Abstract
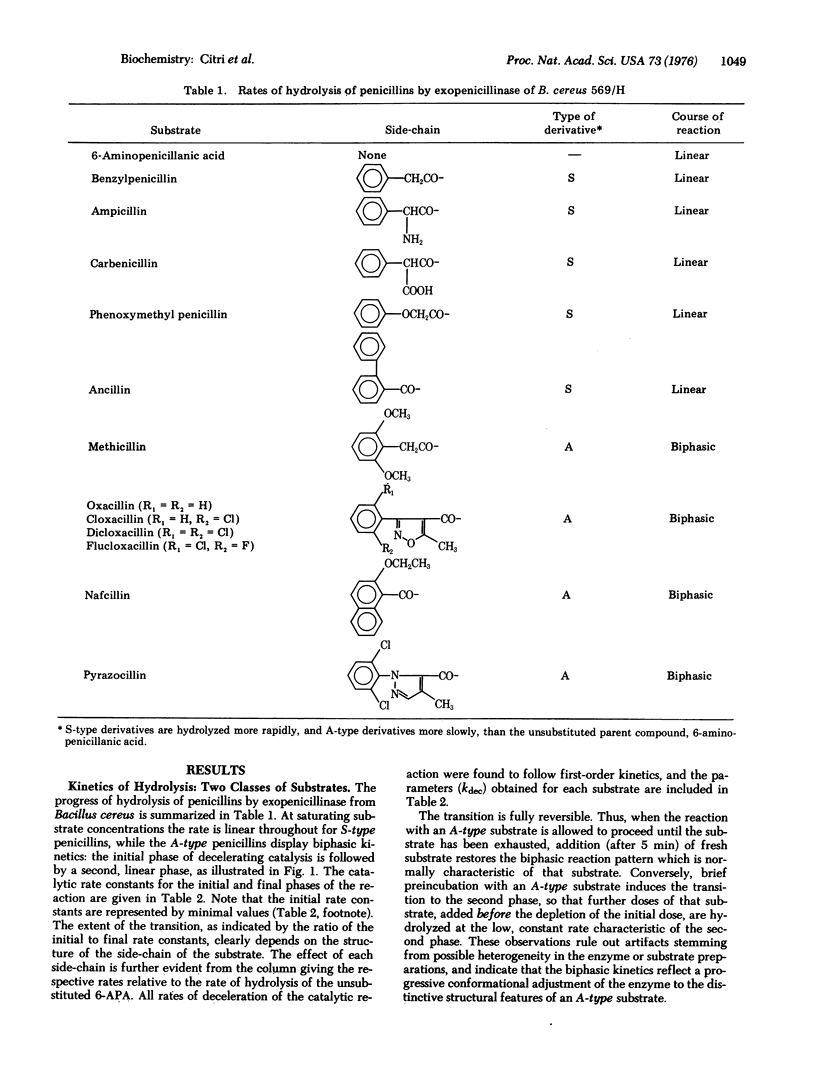
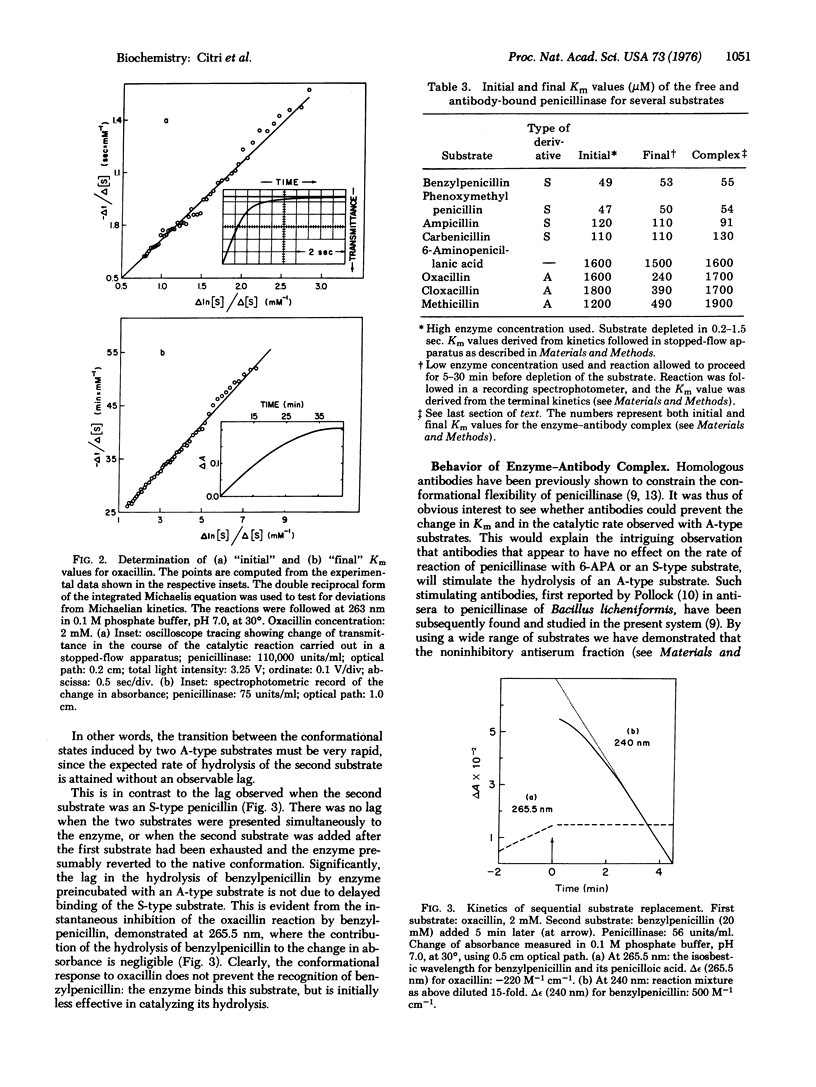
The progress of the catalytic reaction of penicillinase (EC 3.5.2.6; penicillin amido-beta-lactamhydrolase) depends on the structure of the side-chain in derivatives of 6-aminopenicillanic acid (the parent substrate). Side-chains of one class promote the rate of the reaction and cause no deviation from the linear kinetics observed with the parent compound. By contrast, side-chains of the other class induce a time-dependent, reversible change in the parameters of the catalytic reaction. The rate decelerates considerably and then becomes constant; the decrease in kcat is accompanied by a corresponding decrease in Km. The initial parameters of the biphasic reaction, determined by stopped-flow spectrophotometry, approach those of the unsubstituted 6-aminopenicillanic acid. The final parameters, which are specific for each derivative, are not acquired when the native conformation of the enzyme is stabilized by homologous antibodies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainslie G. R., Jr, Shill J. P., Neet K. E. Transients and cooperativity. A slow transition model for relating transients and cooperative kinetics of enzymes. J Biol Chem. 1972 Nov 10;247(21):7088–7096. [PubMed] [Google Scholar]
- BATCHELOR F. R., CAMERON-WOOD J., CHAIN E. B., ROLINSON G. N. STUDIES ON PENICILLINASE PRODUCED BY A STRAIN OF STAPHYLOCOCCUS AUREUS. Proc R Soc Lond B Biol Sci. 1963 Oct 22;158:311–328. doi: 10.1098/rspb.1963.0050. [DOI] [PubMed] [Google Scholar]
- CITRI N., GARBER N., SELA M. The effect of urea and guanidine hydrochloride on activity and optical rotation of penicillinase. J Biol Chem. 1960 Dec;235:3454–3459. [PubMed] [Google Scholar]
- Citri N. Conformational adaptability in enzymes. Adv Enzymol Relat Areas Mol Biol. 1973;37:397–648. doi: 10.1002/9780470122822.ch7. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Csányi V., Mile I., Koczka I., Badár E., Horváth I. A particular conformational change of Bacillus cereus penicillinase under the action of a new penicillin analogue pyrazocillin. Biochim Biophys Acta. 1970 Nov 11;220(2):317–325. doi: 10.1016/0005-2744(70)90016-1. [DOI] [PubMed] [Google Scholar]
- Dyke K. G. Substrate-specific inactivation of staphylococcal penicillinase. Biochem J. 1967 Jun;103(3):641–646. doi: 10.1042/bj1030641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley H. N. Statistical estimations in enzyme kinetics. The integrated Michaelis equation. Eur J Biochem. 1974 Apr 1;43(2):377–378. doi: 10.1111/j.1432-1033.1974.tb03423.x. [DOI] [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- GOUREVITCH A., PURSIANO T. A., LEIN J. Inactivation of staphylococcal penicillinase by reaction with resistant penicillins. Nature. 1962 Aug 4;195:496–497. doi: 10.1038/195496a0. [DOI] [PubMed] [Google Scholar]
- IMSANDE J. NEW ASSAY FOR PENICILLINASE AND SOME RESULTS ON PENICILLINASE INDUCTION. J Bacteriol. 1965 May;89:1322–1327. doi: 10.1128/jb.89.5.1322-1327.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- Rabin B. R. Co-operative effects in enzyme catalysis: a possible kinetic model based on substrate-induced conformation isomerization. Biochem J. 1967 Feb;102(2):22C–23C. doi: 10.1042/bj1020022c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Samuni A., Citri N. Biphasic kinetics induced by modified substrates of penicillinase. Biochem Biophys Res Commun. 1975 Jan 6;62(1):7–11. doi: 10.1016/s0006-291x(75)80397-4. [DOI] [PubMed] [Google Scholar]
- Zyk N., Citri N. The interaction of penicillinase with penicillins. VI. Comparison of free and antibody-bound enzyme. Biochim Biophys Acta. 1968 Jun 4;159(2):317–326. doi: 10.1016/0005-2744(68)90080-6. [DOI] [PubMed] [Google Scholar]
- Zyk N., Citri N. The interaction of penicillinase with penicillins. VII. Effect of specific antibodies on conformative response. Biochim Biophys Acta. 1968 Jun 4;159(2):327–339. doi: 10.1016/0005-2744(68)90081-8. [DOI] [PubMed] [Google Scholar]



