Abstract
Ataxia telangiectasia (AT) is a human genetic disease characterized by radiation sensitivity, impaired neuronal development and predisposition to cancer. Using a genetically defined model cell system consisting of cells expressing a kinase dead or a kinase proficient ATM gene product, we previously reported systemic alterations in major metabolic pathways that translate at the gene expression, protein and small molecule metabolite levels. Here, we report ionizing radiation induced stress response signaling arising from perturbations in the ATM gene, by employing a functional proteomics approach. Functional pathway analysis shows robust translational and post-translational responses under ATM proficient conditions, which include enrichment of proteins in the Ephrin receptor and axonal guidance signaling pathways. These molecular networks offer a hypothesis generating function for further investigations of cellular stress responses.
INTRODUCTION
Cellular responses to ionizing radiation are complex, multi-genetic traits, important in cell survival and genomic stability. Cell cycle arrest, activation of signal transduction pathways and stimulation of DNA repair processes all contribute to the phenotype, which may be categorized in various gradations of radiation sensitivity or radiation resistance. These intrinsic genetic programs allow the cell to elicit DNA damage responses or to activate programmed cell death in a quantitative context of cellular injury (1).
Among the clinical syndromes exhibiting radiation sensitivity as a result of single gene mutations, the human genetic disease, ataxia telangiectasia (AT), has been the subject of intense investigations and the ATM gene that is mutated in AT has been cloned and characterized (2–4). ATM is a member of phosphatidylinositol 3 kinase family of genes, which play roles in multiple signal transduction pathways. Most patients with AT have mutations which result in ATM gene product truncation. These AT patients develop neurological degeneration leading to ataxia, a pattern of cutaneous teleangiectasias, immunological deficiencies and extreme sensitivity to ionizing radiation exposure (5–9). However, the correlation of clinical observations to disruptions in signaling pathways still needs to be fully defined and integrated on a global proteomic scale.
AT5BIVA cells are SV40 immortalized fibroblast derived from an AT-group D patient, resulting in truncation in the carboxyl terminus domain (see Supplementary Fig. S1; http://dx.doi.org/10.1667/RR3198.1.S1). The clonal cell line, ATCL8, was established by introducing a vector expressing the full-length wild-type ATM cDNA into AT5BIVA (10). Previously we reported an integrated “omics” study to characterize the molecular changes at the gene expression, metabolomic and proteomics levels in a baseline comparison of ATCL8 and AT5BIVA cells (11). With a goal to gain insight into post-irradiation changes in protein levels or modifications affected by the presence or absence of functionally intact ATM, we now report here the global proteomic responses in these human AT fibroblasts, following exposure to ionizing radiation. In the study, the focus is on relative changes in protein levels of the irradiated cells, compared to the unirradiated controls. Proteins were identified using mass spectrometry. Western blot analysis was utilized to validate a subset sample of proteins and proteins showing significant expression alterations in response to radiation-induced stress. The post-translational modifications of proteins were investigated using phospho-protein antibody arrays. Our data show distinct patterns of stress response signaling under ATM deficient or proficient conditions.
METHODS
Samples
AT5BIVA cells were obtained from the National Institute of General Medical Sciences (NIGMS). The ATCL8 cell line was established by transfecting AT5BIVA cells with the wild-type, full length “ATM” cDNA in a pcDNA expression vector and selected by screening for the presence of ATM protein expression and correction of radiation sensitivity (12). Cells were maintained in modified Eagle’s medium with 20% fetal bovine serum, 100 U/ penicillin, and 100 pg/ml streptomycin. For this study the cells were grown to 80% confluence and serum starved for 24 h. Logarithmically growing cells were exposed to 5 Gy of γ radiation in air at room temperature using a [137Cs] irradiator (J. L. Shepard Mark I). The cells were incubated at 37°C and washed twice with chilled phosphate buffered saline (PBS) before harvesting at predetermined intervals via scraping and centrifuging (11).
Immunoflourescence Microscopy
Cells were placed on glass cover slips, fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100 in PBS for 10 min, followed by incubation with blocking solution (1% bovine serum albumin in PBS). After incubation with primary antibodies, the cells were incubated with the appropriate secondary antibodies: donkey anti-mouse or anti-rabbit Alexa488 and Alexa566 fluoro-conjugated antibodies (Invitrogen), nuclei were stained with DAPI. The cover slips were washed with PBS, and then mounted on slides with ProLongt Gold Antifade Reagent (Invitrogen). Images were captured with an Olympus IX-70 Laser Confocal Scanning fluorescence microscope with laser lines: Blue Diode (Ex. 405 nm), Argon Multiline (Ex. 488, 514 nm), Green Helium-Neon (Ex. 543 nm), all with AOTF for individual intensity control. Images data for each fluorophore was collected in sequential mode, using Fluoview acquisition software and oil immersion lenses (40× N.A. 1.0, 60× N.A. 1.4).
Two-Dimensional Gel Electrophoresis and Mass Spectrometry
For each proteomic experiment, two 2D gels were run for each of three biological replicates per cell type to yield six independent data sets. Five hundred milligrams of each prepared whole cell lysate were loaded onto IPG ReadyStrips pH 3–10 NL (Bio-Rad), and rehydrated at 50 V for 12 h at 20°C. Isoelectric focusing on the Protean IEF Cell from Bio-Rad was performed as follows: 15 min at 250 V, a rapid voltage ramping to 10,000 V and a final step at 10,000 V up to 80,000 V-hours. The strips were equilibrated and the proteins were resolved in the second dimension on 12% SDS-PAGE gels, fixed and stained with Commassie blue G250, destained in water and imaged. The protein patterns were compared using Dymension imaging software (version 1.5, Syngene). Each of the 2D gels was used for comparison of normalized spot density. Three biological replicates were used in duplicates, for each condition (cell type and post-radiation time point). The protein profiles were highly reproducible among replicates from each sample resulting in 85–93% correlation with respect to the number of common spots present. Although we used a broad pH range (3–10) for protein resolution, most of the protein spots lay within pH 4–7 and molecular weight 25–85 KDa ranges. For a protein spot to qualify as a differentially expressed spot, high stringency cut-off parameters were used. Spots with a fold change of ≥2 with P ≤ 0.05 were considered to be statistically significant and were subsequently selected for identification by mass spectrometry.
In-Gel Tryptic Digestion and Protein Identification by Mass Spectrometry
Briefly, the protein spots of interest were manually excised from the 2D gel, transferred to Montage plate (Millipore) and destained with 50% acetonitrile in 25 mM ammonium bicarbonate, dehydrated with acetonitrile for 5 min and vacuum dried. Gel pieces were then rehydrated with 25 mM ammonium bicarbonate supplemented with trypsin (5 ng/μl, Promega, Madison, WI) at 37°C for 16 h. Subsequently, tryptic peptides were extracted in 0.1% TFA/50% acetonitrile and mixed with an equal volume of 5 mg/ml CHCA (Acros Organics, NJ). Mass spectra were recorded with a matrix assisted laser desorption/ionization–time of flight, time of flight (MALDI-TOF-TOF) spectrometer (4700 Proteomics Analyzer, ABI) set in reflector positive mode by spotting the samples onto a MALDI plate. Peptide masses were compared with the theoretical masses derived from the sequences contained in Swiss-Prot databases using MASCOT for Homo sapiens as the taxonomy filter. The search parameters were set as follows: monoisotopic mass selection for MH+1 peptides with cysteines as carbamidomethyl derivative, one missed cleavage for trypsin allowed, peptide mass error 50 ppm, at least four peptides mass hits were required for protein match.
Western Blotting
Protein extracts of AT5BIVA and ATCL8 cells were resolved by 1D gel electrophoresis on 4–20% Bis-Tris NuPage gels (Invitrogen), transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore Corporation, Bedford, MA). All antibodies were purchased from Santacruz Biotechnology. Anti-phosphorylated serine 1981 ATM antibody was procured from Rockland Chemicals. Western blotting was performed using standard procedures and the blots were developed using Super-signal West Pico Chemiluminiscent substrate (Pierce). Detection was performed with autoradiography films (BioMax Light Film, Eastman Kodak Rochester, NY).
Phospho-Kinase Array
The phospho-proteome profiler kit was purchased from R&D systems, which contained capture antibodies against a panel of 46 kinases spotted in duplicates on nitrocellulose membranes. The blotting was performed per instructions of the manufacturer (R&D Systems). The normalized spot intensity was used to evaluate differential levels of phosphorylation.
Functional Pathway Analysis
IPA-proteomics a capability within the Ingenuity Pathway Analysis (IPA) program was used to automatically generate pathways related to the differentially expressed proteins. Network analysis was performed using the IPA tool, to generate functionally associated networks based on curated literature information of protein interaction, co-expression and genetic regulation.
RESULTS AND DISCUSSION
Validation of the AT Phenotype
The ATM gene product is crucial for mediating cellular response to ionizing radiation exposure and to other agents, which cause double-stranded DNA breaks. Various signaling pathways are initiated leading to DNA repair, stress response and cell survival (13). The ATM gene in AT5BIVA cells is mutated at multiple loci as illustrated in Supplementary Fig. S1 (http://dx.doi.org/10.1667/RR3198.1.S1), leading to a kinase dead gene product. To validate the AT phenotype in AT5BIVA and the restored ATM activity in ATCL8 cells, we determined the characteristic serine 1981 phosphorylation in response to radiation exposure by immune-fluorescence (Fig. 1). The data indicate loss of phosphorylation of ATM in AT5BIVA cells and restoration of serine 1981 phosphorylation ATM in ATCL8 cell line.
FIG. 1.
ATM is phosphorylated at Serine1891 in response to ionizing radiation (5 Gy) within 1 h in ATCL8 cells but not in AT5BIVA. Irradiated (+R) and unirradiated (−R) cells were stained with anti-phosphorylated serine 1981 ATM antibody (red) and analyzed by immunofluorescence microscopy. Calreticulin antibody (green) was used as a control. Nuclear staining for phosphorylated ATM was observed in ATCL8 in response to radiation exposure (+R) but not in AT5BIVA indicating a loss of function in AT5BIVA cells and restoration of ATM activity in ATCL8 cells. Panel B. Quantitative representation of the results in Panel A.
Identification of Differentially Expressed Proteins in Response to Radiation Exposure
We studied the changes in the proteome of ATCL8 and AT5BIVA cells 0.5, 1, 3 and 24 h after radiation exposures in comparison to nonirradiated cells. Whole cell protein extracts were resolved on 2-DGE (Supplementary Figs. S2–S5; http://dx.doi.org/10.1667/RR3198.1.S1). Comparative 2D analysis detected a total of 435 and 630 differentially abundant proteins, representing either changes in relative levels or post-translational modifications, for AT5BIVA and ATCL8 cell lines respectively, across the time course study. Of these, a total of 164 proteins for AT5BIVA and 186 proteins for ATCL8 were identified with high confidence. A subset of these proteins are listed in Tables 1A and 1B, while the rest are listed in Supplementary Tables S1 (A–H) (http://dx.doi.org/10.1667/RR3198.1.S1). Gene ontology analysis showed the differentially abundant proteins to be involved in diverse cellular processes including transcriptional regulation, metabolism, mitochondrial structure and function, cell structure maintenance and signaling among others (Supplementary Fig. S6A and B; http://dx.doi.org/10.1667/RR3198.1.S1). A subset of these proteins was selected for validation by Western blot analysis and confirmed to be consistent with 2D gel analysis findings (Fig. 2).
TABLE 1A.
List of differentially expressed proteins identified by expression profiling in AT5BIVA and ATCL8 cells, respectively. Differentially expressed proteins belonging to functionally different classes are listed. Two-dimensional electrophoresis was performed for AT5BIVA and ATCL8 whole cell lysates with and without radiation exposure. The gel spots from radiation treated samples were compared to those without radiation treatment using Dymension (Syngene) image analysis software. The spots exhibiting differential normalized intensities were analyzed by MALDI-TOF mass spectrometry and identified using MASCOT database search. The listed proteins had confidence intervals greater than 95% and have at least 3 unique peptides associated with the protein identification. A green arrow depicts a down regulation subsequent to radiation treatment while a red arrow depicts an up-regulation.
| AT5BIVA | |||||
|---|---|---|---|---|---|
|
| |||||
| Protein Name | Accession Number | 30 min | 1h | 3h | 24h |
| Cytoskelton protein | |||||
| Vimentin | VIME_HUMAN | - | - |

|

|
| Ezrin | EZRI_HUMAN | - |

|
- | - |
| Actin, cytoplasmic 1 | ACTB_HUMAN | - | - |

|

|
| Tubulin beta–4 chain | TBB4_HUMAN |

|
- |

|

|
| Myosin regulatory light chain 2 | MLRM_HUMAN |

|
- |

|

|
| Cofilin, non-muscle isoform | COF1_HUMAN |

|
- | - | - |
| Transgelin | TAGL_HUMAN | - | - | - |

|
| Annexin A3 | ANXA3_HUMAN | - | - |

|
- |
| Vimentin | VIME_HUMAN | - | - |

|
- |
| Moesin | MOES_HUMAN | - |

|

|
- |
|
| |||||
| Signaling | |||||
|
| |||||
| Adenosine kinase | ADK_HUMAN | - | - |

|
- |
| Adenylate kinase | KAD2_HUMAN |

|
- | - |

|
| cAMP-dependent protein kinase | KAP3_HUMAN | - | - |

|

|
| ALK tyrosine kinase receptor precursor | ALK_HUMAN | - |

|
- | - |
| neutral alpha=glucosidase AB precursor | GANAB_HUMAN | - |

|

|

|
|
| |||||
| Transcription/Traslation/DNA Repair | |||||
|
| |||||
| Structural maintenance of chromosome | SMC1A_HUMAN | - |

|
- | - |
| Elongation factor 2 | EF2_HUMAN | - |

|
- | - |
| Nucleolin | NUCL_HUMAN | - |

|
- | - |
| Cellular nucleic acid binding protein | CNBP_HUMAN | - | - |

|
- |
| ATP dependent DNA helicase II | KU86_HUMAN | - | - |

|
- |
| Splicing factor | SFRS3_HUMAN | - | - |

|

|
| Exostosin-2 | EXT2_HUMAN | - |

|
- | - |
| Zinc finger protein 95 homolog | ZFP95_HUMAN | - |

|
- | - |
|
| |||||
| Cell Cycle/Apoptosis | |||||
|
| |||||
| Cell division protein kinase 6 | CDK6_HUMAN | - | - | - |

|
| 26S protease regulatory subunit 8 | PRS10_HUMAN | - | - |

|

|
| Ubiquitin-like 1 activating enzyme E1A | ULE1A_HUMAN | - | - |

|
- |
| Proteasome subunit alpah type 6 | PSA6_HUMAN |

|
- | - | - |
| Thioredoxin-like protein p19 precursor | TXNL2_HUMAN |

|
- |

|

|
| Cyclin B1 interacting protein 1 | CIP1_HUMAN | - |

|
- |

|
| 14-3-3 protein tau | 1433T_HUMAN | - | - |

|

|
| Heat shock protein HSP 90-alpha/beta | HS90A_HUMAN | - | - |

|
- |
| UV excision repair protein RAD23 homolog | RAD23B_HUMAN |

|
- | - | - |
| Microtubule-actin crosslinking factor 1 | MACF4_HUMAN |

|
- | - | - |
| Glyceraldehyde-3-phosphate dehydrogena | G3P2_HUMAN | - |

|
- | - |
| Superoxide dismutase[Cu-Zn] | SODC_HUMAN | - | - | - |

|
TABLE 1B.
List of differentially expressed proteins identified by expression profiling in AT5BIVA and ATCL8 cells, respectively. Differentially expressed proteins belonging to functionally different classes are listed. Two-dimensional electrophoresis was performed for AT5BIVA and ATCL8 whole cell lysates with and without radiation exposure. The gel spots from radiation treated samples were compared to those without radiation treatment using Dymension (Syngene) image analysis software. The spots exhibiting differential normalized intensities were analyzed by MALDI-TOF mass spectrometry and identified using MASCOT database search. The listed proteins had confidence intervals greater than 95% and have at least 3 unique peptides associated with the protein identification. A green arrow depicts a down regulation subsequent to radiation treatment while a red arrow depicts an up-regulation.
| ATGL8 | |||||
|---|---|---|---|---|---|
|
| |||||
| Protein Name | Accession Number | 30 min | 1h | 3h | 24h |
| Cytoskelton protein | |||||
| Annexin A1 | ANXA1_HUMAN |

|

|
- | - |
| Caldesmon | CALD_HUMAN |

|
- | - |

|
| Rab GDP dissiciation inhibitor alpha | GDIA_HUMAN |

|
- | - | - |
| Supervillin | SVIL_HUMAN | - |

|
- | - |
| Annexin A6 | ANXA6_HUMAN | - |

|

|

|
| Myosin heavy chain, skeletal muscle, fetal | MYH4_HUMAN | - | - |

|

|
| Transgelin-2 | TAGL2_HUMAN | - |

|
- | - |
| WD-repeat protein 22 | WDR22_HUMAN | - | - |

|

|
| Ezrin | EZRI_HUMAN |

|
- | - | |
| Myosin light chain kinase 2, skeletal/cardiac muscle | MYLK2_HUMAN |

|

|

|

|
|
| |||||
| Signaling | - | ||||
|
| |||||
| Protocadherin 15 precursor | PCD15_HUMAN |

|

|

|

|
| Mitigen-activated protein kinase kinase kinase kinase | M4K2_HUMAN | - |

|
- | - |
| Camp-specific 3′,5′-cyclic phosphodiesterase | PDE4B_HUMAN |

|

|
- | - |
| tumor necrosis factor | TNAP1_HUMAN |

|
- |

|
- |
| Phosphatidylinositol-glycan-specific phospholipase | PHL2_HUMAN |

|
- |

|
- |
| RasGAP-activating-like -protein 1 | RASL1_HUMAN |

|

|

|

|
| Protein tyrosine kinase 2 beta | FAK2_HUMAN | - | - |

|
- |
| Signal transducer and activator of transcription 3 | STAT3_HUMAN | - | - |

|
- |
| Guanine nucleotide-binding protein | GNAS_HUMAN | - | - |

|
- |
| Ephrin-B3 precursor | EFNB3_HUMAN | - | - |

|
- |
| Serine/threonine-protein kinase PAK 7 | PAK7_HUMAN | - | - |

|
- |
| Serine/threonine-protein kinase PAK 7 | PAK7_HUMAN |

|

|
||
| 130 kDa leucine-rich protein | LPPRC_HUMAN |

|
- | - | - |
| Vinculin | VINC_HUMAN |

|
- | - | - |
| Glyceraldehyde-3-phosphate dehydrogenase | G3P2_HUMAN | - | - |

|
- |
| ATP-dependent DNA helicase II, 70 kDa subunit | KU70_HUMAN |

|
- | - | - |
| Dedicator of cytokinesis protein 9 | DOCK9_HUMAN |

|
- |

|
- |
| Heat shock cognate 71 kDa protein | HSP7C_HUMAN | - | - |

|

|
|
| |||||
| Transcription/Translation/DNA Repair | |||||
|
| |||||
| Structural maintenance of chromosome 1 | SMCIA_HUMAN | - | - |

|
- |
| Transformation/transcription domain-associated protein | TRRAP_HUMAN | - |

|

|

|
| Chromodomain-helicase-DNA-binding protein 1 | CHD1_HUMAN | - |

|

|

|
| DNA-direct RNA polymerase L largest subunit | RPA1_HUMAN | - |

|

|

|
| RNA 3;-terminal phosphate cyclase-loke protein | RCL1_HUMAN |

|
- |

|

|
| Nucleolar protein | NOP5_HUMAN |

|
- |

|
- |
| Elongation factor 1-gamma | EF1G_HUMAN | - | - |

|
- |
| DNA-lyase | APEX1_HUMAN | - |

|
- | |
| Guanine nucleotide-binding prolein G, alpha subunit | GNA11_HUMAN | - |

|

|

|
| CENP-F kinetochore protein | CENPF_HUMAN | - |

|

|

|
| Nuclear receptor corepressor 1 | NCOR1_HUMAN | - | - |

|
- |
| Nucleosome assembly protein 1-like 4 | NP1L4_HUMAN | - | - |

|

|
|
| |||||
| Cell Cycle/Apoptosis | |||||
|
| |||||
| Proteosome subunit beta type1 | PSB1_HUMAN |

|
- | - | - |
| Ubiquitin ligase protein COP1 | COP1H_HUMAN | - |

|

|
- |
| 26S proteasome-associated protein | S1769_HUMAN | - | - |

|

|
| Ubiquitin-activating enzyme E1 | UBE1_HUMAN | - | - |

|
- |
| Proteasome subunit alpha type 7 | PSA7_HUMAN | - |

|
- | - |
| Programmed cell death 6-interacting protein | PDC6I_HUMAN | - |

|
- | - |
| G2 and S phase expressed protein 1 | GTSE1_HUMAN |

|
- | - | - |
| Angiopoietin-2 precursor | ANGP2_HUMAN | - | - |

|

|
| Stress-70 protein | GRP75_HUMAN | - |

|

|

|
| Nuclear autoantigenic sperm protein | NASP_HUMAN | - |

|

|

|
FIG. 2.
Validation of differential expression using Western blot analysis. ATCL8 (top panel) and AT5BIVA (bottom panel) were either sham treated or exposed to γ rays (5 Gy) and harvested at 30 min or 3 h after radiation exposure. The protein extracts were run on 4–12% Bis-Tris NuPage gels (Invitrogen), transferred on to a PVDF membrane and blotted with the respective antibodies. The band intensity was measured by densitometry using image-J. The bars indicate the target protein intensity normalized to the band intensity of GAPDH. The error bars represent the standard deviation. P values represent the significant of change in the expression levels of proteins after 30 min and 3 h after radiation exposure compared to the sham treated cells. The significance was determined using t test (Graph pad Prism V5.0). ***P < 0.001, **P < 0.01, *P < 0.05.
To understand pathway based responses to radiation exposure, we selected 30 min and 3 h as early and late response, post-radiation time points. Network modeling revealed enrichment of proteins participating in cell cycle, cellular assembly and organization, cell death and proliferation pathways in both cell lines; however the interacting protein nodes were different (Figs. 3 and 4; and Supplementary Fig. S7; http://dx.doi.org/10.1667/RR3198.1.S1). This indicated that although the same pathways were impacted in AT5BIVA and ATCL8, the patterns of responses were distinct and ATM dependent. As an example, ATCL8 cells showed an up-regulation of PAK family kinases at the early response time point of 30 min, consistent with regulation of cell survival and apoptosis through the activation of AKT (14). In general, signaling pathways promoting proliferation, transcription and translational processes as well as cytoskeletal proteins were down regulated 30 min after radiation exposure. In ATCL8 cells, concomitant increases in proteins promoting degradation and apoptosis were observed, including ubiquitin activating enzyme, proteasomes, and ubiquitin ligase. Three hours after radiation treatment, NCOR1, a protein reported to facilitate cellular recovery from DNA double-strand breaks was found to be upregulated in ATCL8, while STAT3, a protein known to trigger cell proliferation or apoptosis in appropriate cellular context was downregulated (Supplementary Table 1B and Fig. S9A; http://dx.doi.org/10.1667/RR3198.1.S1). ATCL8 also showed upregulation of the MAP2K3 based apoptosis signaling pathway, GNAS complex and phosphinositide 3-kinase, which regulates cellular events that include protein folding and intracellular targeting. By contrast, AT5BIVA showed a downregulation of RAD23 and upregulation of microtubule actin cross-linking factor (MACF1) and GLRX3, which regulate the cellular redox state and have been shown to be upregulated in lung and colon cancer (15) (Fig. 2B). In contrast to ATCL8, AT5BIVA cells did not show any changes in the expression of proteins participating in cell cycle, cell signaling or apoptosis up to 1 h after radiation exposure. Notably, Vimentin, a cytoskeletal protein that is responsible for maintaining cellular integrity (16) was found to be down-regulated in AT5BIVA in response to radiation exposure while Ezrin was upregulated 1 h after irradiation. Increased expression of Ezrin has been associated with cellular transformation and cellular redistribution in Rat-1 fibroblasts (17). Taken together, these data emphasize the requirement of functional ATM for triggering cell cycle checkpoints and DNA repair mechanisms.
FIG. 3.
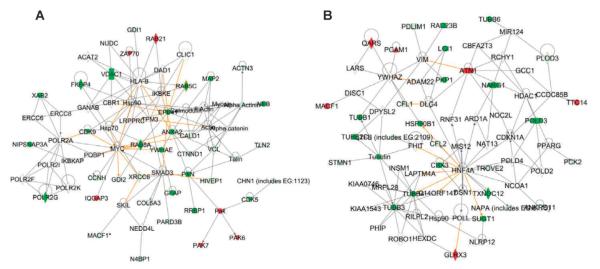
Network modeling reveals differential stress response signaling in an ATM dependent manner, 30 min after radiation exposure. Differential protein expression was determined by comparing samples that were harvested after radiation exposure to control samples that were not subjected irradiated for each cell type. Panels A and B: Molecular networks modules for ATCL8 and AT5BIVA respectively, 30 min after irradiation showing a distinct difference in the participating proteins. In each network module, a solid line indicates direct interaction; a dashed line indicates indirect interaction; a line without arrowhead indicates binding; an arrow from protein A to protein B indicates A acts on B. Node shapes are indicative: triangle, kinase; diamond, enzyme; hexagon, translation regulator; trapezoid, transporter; oval (horizontal), transcription regulator; oval (vertical), trans-membrane receptor. Proteins that are upregulated are marked in red while those that are down regulated are marked in green.
FIG. 4.
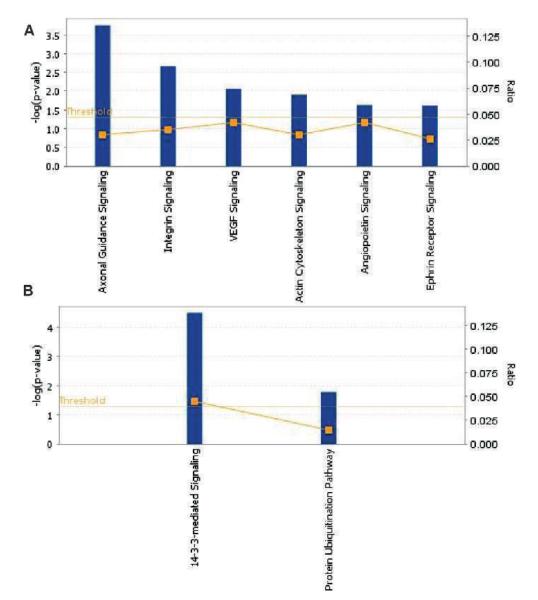
Functional pathway analysis reveals differential stress response signaling in an ATM dependent manner 30 min after irradiation. Differential protein expression was determined by comparing samples that were harvested after radiation treatment to unirradiated samples for each cell type. Canonical pathway enrichment analysis shows a robust oxidative stress response signaling by ATCL8 with an enrichment of proteins in integrin, VEGF and Ephrin receptor signaling (Panel A) in contrast to AT5BIVA (Panel B).
Analysis of the canonical pathways in ATCL8 showed the enrichment of axonal guidance, actin cytoskeletal and Ephrin receptor signaling as the major pathways, consistently at 30 min and 3 h with Ephrin B3 and PAK family of kinases as major participants (Figs. 3A and 4A and Supplementary Fig. S8A and C; http://dx.doi.org/10.1667/RR3198.1.S1). ATM deficient AT5BIVA at 30 min was a slow responder with few major pathways triggered in response to radiation exposure (Fig. 4B). At 3 h, purine metabolism, 14-3-3 and VEGF signaling were found to predominate in AT5BIVA (Supplementary Fig. S7D; http://dx.doi.org/10.1667/RR3198.1.S1).
In summary, ATCL8 showed down-regulation of metabolic pathways and those which facilitate cell division, proliferation and differentiation. This is complemented by the upregulation of stress pathways including cell-cycle arrest, protein degradation and cellular apoptosis concomitant to radiation exposure. In contrast to the stress response signaling in ATCL8 as early as 30 min after radiation exposure, proteomic profiling in AT5BIVA revealed upregulation of proteins involved in metabolic pathways including protein biosynthesis at this time point. At 3 h after radiation exposure, proteins such as cAMP dependent protein kinase and HSP 90 were upregulated in AT5BIVA. These proteins have been shown to promote cell proliferation by inhibiting stress-induced apoptosis (18).
Detection of Protein Modifications
We interpret the observed changes in proteins that lead to differential fold changes are a direct reflection of gene expression, protein stability or changes in the post translational modification (PTM) status. Hence, we used an antibody array to characterize the phospho-proteome before and after radiation exposure in ATCL8 and AT5BIVA. The levels and pattern of protein phosphorylations in response to radiation were found to differ significantly in the two cell lines in an ATM dependent fashion. A basal level comparison of AT5BIVA and ATCL8 showed a significant elevation in phosphorylation of p38alpha T180/T182, JNK T183/Y185 and AKT S473 in ATCL8 (Fig. 5A). Phosphorylation of p38 regulates the base excision mediated DNA repair (19). Our findings are consistent with reports suggesting that ATM-dependent signaling through the RAF/MEK/ERK pathway is critical for efficient homologous recombination and for radiation-induced ATM activation, implicating a regulatory feedback loop between ERK and ATM (20). There was a significant increase in phosphorylation levels of GSK-3alpha/beta S21/29 in ATCL8, 1 h after irradiation compared to AT5BIVA (Fig. 5B). AKT is known to be phosphorylated by ATM, which in turn phosphorylates GSK3 leading to its inactivation (21). The AKT-insulin pathway has been implicated in mediating the DNA damage response (22). We also found ATM dependent phosphorylation of cAMP response element binding protein (CREB) at serine 133 in ATCL8, which is consistent with earlier reports (23). Using kinase prediction enrichment analysis, Bensimon et al. reported the alteration in GSK3beta phosphorylation status in human melanoma cell analyzed for ATM dependent and independent phosphorylation events (24). In addition, we found an increase in phosphorylation of HSP27 at serine residues 78 and 82 in cells which has been reported to be catalyzed by MAP kinase5 (MK5) (25) (Fig. 5B). Choi et al. reported a phospho-proteomics study which revealed alterations in the phosphorylation levels of several proteins in the presence of an ATM inhibitor including heat shock proteins (26). Previously, Kim et al. reported the identification of signaling molecules under low-dose ionizing radiation by using quantum dot nano-technology to determine changes in patterns of kinase expression levels in AT cells. In this study, we interpret these modifications to impact cellular processes like DNA damage and repair, cell cycle and proliferation.
FIG. 5.
Panel A: Antibody array analysis shows increased levels of phosphorylation in p38alpha T180/T182, JNK T183/Y185 and AKT S473 in ATCL8 cells. Nonirradiated AT5BIVA and ATCL8 protein extracts were incubated overnight with the human phospho-kinase array. The arrays were washed to remove unbound proteins, followed by incubation with a cocktail of biotinylated detection antibodies. Streptavidin-HRP was used for chemiluminescent detection. Normalized spot intensities were used to calculate fold changes in phosphorylation levels. Panel B: AT5BIVA and ATCL8 show differential phosphorylation patterns in response to radiation treatment. Protein extracts from the two cell lines, irradiated and controls, were processed as described above. The levels and patterns of phosphorylations in response to radiation were found to differ significantly in the two cell lines in an ATM dependent fashion.
DISCUSSION
Since ionizing radiation mediated cellular responses are complex, a pathway enrichment analysis offers a pragmatic approach towards furthering for enhancing our understanding of molecular events that accompany radiation injury and also those that are a result of single gene perturbations. A goal of global “omics” studies is to understand physiological processes as an integrated result of alterations in molecular networks. Analysis of high throughput data to yield meaningful biological information and its correlation with the physiological status of the system under study presents a challenge. The AT clinical syndrome results in a myriad of patho-physiological symptoms including extreme radiation sensitivity and predisposition to cancer. Our comprehensive proteomic approach includes the use of 2D gel proteomics and antibody arrays to determine changes in protein levels or PTM status in AT5BIVA and ATCL8 cell lines in a radiation response time course study.
ATM Dependent Differential Response
Our results underscore the differential responses to radiation arising from perturbations in a single gene. Ephrin signaling was found to be significantly enriched in ATCL8 cells at 30 min and 3 h after radiation exposure. Our results therefore show not only anticipated roles for Intersectin-1 and STAT3 which are known to be involved in cell survival and proliferation but a potential role for Ephrin B3 (EphB3) in these processes. The Ephrin signaling pathway has been reported to regulate multiple cellular development and signaling processes and our data now suggest Ephrin 3 plays a role in cell survival and cell growth (27, 28). Ephrins have also been reported to have critical function in neuronal development (27). Axonal guidance signaling was another predominant pathway in ATCL8 with twelve focus molecules including paxillin, Netrin, CDK5, EphB3, ITSN1 and PAK6 (Supplementary Fig. S7A and D; http://dx.doi.org/10.1667/RR3198.1.S1).
AT5BIVA showed an attenuated response to radiation exposure with 14-3-3 mediated signaling and protein ubiquitination pathways as being significantly perturbed. 14-3-3 mediated signaling has been implicated in radiation resistance in NSCLC cells and could partly explain the failure of AT5BIVA cells to elicit a stress response in the absence of ATM (29, 30). The focus molecules involved in the protein ubiquination pathway included proteasomes, USP7 and SUG-T1. Activated ATM phosphorylates and activates PPM1G, in response to DNA damage, which, in turn, dephosphorylates USP7S and promotes its degradation. Inactivation of USP7S results in Mdm2 self-ubiquitylation and degradation that leads to p53 upregulation (31). We also found an up-regulation of nucleolin in AT5BIVA, 30 min after radiation treatment, while the levels were unaltered in ATCL8. Nucleolin is an abundant factor critical for pre-RNA processing and has been reported to be antiapoptotic (32). It has also been shown to mediate the antiapoptotic response of HSP70 under oxidative stress conditions (33). The apparent up-regulation of nucleolin as an early response to radiation induced stress may be indicative of an aberrant response whereby cells continue to progress through the cell cycle. On the other hand, we detected phosphorylation of SMC1 and M4K4 in response to ionizing radiation in ATCL8. SMC1 phosphorylation has been reported to be ATM dependent in response to radiation exposure, consistent with our previous findings (34).
Network Modeling
Network modeling focused on proteins involved in cell cycle, signaling, transcription and cytoskeleton regulation. The participating proteins in each interacting network module differed significantly by the presence of a functional ATM. For example, AT5BIVA cells showed decreased expression of Vimentin 3 h after radiation exposure. Vimentin is a cytoskeletal component responsible for maintaining cell integrity (16). An increase in the levels of Ezrin was observed in AT5BIVA after radiation exposure, which has been implicated in cellular transformation in Rat-1 fibroblasts (17). It has been reported that cells with a functional ATM exhibit cell cycle check points in response to radiation treatment while AT cells continue to progress into the cell cycle and undergo defective cell divisions (35). Consistent with earlier reports, we observed a down regulation of proteins like NP1L4, CHD1 and EF1-G that mediate cell division and proliferation in ATCL8.
Delayed Response to Radiation in AT Cells
AT5BIVA was a slow responder to radiation exposure at 30 min. Significant differential protein expression was seen at 3 and 24 h, underscoring an attenuated stress response in the absence of ATM. Hence we performed time wise comparisons of differential protein expression patterns in AT5BIVA and ATCL8. We found little overlap in the proteome map of the differentially expressed proteins for the same time points for the two cell types (Supplementary Fig. S8A–D; http://dx.doi.org/10.1667/RR3198.1.S1). For example, at 1 h, Zinc finger protein 44, Nebulin, 14-3-3 protein epsilon and SP 110 nuclear protein were found to be differentially expressed in both the cell lines; however the patterns of regulation were opposite. Of the total number of proteins that were differentially expressed only 1, 4, 6 and 12 proteins were common for 30 min, 1, 3 and 24 h comparisons, respectively.
CONCLUSIONS
This study demonstrates the utility of using a genetically defined model cell system to elucidate global alterations in cellular networks resulting from perturbation in the ATM gene. The presence of the ATM resulted in a robust response to ionizing radiation injury in terms of time and number of signaling pathways that were activated. Our results show a distinctive radiation response in the two isogenic cell lines with respect to protein expression and post-translational modifications, emphasizing the value of this model system for “systems biology” studies. These changes are integrated with the metabolomics responses that we reported previously (36). We have also reported a cross-platform systems biology approach, which integrates common responses seen at RNA, protein and metabolite expression level (11). This approach provided a pathway based response where alteration of network modules is likely to result in perturbation of downstream signaling networks thereby causing altered radiation sensitivity, cellular morphology and cell mortality all of which are characteristics of AT cells. Interestingly, there was an unanticipated involvement of the Ephrin signaling pathway in mediating the radiation induced stress response in ATCL8 cells. Previously, microarray analysis showed an upregulation of EphB1 in ATCL8 cells prior to radiation exposure (11). This has translated into differential expression of multiple proteins involved in the Ephrin signaling pathway as determined by proteomics analysis in this study. Additionally, metabolomics (34) and proteomics studies performed using the same model system show pathway based correlations including purine and phospholipid metabolism, providing an orthogonal validation. The consistency between the findings from different “omics” data provides an in-silico validation for one of several hypothesis generating results from this study. Thus our results illustrate the complexity of the proteomic landscape underlying the overall response to radiation exposure. It is evident from our results that these networks affect a broad range of cellular processes, which may help explain the myriad of symptoms associated with the ataxia syndrome. The precise biological roles of these pathway perturbations in this model system are appropriate subjects for follow-up investigations including the investigation of the roles of axonal guidance signaling (Ephrins), Integrin signaling (Cadherin) and the role of 14-3-3 mediated signaling in nuclear translocation of histone deacetylases (37). Further investigations of the proteins and their modifications may provide novel insights into stress signaling and serve as a vehicle to define biomarkers of radiation exposure.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant PO1CA74175. The authors would like to thank Alfredo Velena and Jenny Tutera for technical assistance. The authors also wish to acknowledge the support of the Georgetown Lombardi Cancer Center Proteomics and Metabolomics Shared Resource NIH P30 CA5100.
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/RR3198.1) contains supplementary information that is available to all authorized users.
REFERENCES
- 1.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268(5218):1749–53. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 3.Savitsky K, Sfez S, Tagle DA, Ziv Y, Sartiel A, Collins FS, et al. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet. 1995;4(11):2025–32. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- 4.Pollard JM, Gatti RA. Clinical radiation sensitivity with DNA repair disorders: an overview. Int J Radiat Oncol Biol Phys. 2009;74(5):1323–31. doi: 10.1016/j.ijrobp.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair (Amst) 2004;3(8–9):1187–96. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Tichy A, Vavrova J, Pejchal J, Rezacova M. Ataxia-telangiectasia mutated kinase (ATM) as a central regulator of radiation-induced DNA damage response. Acta Medica (Hradec Kralove) 2010;53(1):13–7. doi: 10.14712/18059694.2016.57. [DOI] [PubMed] [Google Scholar]
- 7.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair (Amst) 2002;1(1):3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 8.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9(10):759. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 9.Concannon P, Gatti RA. Diversity of ATM gene mutations detected in patients with ataxia-telangiectasia. Hum Mutat. 1997;10(2):100–7. doi: 10.1002/(SICI)1098-1004(1997)10:2<100::AID-HUMU2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Jung M, Timofeeva O, Cheema AK, Varghese R, Ressom H, Dritschilo A. Human fibroblasts for large-scale “omics” investigations of ATM gene function. Adv Exp Med Biol. 2011;720:181–90. doi: 10.1007/978-1-4614-0254-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheema AK, Timofeeva O, Varghese R, Dimtchev A, Shiekh K, Shulaev V, et al. Integrated analysis of ATM mediated gene and protein expression impacting cellular metabolism. J Proteome Res. 2011;10(5):2651–7. doi: 10.1021/pr101243j. [DOI] [PubMed] [Google Scholar]
- 12.Jung M, Zhang Y, Lee S, Dritschilo A. Correction of radiation sensitivity in ataxia telangiectasia cells by a truncated I kappa B-alpha. Science. 1995;268(5217):1619–21. doi: 10.1126/science.7777860. [DOI] [PubMed] [Google Scholar]
- 13.Lavin MF, Kozlov S. DNA damage-induced signalling in ataxia-telangiectasia and related syndromes. Radiother Oncol. 2007;83(3):231–7. doi: 10.1016/j.radonc.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Giroux V, Iovanna J, Dagorn JC. Probing the human kinome for kinases involved in pancreatic cancer cell survival and gemcitabine resistance. FASEB J. 2006;20(12):1982–91. doi: 10.1096/fj.06-6239com. [DOI] [PubMed] [Google Scholar]
- 15.Cha MK, Kim IH. Preferential overexpression of glutaredoxin3 in human colon and lung carcinoma. Cancer Epidemiol. 2009;33(3–4):281–7. doi: 10.1016/j.canep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134(4):971–83. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb RF, Ozanne BW, Roy C, McGarry L, Stipp C, Mangeat P, et al. Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr Biol. 1997;7(9):682–8. doi: 10.1016/s0960-9822(06)00295-8. [DOI] [PubMed] [Google Scholar]
- 18.Khong T, Spencer A. Targeting HSP 90 induces apoptosis and inhibits critical survival and proliferation pathways in multiple myeloma. Mol Cancer Ther. 2011;10(10):1909–17. doi: 10.1158/1535-7163.MCT-11-0174. [DOI] [PubMed] [Google Scholar]
- 19.Tsai MS, Weng SH, Chen HJ, Chiu YF, Huang YC, Tseng SC, et al. Inhibition of p38 MAPK-dependent excision repair cross-complementing 1 expression decreases the DNA repair capacity to sensitize lung cancer cells to etoposide. Mol Cancer Ther. 2012;11(3):561–71. doi: 10.1158/1535-7163.MCT-11-0684. [DOI] [PubMed] [Google Scholar]
- 20.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67(3):1046–53. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 21.Gong R, Rifai A, Dworkin LD. Activation of PI3K-Akt-GSK3beta pathway mediates hepatocyte growth factor inhibition of RANTES expression in renal tubular epithelial cells. Biochem Biophys Res Commun. 2005;330(1):27–33. doi: 10.1016/j.bbrc.2005.02.122. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316(5828):1160–6. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes ND, Sun Y, Price BD. Activation of the kinase activity of ATM by retinoic acid is required for CREB-dependent differentiation of neuroblastoma cells. J Biol Chem. 2007;282(22):16577–84. doi: 10.1074/jbc.M609628200. [DOI] [PubMed] [Google Scholar]
- 24.Bensimon A, Schmidt A, Ziv Y, Elkon R, Wang SY, Chen DJ, et al. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci Signal. 2010;3(151):rs3. doi: 10.1126/scisignal.2001034. [DOI] [PubMed] [Google Scholar]
- 25.Kostenko S, Johannessen M, Moens U. PKA-induced F-actin rearrangement requires phosphorylation of Hsp27 by the MAP-KAP kinase MK5. Cell Signal. 2009;21(5):712–8. doi: 10.1016/j.cellsig.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Choi S, Srivas R, Fu KY, Hood BL, Dost B, Gibson GA, et al. Quantitative proteomics reveal ATM kinase-dependent exchange in DNA damage response complexes. J Proteome Res. 2012;11(10):4983–91. doi: 10.1021/pr3005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soskis M, Salogiannis J, Greenberg M. EphrinBs send mixed messages. Nat Neurosci. 2011;14(11):1356–8. doi: 10.1038/nn.2968. [DOI] [PubMed] [Google Scholar]
- 28.Ricard J, Salinas J, Garcia L, Liebl DJ. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31(4):713–22. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim W, Youn H, Seong KM, Yang HJ, Yun YJ, Kwon T, et al. PIM1-activated PRAS40 regulates radioresistance in non-small cell lung cancer cells through interplay with FOXO3a, 14-3-3 and protein phosphatases. Radiat Res. 2011;176(5):539–52. doi: 10.1667/rr2609.1. [DOI] [PubMed] [Google Scholar]
- 30.Rajendran P, Delage B, Dashwood WM, Yu TW, Wuth B, Williams DE, et al. Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: competing actions of 14-3-3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly. Mol Cancer. 2011;10:68. doi: 10.1186/1476-4598-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoronenkova SV, Dianov GL. Regulation of USP7/HAUSP in response to DNA damage: Yet another role for ATM. Cell Cycle. 2012;11(13):2409–10. doi: 10.4161/cc.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Wang H, Jiang B, Liang P, Liu M, Deng G, et al. Nucleolin/C23 is a negative regulator of hydrogen peroxide-induced apoptosis in HUVECs. Cell Stress Chaperones. 2010;15(3):249–57. doi: 10.1007/s12192-009-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang B, Zhang B, Liang P, Song J, Deng H, Tu Z, et al. Nucleolin/C23 mediates the antiapoptotic effect of heat shock protein 70 during oxidative stress. FEBS J. 2010;277(3):642–52. doi: 10.1111/j.1742-4658.2009.07510.x. [DOI] [PubMed] [Google Scholar]
- 34.Kitagawa R, Kastan MB. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb Symp Quant Biol. 2005;70:99–109. doi: 10.1101/sqb.2005.70.002. [DOI] [PubMed] [Google Scholar]
- 35.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15(17):2177–96. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 36.Varghese RS, Cheema A, Cheema P, Bourbeau M, Tuli L, Zhou B, et al. Analysis of LC-MS data for characterizing the metabolic changes in response to radiation. J Proteome Res. 2010;9(5):2786–93. doi: 10.1021/pr100185b. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Chen J, Ricupero CL, Hart RP, Schwartz MS, Kusnecov A, et al. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nat Med. 2012;18(5):783–90. doi: 10.1038/nm.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.