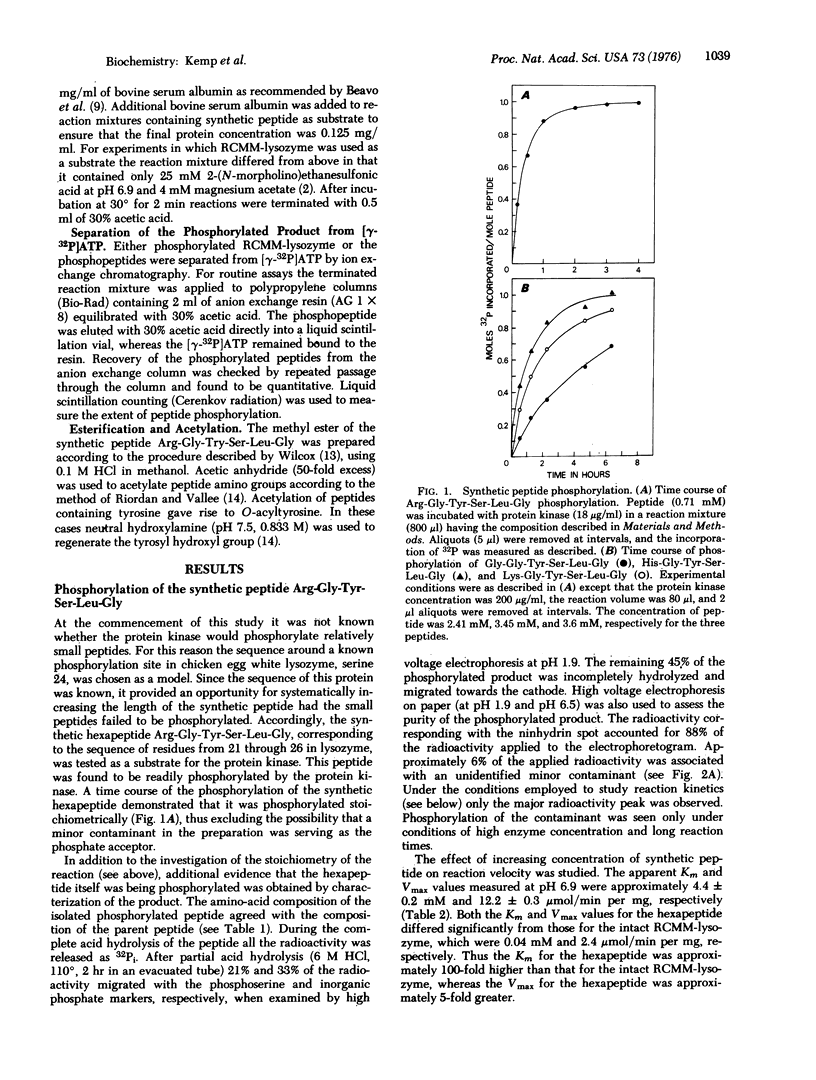
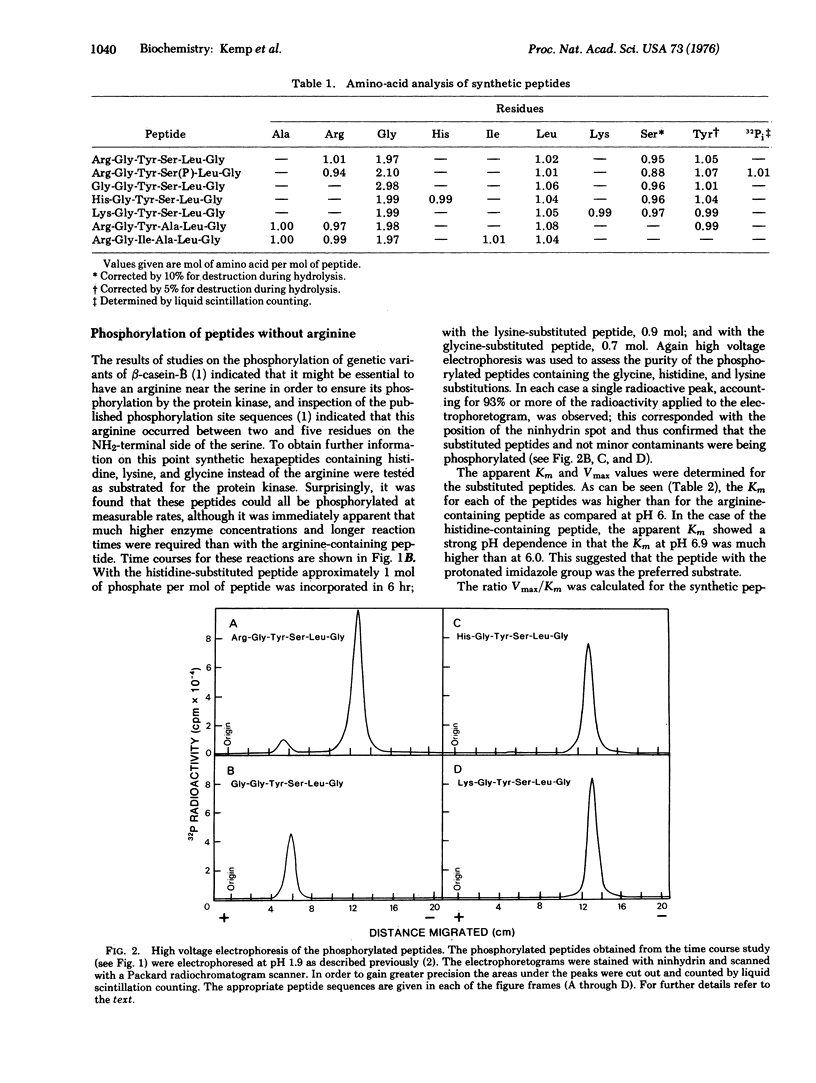
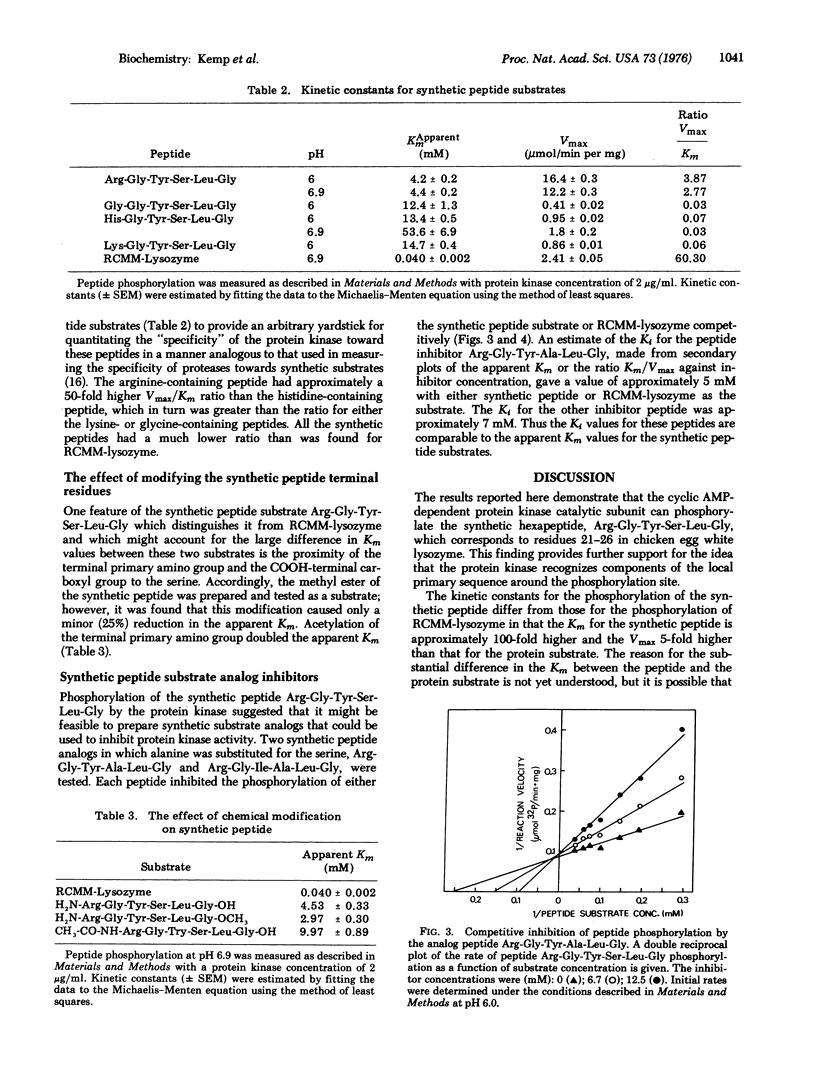
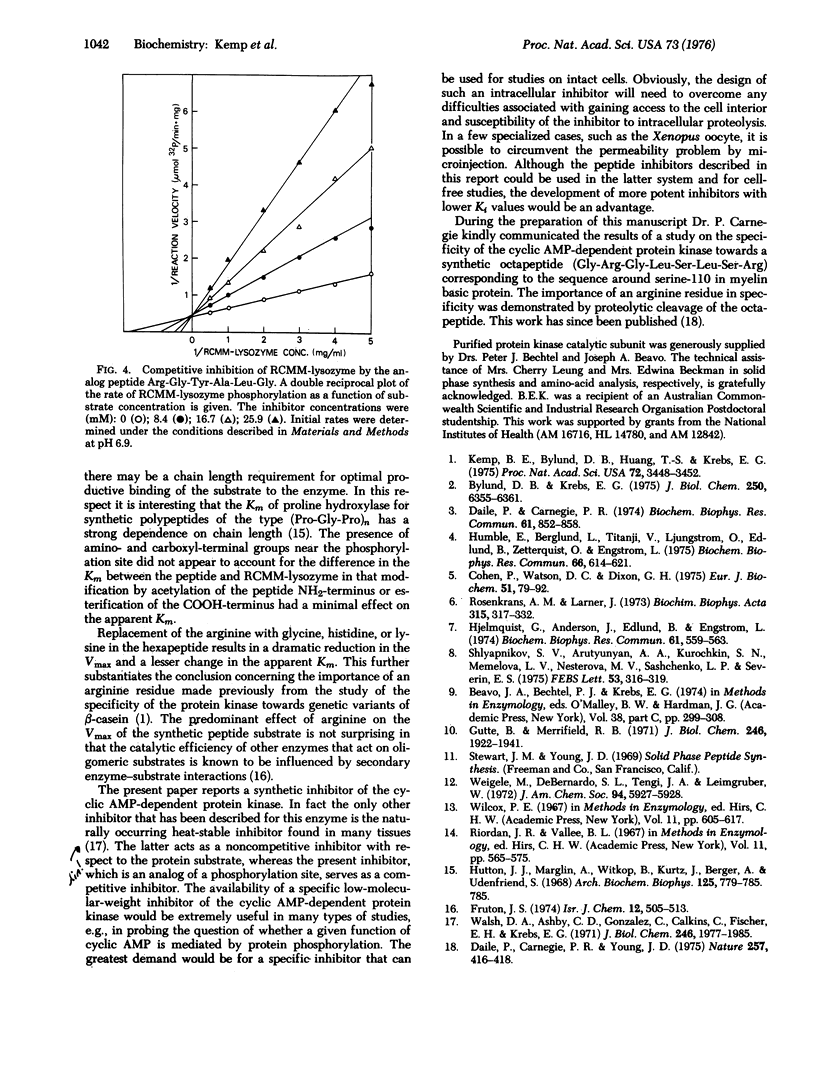
The substrate specificity of the catalytic subunit of rabbit skeletal muscle 3': 5'-cyclic AMP-dependent protein kinase (EC 2.7.1.37; ATP: protein phosphotransferase) has been studied using the synthetic peptide Arg-Gly-Tyr-Ser-Leu-Gly corresponding to the sequence around serine 24, a phosphorylation site in reduced, carboxymethylated, maleylated (RCMM) chicken egg white lysozyme. This peptide served as a substrate for the enzyme and exhibited a 6-fold higher Vmax and a 100-fold higher Km than RCMM-lysozyme. Replacement of the arginine with glycine, histidine, or lysine resulted in a dramatic reduction in the Vmax. These results support the concept that arginine is an important residue in determining the substrate specificity of the protein kinase, predominantly influencing the Vmax of the phosphorylation reaction. Two synthetic peptides in which serine was replaced by an alanine acted as competitive inhibitors of phosphorylation of the synthetic peptide substrate and RCMM-lysozyme.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bylund D. B., Krebs E. G. Effect of denaturation on the susceptibility of proteins to enzymic phosphorylation. J Biol Chem. 1975 Aug 25;250(16):6355–6361. [PubMed] [Google Scholar]
- Cohen P., Watson D. C., Dixon G. H. The hormonal control of activity of skeletal muscle phosphorylase kinase. Amino-acid sequences at the two sites of action of adenosine-3':5'-monophosphate-dependent protein kinase. Eur J Biochem. 1975 Feb 3;51(1):79–92. doi: 10.1111/j.1432-1033.1975.tb03909.x. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R. Peptides from myelin basic protein as substrates for adenosine 3', 5'-cyclic monophosphate-dependent protein kinases. Biochem Biophys Res Commun. 1974 Dec 11;61(3):852–858. doi: 10.1016/0006-291x(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R., Young J. D. Synthetic substrate for cyclic AMP-dependent protein kinase. Nature. 1975 Oct 2;257(5525):416–418. doi: 10.1038/257416a0. [DOI] [PubMed] [Google Scholar]
- Gutte B., Merrifield R. B. The synthesis of ribonuclease A. J Biol Chem. 1971 Mar 25;246(6):1922–1941. [PubMed] [Google Scholar]
- Hjelmquist G., Andersson J., Edlund B., Engstroöm L. Amino acid sequence of a (32P) phosphopeptide from pig liver pyruvate kinase phosphorylated by cyclic 3',5'-AMP-stimulated protein kinase and gamma-(32P)ATP. Biochem Biophys Res Commun. 1974 Nov 27;61(2):559–563. doi: 10.1016/0006-291x(74)90993-0. [DOI] [PubMed] [Google Scholar]
- Humble E., Berglund L., Titanji V., Ljungström O., Edlund B., Zetterqvist O., Engström L. Non-dependence on native structure of pig liver pyruvate kinase when used as a substrate for cyclic 3',5'-AMP-stimulated protein kinase. Biochem Biophys Res Commun. 1975 Sep 16;66(2):614–621. doi: 10.1016/0006-291x(75)90554-9. [DOI] [PubMed] [Google Scholar]
- Hutton J. J., Jr, Witkop B., Kurtz J., Berger A., Udenfriend S. Synthetic polypeptides as substrates and inhibitors of collagen proline hydroxylase. Arch Biochem Biophys. 1968 Jun;125(3):779–785. doi: 10.1016/0003-9861(68)90514-6. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyapnikov S. V., Arutyunyan A. A., Kurochkin S. N., Memelova L. V., Nesterova M. V., Sashchenko L. P., Severin E. S. Investigation of the sites phosphorylated in lysine-rich histones by protein kinase from pig brain. FEBS Lett. 1975 May 15;53(3):316–319. doi: 10.1016/0014-5793(75)80045-7. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]



