Abstract
Background
The mechanisms of general anesthesia by volatile drugs remain largely unknown. Mitochondrial dysfunction and reduction in energy levels have been suggested to be associated with general anesthesia status. 2-Deoxy-d-glucose (2-DG), an analog of glucose, inhibits hexokinase and reduces cellular levels of adenosine triphosphate (ATP). 3-Nitropropionic acid is another compound which can deplete ATP levels. In contrast, idebenone and L-carnitine could rescue deficits of energy. We therefore sought to determine whether 2-DG and/or 3-nitropropionic acid can enhance the anesthetic effects of isoflurane, and whether idebenone and L-carnitine can reverse the actions of 2-DG.
Methods
C57BL/6J mice (8 months old) received different concentrations of isoflurane with and without the treatments of 2-DG, 3-nitropropionic acid, idebenone, and L-carnitine. Isoflurane-induced loss of righting reflex (LORR) was determined in the mice. ATP levels in H4 human neuroglioma cells were assessed after these treatments. Finally, 31P-magnetic resonance spectroscopy was used to determine the effects of isoflurane on brain ATP levels in the mice.
Results
2-DG enhanced isoflurane-induced LORR (P = 0.002, N = 15). 3-nitropropionic acid also enhanced the anesthetic effects of isoflurane (P = 0.005, N = 15). Idebenone (Idebenone + saline versus Idebenone + 2-DG: P = 0.165, N = 15), but not L-cartinine (L-carnitine + saline versus L-carnitine + 2-DG: P < 0.0001, N = 15), inhibited the effects of 2-DG on enhancing isoflurane-induced LORR in mice, as evidenced by 2-DG not enhancing isoflurane-induced LORR in mice pretreated with idebenone. Idebenone (Idebenone + saline versus Idebenone + 2-DG: P = 0.177, N = 6), but not L-cartinine (L-carnitine + saline versus L-carnitine + 2-DG: P = 0.029, N = 6), also mitigated the effects of 2-DG on reducing ATP levels in cells, as evidenced by 2-DG not decreasing ATP levels in the cell pretreated with idebenone. Finally, isoflurane decreased ATP levels in both cultured cells and mouse brains (-ATP: P = 0.003, N = 10; -ATP/phosphocreatine: P = 0.006, N = 10; -ATP/inorganic phosphate: P = 0.001, N = 10).
Conclusions
These results from our pilot studies have established a system and generated a hypothesis that 2-DG enhances anesthetic effects via reducing energy levels. These findings should promote further studies to investigate anesthesia mechanisms.
Introduction
Volatile anesthetics are widely used clinically. However, their mechanisms of action remain uncertain1–3,4,5. Several studies have suggested that mitochondrial function and energy levels may regulate sensitivity to anesthetics.6–9 Mutations in complex I of mitochondria increase sensitivity to volatile anesthetics in Caenorhabditis elegans (C. elegans).6 Similarly, knockout of a major complex I protein, Ndufs4, sensitizes mice to volatile anesthetics.10 It has been reported that rates of oxidative phosphorylation and changes in adenosine triphosphate (ATP) concentrations in the C. elegans gas-1 and other mutants may also contribute to the altered sensitivity to volatile anesthetics in C. elegans.7,9 At the same time, inhaled anesthetics have been reported to affect mitochondrial function.11–15 Halothane, isoflurane, and sevoflurane can inhibit complex I (NADH:ubiquinone oxidoreductase) of the electron transport chain of mitochondria in myocytes of guinea pigs.12 Isoflurane has also been reported to induce opening of the mitochondrial permeability transition pore, increase levels of reactive oxygen species, and reduce levels of mitochondrial membrane potential in vitro.14,15 However, it still remains largely unknown whether changes in energy levels could affect sensitivity to general anesthetics.
2-Deoxy-d-glucose (2-DG), an analog of glucose, is a competitive inhibitor of hexokinase.16 2-DG is phosphorylated by hexokinase to 2-DG-P, which inhibits glycolysis mainly at the step of phosphorylation of glucose by hexokinase, leading to a depletion of cellular ATP.17 We have previously shown that 2-DG affects isoflurane-induced cellular toxicity in vitro.18,19 3-Nitropropionic acid (3-NP) inhibits the Krebs cycle and electron transport chain enzyme succinate dehydrogenase, also leading to ATP depletion.20 In the current study, we tested a hypothesis that 2-DG enhances the anesthetic effects of isoflurane via reduction in energy levels.
3-NP inhibits the Krebs cycle and electron transport chain enzyme succinate dehydrogenase, leading to ATP depletion.20 We therefore assessed whether 3-NP could also enhance the anesthetic effects of isoflurane.
Coenzyme Q plays an important role as a mobile electron carrier in the respiratory chain function, and enhances cellular energy.21 Idebenone is a synthetic analog of coenzyme Q,22 and has been reported to mitigate the reduction in ATP levels associated with mitochondrial dysfunction.21–25 Mitochondrial -oxidation is involved in energy production, and L-carnitine contributes to -oxidation of quarried fatty acids from the mitochondrial membrane.26 We therefore also assessed whether idebenone and L-carnitine could regulate the effects of 2-DG on the action ofisoflurane anesthesia.
Materials and Methods
The study protocol was approved by the Standing Committee on Animals at Massachusetts General Hospital, Boston, Massachusetts. Wild-type C57BL/6J mice (8 months old, The Jackson Laboratory, Bar Harbor, ME) were randomly assigned to the anesthesia or control group, and then were further divided into saline and treatment (e.g., 2-DG) groups. Mice were housed in a controlled environment (20 – 22°C; 12 hour light: dark on a reversed light cycle) for 1 week before the studies. The maintenance and handling of mice were consistent with the guidelines of the National Institute of Health, and all efforts were made to minimize the number of animals studied. Power analyses used to establish experimental group sizes are described below in Statistics.
Mice inhaled different concentrations of isoflurane (0.6% to 1.4%) in 100% oxygen, with increments of 0.1% introduced every 15 minutes. The anesthetizing chamber temperature was controlled (DC Temperature Control System; FHC, Bowdoinham, Maine) in order to maintain rectal temperature at 37±0.5°C during anesthesia. Mice were placed in an empty home cage after anesthesia for 60-second measurement of loss of righting reflex (LORR), where there was no heating system. The body temperature of the mice slightly decreased (less than 2 °C) during the 60-second measurement. A mouse-tail blood pressure cuff (Kent Scientific Cooperation, Torrington, CT) was used to measure blood pressure, and blood gases were assayed with a blood gas machine (Trupoint, ITC, Edison, NJ) as described in our previous studies.27,28
2-DG (1000 mg/kg),29 3-NP (50 mg/kg),30 or saline was injected [intraperitoneally (ip)] 5 minutes before isoflurane anesthesia. Idebenone (200 mg/kg)31 or L-carnitine (100 mg/kg)32 was injected 15 minutes before isoflurane anesthesia (10 minutes before treatment with 2-DG or 3-NP).
Mice were put into the empty home cage, and the cage was rotated 180 degrees to place the mouse on its back. LORR was scored positive if the mouse remained on its back with at least 3 paws in the air for 60 seconds.
We used H4 human neuroglioma cells in the experiments. The cells were cultured in Dulbecco's Modified Eagle Media containing 9% heat-inactivated fetal calf serum, 100 units/ml penicillin, 100 g/ml streptomycin, and 2 mM L-glutamine. The cells were treated with 2% isoflurane for different time periods (30, 60, 90, and 180 minutes) as described in our previous studies.33 For the interaction studies, the cells were treated with 2-DG (25 mM),34 L-carnitine (50 nM),35 or idebenone (25 nM),36 15 minutes before treatment with isoflurane.
We used a bioluminescence-based ATP Determination Kit (Invitrogen, Carlsbad, CA) in the experiments to detect ATP levels. Briefly, H4 human neuroglioma cells were placed in a 6-well plate overnight in an incubator. The cells were then exposed to treatments with isoflurane, 2-DG, L-carnitine, or idebenone, for 3 hours. Treatment with 2% isoflurane for 3 hours did not induce apoptosis.37 At the end of treatment, the amount of fluorescence was measured and the levels of ATP in the experimental samples were calculated from a standard curve made from known amounts of ATP.
31P-Magnetic resonance spectroscopy (31P-MRS) was used to measure brain ATP levels as described in previous studies38–40 with modifications. Briefly, the mice under the control condition or isoflurane anesthesia were gently restrained to a plastic warm water bag (37 C°) in prone position, using paper tape. Each mouse was then placed in the MRS machine to measure brain ATP levels. The measurement took about 5 minutes. Isoflurane anesthesia was maintained by putting a paper cone breathing device to the mouths of the mice to continuously provide isoflurane during the MRS studies. 31P-MRS was used to measure the peak areas of the following metabolites: phosphomonoesters (PME), inorganic phosphate (Pi), phosphodiesters (PDE), phosphocreatine (Pcr), and ATP (3 peaks: α-, β- and γ-ATP). In the current studies, only β-ATP levels were determined because the signals of α-ATP and γ-ATP could have overlapped with other compounds.
Statistics
Data are expressed as mean + standard deviation (SD). The number of samples varied from 10 to 15 in each group for the experiments, and 6 in each group for the in vitro studies.
We measured LORR after isoflurane anesthesia in mice (Figure 1 and Figure 2) and assessed the ATP levels after isoflurane treatment in cultured cells in our preliminary studies. The power calculation was performed using information we collected from a preliminary study that was conducted under the same conditions. Based on the preliminary data, assuming a two-sided Student-t test, a sample of 6 and 10 for each control and treatment group for ATP measurement and LORR studies, respectively, would lead to 90% power and 95% significance.
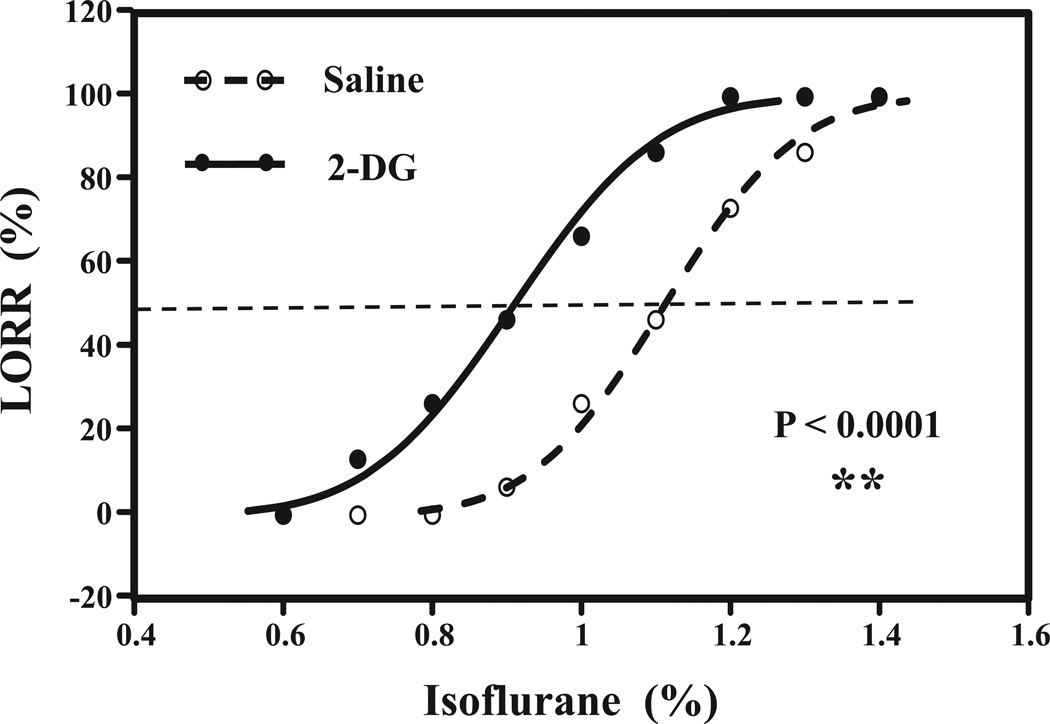
Figure 1. 2-DG enhances isoflurane-induced loss of righting reflex (LORR) in mice.
Concentration effect relationship curve for the interaction of 2-DG or saline with isoflurane. Open circle indicates the mice after the treatment with saline plus isoflurane, and closed circle indicates mice received treatment with 2-DG plus isoflurane. 2-DG significantly shifts the isoflurane-induced LORR curve to the left as compared to saline (P =0.002). N = 15 per group. 2-DG, 2-Deoxyglucose.
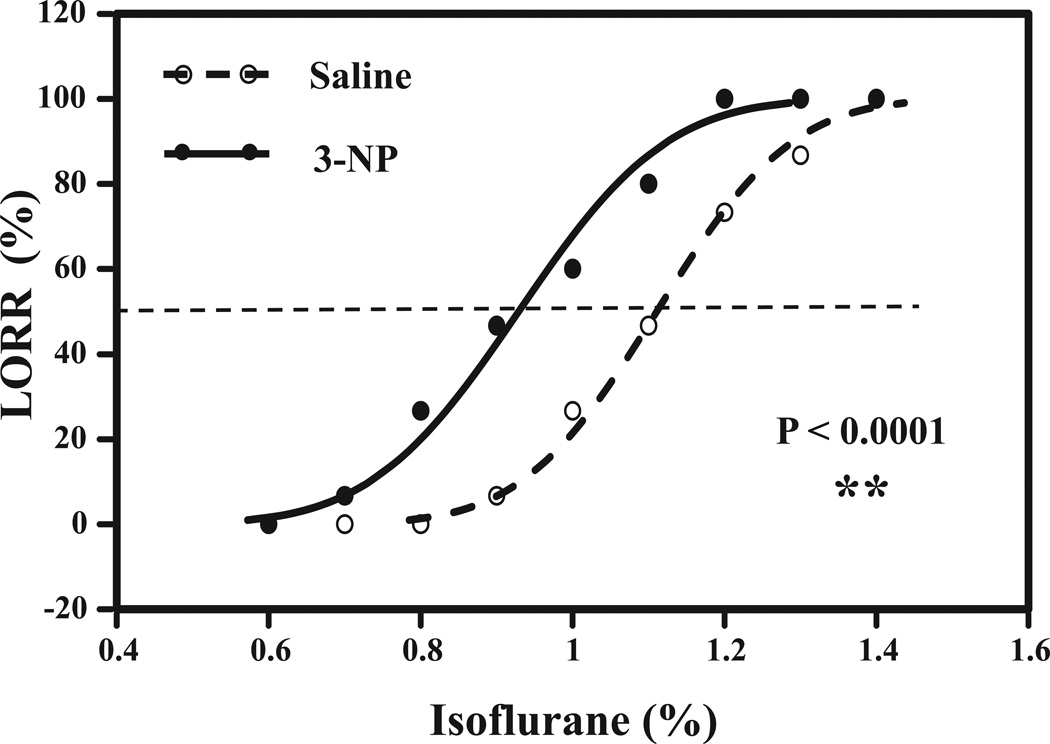
Figure 2. 3-NP enhances isoflurane-induced LORR in mice.
Concentration effect relationship curve for the interaction of 3-NP or saline with isoflurane. Open circle indicates the mice after treatment with saline plus isoflurane, and closed circle indicates mice received treatment with 3-NP plus isoflurane. 3-NP significantly shifts the isoflurane-induced LORR curve to left as compared to saline (P = 0.005). N = 15 per group. 2-DG, 2-Deoxyglucose.
Two-way ANOVA was used to assess the interaction of 2-DG or 3-NP with idebenone or L-cartinine to test a hypothesis that idebenone and L-cantinine would mitigate the effects of 2-DG on enhancing isoflurane-induced LORR in the mice. Post hoc analyses were conducted should the main effects have been found to be statistically significant. The Wald test was used when examining the hypotheses of whether 2-DG or 3-NP was able to decrease the EC50 of isoflurane on inducing LORR in mice. The cut-off p-value was Bonferroni adjusted to correct for sub-set analysis, e.g., comparing EC50 of isoflurane between the treatment of idebenone plus saline and the treatment of Idebenone plus 2-DG (Figure 3).
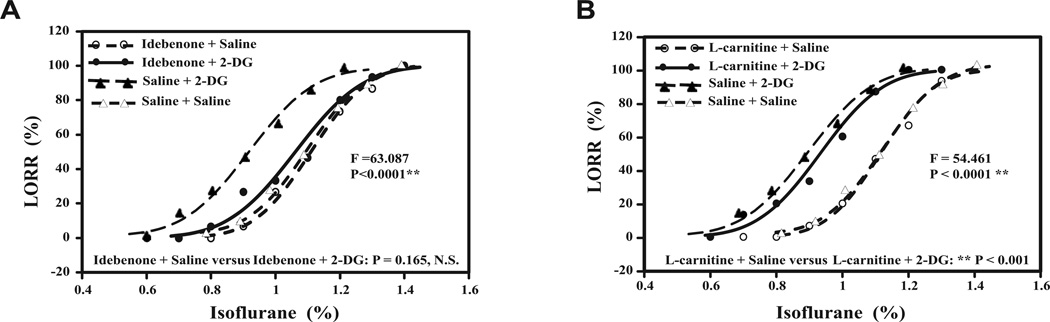
Figure 3. Idebenone, but not L-carnitine, inhibits the effects of 2-DG on the isoflurane-induced LORR.
Concentration effect relationship curve of isoflurane in the mice with treatment of idebenone or L-carnitine plus 2-DG or saline. Open circle indicates the mice after treatment with idebenone or L-carnitine plus isoflurane and saline, closed circle indicates mice received treatment with idebenone or L-carnitine plus isoflurane and 2-DG; closed triangle indicates mice received treatment with saline and 2-DG, and open triangle indicates mice received treatment with saline plus saline. The hill slope of each curve is demonstrated after the legend of each of the treatments. Two-way ANOVA with post-hoc test shows that: A. In mice pretreated with idebenone, the 2-DG treatment does not shift the curve to left as compared to saline treatment: P = 0.165. B. In mice pretreated with L-carnitine, 2-DG treatment still shifts the curve to left as compared to saline treatment: ** P < 0.001. N = 15 per group. ANOVA, Analysis of variance; 2-DG, 2-Deoxyglucose.
Student t-test was used to assess the difference in ATP levels in H4 human neuroglioma cells and in the brains of mice between isoflurane treatment and the control condition.
Student t-test was also used to assess the difference in ATP levels between the 2 groups: one group was treated with idebenone plus saline and the other group was treated with idebenone plus 2-DG. Similarly, Student t-test was used to assess the difference in ATP levels between treatment with L-carnitine plus saline and treatment with L-carnitine plus 2-DG. The hypothesis that L-carnitine or idebenone was able to mitigate 2-DG-induced reduction in ATP levels in cultured cells was tested.
The hypothesis testing was two-tailed. P values less than 0.05 were considered statistically significant. For the data presented in Figure 4, we performed the Normality test to determine whether the N = 12 residuals in the studies were normally distributed with unknown parameters.
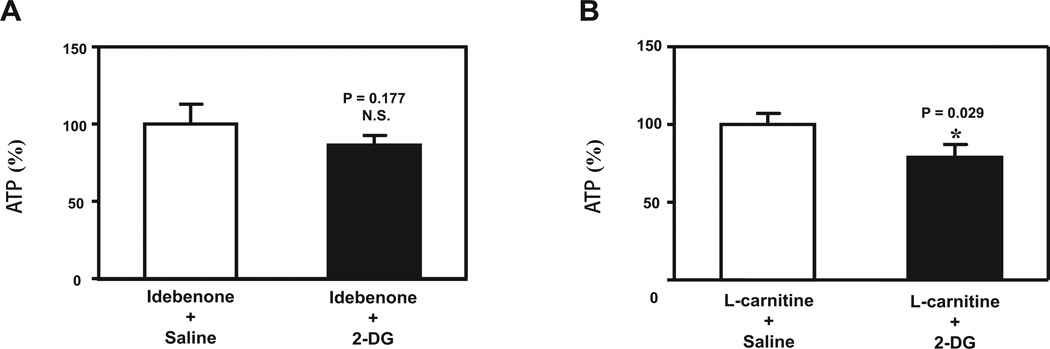
Figure 4. Idebenone, but not L-carnitine, inhibits the effects of 2-DG on ATP levels in H4 human neuroglioma cells.
A. 2-DG does not decrease ATP levels in cells pretreated with idebenone. The N=12 residuals are normally distributed (P = 0.948). B. 2-DG decreases ATP levels in cells pretreated with L-carnitine. The N=12 residuals are normally distributed (P = 0.991). N = 6 per group. 2-DG, 2-Deoxyglucose
SAS software (Cary, NC) and Prism 6 software (La Jolla, CA) were used to analyze the data.
Results
2-DG enhanced isoflurane-induced LORR in mice
Mice receiving 1.4% isoflurane anesthesia did not show significant changes in blood pressure and blood gas as compared to mice in the control group (Table 1). The body temperature of the mice was maintained at 37 ± 0.5°C during anesthesia. The body temperature of mice only slightly decreased (less than 2 °C) during the 60-second measurement of LORR in an animal test cage, which did not have a heating system.
Table 1.
Blood pressure and blood gas in mice under isoflurane anesthesia
| Control* | 1.4% Isoflurane (N = 15) |
|
|---|---|---|
| MAP | 107.7 ± 2.5 mmHg | 94 ± 11 mmHg |
| pH | 7.31 ± 0.04 | 7.40 ± 0.2 |
| PvO2 | 226 ± 55 mmHg | 210 ± 27.1 mmHg |
| PvCO2 | 42.2 ± 4.1 mmHg | 47.7 ± 12.6 mmHg |
Baseline blood pressure in C57 mice with 21% O2 (Campen et al., 2005) and baseline blood gas in C57 mice with 100% O2.
Blood pressure and blood gas in C57 mice during the 1.4% isoflurane anesthesia in 100% O2.
MAP, mean arterial blood pressure; PvO2, partial pressure of oxygen in venous blood; PvCO2, partial pressure of carbon dioxide in venous blood.
The Mice were pretreated with saline or 2-DG (1000 mg/kg). The mice were then exposed to different concentrations of isoflurane (0.6% to 1.4%). The relationship curve between the percentage of mice with LORR and the concentrations of isoflurane was determined in the mice pretreated with saline or 2-DG. 2-DG treatment shifted the curve to the left. The Wald test showed that there was a significant difference between 2 EC50s (P = 0.002) (Figure 1). These data suggested that 2-DG was able to enhance the anesthetic effects of isoflurane by enhancing isoflurane-induced LORR.
3-NP enhanced isoflurane-induced LORR in mice
Both 2-DG17 and 3-NP20 have been reported to decrease ATP levels. Therefore, we asked whether 3-NP was also able to affect the anesthetic effects of isoflurane. Treatment with 3-NP (50 mg/kg) shifted the relationship curve between the percentage of mice with LORR and the concentration of isoflurane to the left. The Wald test showed that the difference between 2 EC50s was significantly different (P = 0.005) (Figure 2). These data suggested that 3-NP was also able to enhance the anesthetic effects of isoflurane by enhancing isoflurane-induced LORR in mice. Collectively, these findings suggested that a reduction of energy levels could enhance the anesthetic effects in mice.
Idebenone inhibited the effects of 2-DG on isoflurane-induced LORR in mice
Idebenone is an analogue of coenzyme Q10 and has been reported to rescue ATP levels in mitochondrial dysfunction.21–25 Next, we assessed whether idebenone was able to inhibit the effects of 2-DG on the anesthetic effects. The relationship curve between the percentage of mice with LORR and the concentrations of isoflurane was determined in the mice pretreated with idebenone plus saline or idebenone plus 2-DG. We were able to show that idebenone inhibited the enhancing effects of 2-DG on isoflurane-induced LORR (Figure 3A). Two-way ANOVA showed a significant interaction among the treatments with idebenone, 2-DG, and saline on isoflurane-induced LORR in mice (F = 63.087, P < 0.0001). However, a post hoc test suggested that there was no significant difference between idebenone plus saline and idebenone plus 2-DG on isoflurane-induced LORR in mice (P = 0.165). There was a significant difference between saline plus 2-DG and saline plus saline (P < 0.0001), idebenone plus saline (P < 0.0001) or idebenone plus 2-DG (P = 0.0003) on isoflurane-induced LORR.
Two-way ANOVA showed significant interaction among the treatments with L-carnitine, 2-DG, and saline on isoflurane-induced LORR in the mice (F = 54.461, P < 0.0001). A post hoc test suggested that there was a significant difference between L-carnitine plus saline and L-carnitine plus 2-DG on isoflurane-induced LORR (P = 0.0002). These data suggested that L-carnitine, which contributes to β-oxidation of quarried fatty acid from the mitochondrial membrane,26 was not able to inhibit the effects of 2-DG on isoflurane anesthesia because 2-DG was still able to shift the relationship curve to the left compared to saline in the mice pretreated with L-carnitine (Figure 3B). Collectively, these data suggested that idebenone, but not L-carnitine, was able to inhibit the enhancing effects of 2-DG on isoflurane-induced LORR.
Idebenone, but not L-carnitine, preserved ATP levels in H4 human neuroglioma cells exposed to 2-DG
Given the findings that idebenone, but not L-carnitine, was able to attenuate the 2-DG effects on enhancing isoflurane-induced anesthesia, we next asked whether idebenone and L-carnitine might differently affect the effects of 2-DG on ATP levels in cultured cells. We found that 2-DG did not decrease ATP levels in the cells pretreated with idebenone (Figure 4A), but 2-DG still decreased ATP levels in the cells pretreated with L-carnitine (Figure 4B). Thus, similar interactions between 2-DG and idebenone versus L-carnitine were observed in both LORR studies and cellular ATP assays. Taken together, these findings suggested that 2-DG might enhance isoflurane-induced anesthetic effects via reduction in energy (e.g., ATP) levels.
Isoflurane decreases ATP levels in H4 human neuroglioma cells and in mouse brains
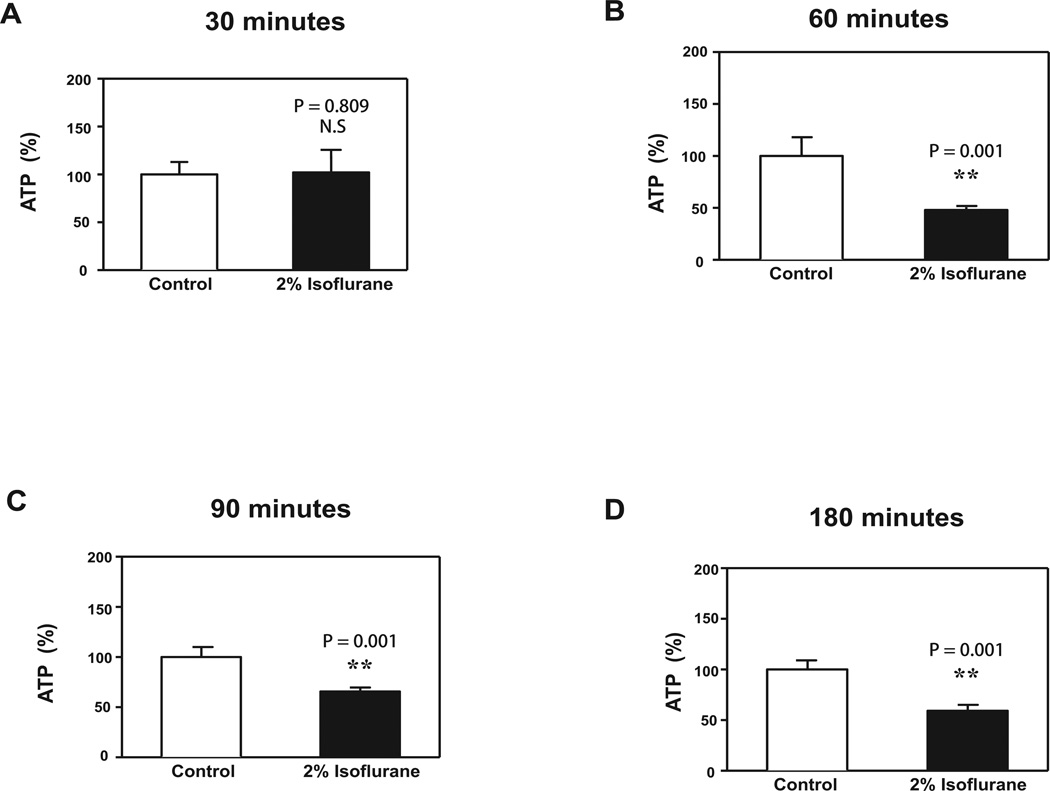
Given the findings that 2-DG might enhance isoflurane-induced anesthetic effects via reduction in energy (e.g., ATP) levels, we then determined the effects of isoflurane on ATP levels in vitro and in vivo. Treatment with 2% isoflurane for 60 minutes (Figure 5B, P = 0.001), 90 minutes (Figure 5C, P = 0.001), and 180 minutes (Figure 5D, P = 0.001), but not 30 minutes (Figure 5A, P = 0.809), significantly decreased ATP levels in H4 human neuroglioma cells.
Figure 5. Isoflurane decreases ATP levels in H4 human neuroglioma cells.
A. Treatment with 2% isoflurane for 30 minutes does not reduce ATP levels as compared to control conditions. B. Treatment with 2% isoflurane for 60 minutes reduces ATP levels as compared to control conditions. C. Treatment with 2% isoflurane for 90 minutes reduces ATP levels as compared to control conditions. D. Treatment with 2% isoflurane for 180 minutes reduces ATP levels as compared to control condition. N = 6 per group.
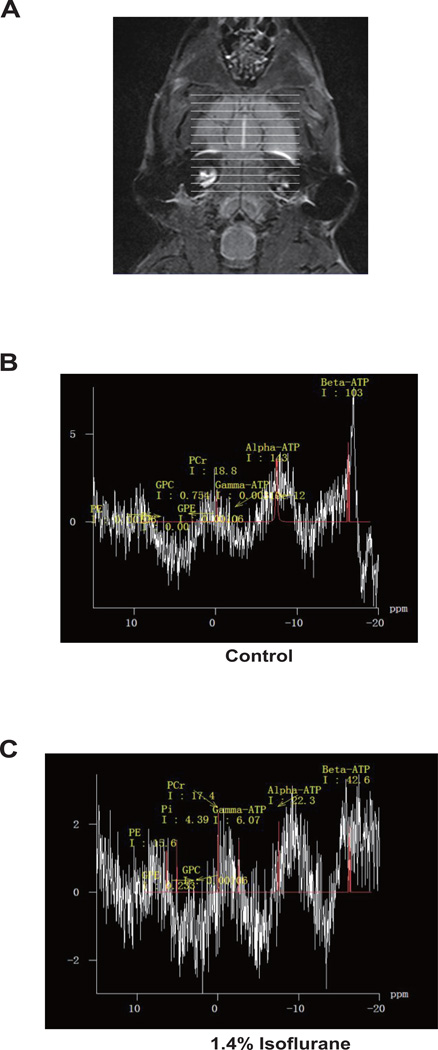
In the in vivo studies, we measured the effects of isoflurane on brain ATP levels.31 P-MRS was used to assess ATP levels in brains (Figure 6A) of both control mice (Figure 6B) and mice anesthetized with 1.4% isoflurane (Figure 6C). The brain ATP levels were then determined by the area under the31 P-MRS curves (Figure 6). These studies revealed a significant difference in the levels of β-ATP (P = 0.003), β-ATP/Pcr ratio (P = 0.006), and β-ATP/Pi ratio (P = 0.0001) between control and anesthetized mice (Table 2, Student’s t-test). These data suggested that isoflurane could reduce ATP levels in brain tissues of mice.
Figure 6. Isoflurane decreases brain ATP levels in mice.
31P magnetization transfer spectroscopy (A) was used to detect brain ATP levels in mice in control conditions (B) and 1.4% isoflurane (C). ATP levels are represented by the area under the curve. N = 10 per group.
Table 2.
Isoflurane decreases brain ATP levels in mice
| Control (N = 10) |
Isoflurane (N = 10) |
P values | |
|---|---|---|---|
| Body weight | 24.2 ± 2.90 | 24.4 ± 2.57 | 0.822 |
| Pcr | 17.3 ± 3.72 | 19.6 ± 4.13 | 0.211 |
| Pi | 1.3 + 0.21 | 1.4 ± 0.18 | 0.05 |
| β-ATP | 112.8 ± 23.52 | 23.1 ± 4.37 | P=0.003 |
| β-ATP/Pcr | 5.5 ± 1.32 | 1.3 ± 0.45 | P=0.006 |
| β-ATP/Pi | 68.7 ± 13.6 | 26.4 ± 4.7 | P=0.001 |
Body weight is not significantly different between the mice in control conditions and mice under isoflurane anesthesia. There is a significant difference in the levels of β-ATP, β-ATP/Pcr and β-ATP/Pi between mice in control conditions and mice under isoflurane anesthesia. Isoflurane anesthesia decreases brain ATP levels as compared to control conditions in mice. ATP, adenosine triphosphate; Pcr, phosphocreatine; Pi, inorganic phosphate.
Discussion
In this proof of concept study, we assessed whether inhibition of energy (e.g., ATP) levels could enhance isoflurane-induced LORR in mice. We found that both 2-DG and 3-NP made mice more sensitive to isoflurane-induced LORR (Figures 1 and 2). 2-DG is a competitive inhibitor of hexokinase16 and decreases intracellular ATP levels.17 3-NP is an inhibitor of succinate dehydrogenase in the Krebs cycle and electron transport chain, and also depletes ATP.20 Therefore, our data showed that acute reduction of energy levels might sensitize mice to isoflurane-induced LORR. These results are in agreement with evidence from animal models and patients with chronic mitochondrial complex I dysfunction.6–9,11–13
In addition to inhibiting hexokinase and decreasing ATP levels, 2-DG produces many other effects. These include decreasing reactive oxygen species generation, increasing cytosolic calcium,41,42 cytotoxicity,41 and endoplasmic reticulum stress.43,44 Therefore, 2-DG could enhance isoflurane-induced LORR via 1 or more of these other cellular and molecular mechanisms in addition to reducing energy (e.g., ATP) levels.
Idebenone, an analogue of coenzyme Q10, that can rescue depletion of ATP levels associated with mitochondrial dysfunction,21–25 reversed the effects of 2-DG on isoflurane-induced LORR (Figure 3A). Interestingly, L-carnitine, a compound which contributes to β-oxidation of quarried fatty acid from mitochondrial membrane,26 did not reverse the effects of 2-DG on isoflurane-induced LORR (Figure 3B). Consistent with the LORR results, idebenone, but not L-carnitine, inhibited 2-DG-induced reduction in ATP levels in cultured cells (Figure 4A and 4B). Both L-carnitine and idebenone have been shown to sustain mitochondrial function. Why idebenone but not L-carnitine attenuated the effects of 2-DG in enhancing isoflurane-induced LORR remains uncertain. We speculate that idebenone better restores the glycolitic pathway that is inhibited by 2-DG than L-carnitine. Idebenone and L-carnitine may also have different effects on regulating ATP or other metabolite levels.45,46 Future studies to test these hypotheses are needed.
We also found that isoflurane reduced ATP levels in cultured neural-derived cells (Figure 5) and in the brains of mice (Figure 6 and Table 2). These results suggested that reducing brain energy (ATP) levels might sensitize the effects of isoflurane anesthesia in the mice. However, neither idebenone nor L-carnitine affected isoflurane-induced LORR in the absence of 2-DG. These findings implied that mitochondria might not be directly involved in isoflurane-induced LORR. Further examination of the cellular and brain metabolic effects of isoflurane combined with idebenone or L-carnitine are needed to address whether mitochondrial metabolism contributes to isoflurane anesthesia.
It is likely that volatile anesthetics act via a number of other molecular targets. Many studies show that these anesthetics both inhibit excitatory synaptic transmission and enhance inhibitory signaling.47,48,4 In particular, γ-aminobutyric acid type A and N-methyl-D-aspartate receptors are potential targets [49,4]. Based on transgenic animal studies, potassium channels50 and subunits of mitochondrial complex I10 have also been suggested as volatile anesthetic targets. However, the precise role of mitochondrial defects in anesthesia is unclear. In a study of C. elegans7, Kayser et al. reported that 4.9% halothane (EC50) only modestly decreased ATP levels in wild-type animals, while in anesthetic-sensitive gas-1(fc21) mutant worms, 1.9% halothane produced a much larger decrease in ATP levels.7 Compared with wild-type C. elegans in halothane, we found significantly larger effects of isoflurane on ATP levels in neuronal-derived cells and brain tissue. This might reflect the different anesthetic used or that our measurements were made in neural cells and tissue, rather than whole worms.
Our study has several limitations. First, we did not measure whether 2-DG altered the concentration of isoflurane in mouse brains in the current studies. It remains possible that 2-DG could enhance isoflurane-induced LORR via a pharmacokinetic mechanism rather than a pharmacodynamic mechanism. We therefore did not conclude that 2-DG enhanced the anesthetic effects of isoflurane exclusively through reduction in brain ATP levels. Second, we did not assess the dose-dependent effects of 2-DG, 3-NP, idebenone, or L-carnitine on isoflurane-induced LORR. We chose fixed doses previously reported in the literature for these exploratory studies, and plan to examine dose-dependent effects in future studies. Third, by reducing metabolism, 2-DG treatment can decrease body temperature in rodents,51 enhancing sensitivity to anesthetics. However, we maintained animal temperatures near normal in LORR experiments. The slight hypothermia (less than 2 °C) could still occur during the 60-second measurement of LORR. Nevertheless, future studies need to investigate whether 2-DG can enhance anesthesia via 2-DG-induced hypothermia or via 2-DG-induced reduction in energy (e.g., ATP) levels.
In conclusion, we found that 2-DG and 3-NP enhanced isoflurane-induced LORR in mice. Idebenone, but not L-carnitine, inhibited the effects of 2-DG on enhancing isoflurane-induced LORR and on reducing ATP levels. Neither idebenone nor L-carnitine alone altered isoflurane-induced LORR. Finally, isoflurane significantly reduced ATP levels in cultured neural-derived cells and in brains of mice. These results suggested that acute metabolic inhibition of energy (e.g., ATP) levels might sensitize animals to general anesthetics. While our current results do not directly address the role of mitochondrial inhibition as a mechanism of isoflurane anesthesia, more studies on the metabolic effects of anesthetics combined with mitochondrial inhibiting and enhancing drugs could address this important hypothesis. These findings will hopefully promote more studies investigating anesthetic mechanisms.
Acknowledgements
The authors would like to thank Dr. Hui Zheng, the Assistant Professor in Medicine and statistician in Massachusetts General Hospital and Harvard Medical School, for the advice regarding statistic analysis of the data in the studies. The authors would like to thank Dr. Stuart Forman, the Associate Professor of Anesthesia in Massachusetts General Hospital and Harvard Medical School, for the scientific discussion and constructive comments for the manuscript. The costs of isoflurane were generously provided by the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital and Harvard Medical School. This manuscript was attributed to the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital and Harvard Medical School.
This manuscript was handled by: Markus W. Hollmann, MD, PhD, DEAA
Funding: This research was supported by R21AG038994, R01 GM088801 and R01 AG041274 from National Institutes of Health, Bethesda, Maryland, Investigator-initiated Research grant from Alzheimer’s Association, Chicago, Illinois, and Cure Alzheimer’s Fund, Wellesley, Massachusetts (to Z. X.), and China National Science Foundation Overseas Young Scholars Collaboration Research Award NSF30928026 (to Y.Y. and Z.X.).
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES
Name: Hui Wang, MD, PhD
Contribution: This author helped conduct the study and analyze the data
Attestation: Hui Wang has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Zhipeng Xu, MD, PhD
Contribution: This author helped design the study, conduct the study, and analyze the data
Attestation: Zhipeng Xu has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Anshi Wu, MD, PhD
Contribution: This author helped design the study
Attestation: Anshi Wu has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Yuanlin Dong, MD, MS
Contribution: This author helped conduct the study
Attestation: Yuanlin Dong has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Yiying Zhang, MD, MS
Contribution: This author helped design the study and write the manuscript
Attestation: Yiying Zhang has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Yun Yue, MD, MS
Contribution: This author helped design the study
Attestation: Yun Yue has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Zhongcong Xie, MD, PhD
Contribution: This author helped design the study and write the manuscript
Attestation: Zhongcong Xie has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Contributor Information
Hui Wang, Geriatric Anesthesia Research Unit, Department of Anesthesia, Critical Care and Pain Medicine; Massachusetts General Hospital and Harvard Medical School, Charlestown, Massachusetts.
Zhipeng Xu, Geriatric Anesthesia Research Unit, Department of Anesthesia, Critical Care and Pain Medicine; Massachusetts General Hospital and Harvard Medical School, Charlestown, Massachusetts.
Anshi Wu, Department of Anesthesia, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China.
Yuanlin Dong, Geriatric Anesthesia Research Unit, Department of Anesthesia, Critical Care and Pain Medicine; Massachusetts General Hospital and Harvard Medical School, Charlestown, Massachusetts.
Yiying Zhang, Geriatric Anesthesia Research Unit, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Charlestown, Massachusetts.
Yun Yue, Department of Anesthesia, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China.
Zhongcong Xie, Geriatric Anesthesia Research Unit, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Charlestown, Massachusetts.
References
- 1.Gomez RS, Guatimosim C. Mechanism of action of volatile anesthetics: involvement of intracellular calcium signaling. Current drug targets. CNS and neurological disorders. 2003;2:123–129. doi: 10.2174/1568007033482940. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey JA, Sedensky MM, Morgan PG. Understanding anesthesia: making genetic sense of the absence of senses. Human molecular genetics. 2002;11:1241–1249. doi: 10.1093/hmg/11.10.1241. [DOI] [PubMed] [Google Scholar]
- 3.Morgan PG, Sedensky M, Meneely PM. Multiple sites of action of volatile anesthetics in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2965–2969. doi: 10.1073/pnas.87.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 5.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. The New England journal of medicine. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayser EB, Morgan PG, Sedensky MM. GAS-1: a mitochondrial protein controls sensitivity to volatile anesthetics in the nematode Caenorhabditis elegans. Anesthesiology. 1999;90:545–554. doi: 10.1097/00000542-199902000-00031. [DOI] [PubMed] [Google Scholar]
- 7.Kayser EB, Morgan PG, Sedensky MM. Mitochondrial complex I function affects halothane sensitivity in Caenorhabditis elegans. Anesthesiology. 2004;101:365–372. doi: 10.1097/00000542-200408000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Morgan PG, Hoppel CL, Sedensky MM. Mitochondrial defects and anesthetic sensitivity. Anesthesiology. 2002;96:1268–1270. doi: 10.1097/00000542-200205000-00036. [DOI] [PubMed] [Google Scholar]
- 9.Kayser EB, Sedensky MM, Morgan PG. The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mechanisms of ageing and development. 2004;125:455–464. doi: 10.1016/j.mad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Quintana A, Morgan PG, Kruse SE, Palmiter RD, Sedensky MM. Altered anesthetic sensitivity of mice lacking Ndufs4, a subunit of mitochondrial complex I. PLoS One. 2012;7:e42904. doi: 10.1371/journal.pone.0042904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miro O, Barrientos A, Alonso JR, Casademont J, Jarreta D, Urbano-Marquez A, Cardellach F. Effects of general anaesthetic procedures on mitochondrial function of human skeletal muscle. European journal of clinical pharmacology. 1999;55:35–41. doi: 10.1007/s002280050589. [DOI] [PubMed] [Google Scholar]
- 12.Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. The Journal of physiology. 2002;544:687–693. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen PJ. Effect of anesthetics on mitochondrial function. Anesthesiology. 1973;39:153–164. doi: 10.1097/00000542-197308000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–4037. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism: clinical and experimental. 1962;11:1098–1112. [PubMed] [Google Scholar]
- 17.Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer chemotherapy and pharmacology. 2004;53:116–122. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Dong Y, Xu Z, Zhang Y, Pan C, McAuliffe S, Ichinose F, Yue Y, Liang W, Xie Z. 2-deoxy-D-glucose attenuates isoflurane-induced cytotoxicity in an in vitro cell culture model of H4 human neuroglioma cells. Anesth Analg. 2011;113:1468–1475. doi: 10.1213/ANE.0b013e31822e913c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. British journal of anaesthesia. 2013;110(Suppl 1):i82–i91. doi: 10.1093/bja/aet115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coles CJ, Edmondson DE, Singer TP. Inactivation of succinate dehydrogenase by 3-nitropropionate. The Journal of biological chemistry. 1979;254:5161–5167. [PubMed] [Google Scholar]
- 21.Kroger A, Klingenberg M. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. European journal of biochemistry / FEBS. 1973;34:358–368. doi: 10.1111/j.1432-1033.1973.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 22.Rauchova H, Drahota Z, Bergamini C, Fato R, Lenaz G. Modification of respiratory-chain enzyme activities in brown adipose tissue mitochondria by idebenone (hydroxydecyl-ubiquinone) Journal of bioenergetics and biomembranes. 2008;40:85–93. doi: 10.1007/s10863-008-9134-1. [DOI] [PubMed] [Google Scholar]
- 23.Erb M, Hoffmann-Enger B, Deppe H, Soeberdt M, Haefeli RH, Rummey C, Feurer A, Gueven N. Features of idebenone and related short-chain quinones that rescue ATP levels under conditions of impaired mitochondrial complex I. PLoS One. 2012;7:e36153. doi: 10.1371/journal.pone.0036153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeffer G, Horvath R, Klopstock T, Mootha VK, Suomalainen A, Koene S, Hirano M, Zeviani M, Bindoff LA, Yu-Wai-Man P, Hanna M, Carelli V, McFarland R, Majamaa K, Turnbull DM, Smeitink J, Chinnery PF. New treatments for mitochondrial disease-no time to drop our standards. Nat Rev Neurol. 2013;9:474–481. doi: 10.1038/nrneurol.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orsucci D, Mancuso M, Ienco EC, LoGerfo A, Siciliano G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr Med Chem. 2011;18:4053–4064. doi: 10.2174/092986711796957257. [DOI] [PubMed] [Google Scholar]
- 26.Yano H, Oyanagi E, Kato Y, Samejima Y, Sasaki J, Utsumi K. L-carnitine is essential to beta-oxidation of quarried fatty acid from mitochondrial membrane by PLA(2) Molecular and cellular biochemistry. 2010;342:95–100. doi: 10.1007/s11010-010-0472-z. [DOI] [PubMed] [Google Scholar]
- 27.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, Tanzi RE, Zhang Y, Xie Z. Peripheral surgical wounding and age-dependent neuroinflammation in mice. PLoS One. 2014;9:e96752. doi: 10.1371/journal.pone.0096752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohapatra R, Ramesh A, Jayaraman G, Santhiya ST, Gopinath PM. Modulatory action of 2-deoxy-D-glucose on mitomycin C-and 4-nitroquinoline-1-oxide-induced genotoxicity in Swiss albino mice in vivo. Journal of cancer research and therapeutics. 2009;5(Suppl 1):S53–S56. doi: 10.4103/0973-1482.55144. [DOI] [PubMed] [Google Scholar]
- 30.Lim S, Chesser AS, Grima JC, Rappold PM, Blum D, Przedborski S, Tieu K. D-beta-hydroxybutyrate is protective in mouse models of Huntington's disease. PLoS One. 2011;6:e24620. doi: 10.1371/journal.pone.0024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed MA. Neuroprotective Effects of Idebenone Against Pilocarpine-Induced Seizures: Modulation of Antioxidant Status, DNA Damage and Na(+), K (+)-ATPase Activity in Rat Hippocampus. Neurochemical research. 2014;39:394–402. doi: 10.1007/s11064-014-1236-z. [DOI] [PubMed] [Google Scholar]
- 32.Keung W, Ussher JR, Jaswal JS, Raubenheimer M, Lam VH, Wagg CS, Lopaschuk GD. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes. 2013;62:711–720. doi: 10.2337/db12-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngamsiri P, Watcharasit P, Satayavivad J. Glycogen synthase kinase-3 (GSK3) controls deoxyglucose-induced mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells. Mitochondrion. 2014;14:54–63. doi: 10.1016/j.mito.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 35.He MD, Xu SC, Lu YH, Li L, Zhong M, Zhang YW, Wang Y, Li M, Yang J, Zhang GB, Yu ZP, Zhou Z. L-carnitine protects against nickel-induced neurotoxicity by maintaining mitochondrial function in Neuro-2a cells. Toxicology and applied pharmacology. 2011;253:38–44. doi: 10.1016/j.taap.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Hirai K, Hayako H, Kato K, Miyamoto M. Idebenone protects hippocampal neurons against amyloid beta-peptide-induced neurotoxicity in rat primary cultures. Naunyn-Schmiedeberg's archives of pharmacology. 1998;358:582–585. doi: 10.1007/pl00005296. [DOI] [PubMed] [Google Scholar]
- 37.Xie Z, Dong Y, Maeda U, Moir R, Inouye SK, Culley DJ, Crosby G, Tanzi RE. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 38.Du F, Cooper A, Lukas SE, Cohen BM, Ongur D. Creatine kinase and ATP synthase reaction rates in human frontal lobe measured by (3)(1)P magnetization transfer spectroscopy at 4T. Magnetic resonance imaging. 2013;31:102–108. doi: 10.1016/j.mri.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banerjee B, Sharma U, Balasubramanian K, Kalaivani M, Kalra V, Jagannathan NR. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: a randomized, placebo-controlled 31P MRS study. Magnetic resonance imaging. 2010;28:698–707. doi: 10.1016/j.mri.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Lazeyras F, Buhler L, Vallee JP, Hergt M, Nastasi A, Ruttimann R, Morel P, Buchs JB. Detection of ATP by "in line" 31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs' kidneys. Magma. 2012;25:391–399. doi: 10.1007/s10334-012-0319-6. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 43.Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Critical reviews in eukaryotic gene expression. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 44.Kang HT, Hwang ES. 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life sciences. 2006;78:1392–1399. doi: 10.1016/j.lfs.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Haefeli RH, Erb M, Gemperli AC, Robay D, Courdier Fruh I, Anklin C, Dallmann R, Gueven N. NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels. PLoS One. 2011;6:e17963. doi: 10.1371/journal.pone.0017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcovina SM, Sirtori C, Peracino A, Gheorghiade M, Borum P, Remuzzi G, Ardehali H. Translating the basic knowledge of mitochondrial functions to metabolic therapy: role of L-carnitine. Translational research : the journal of laboratory and clinical medicine. 2013;161:73–84. doi: 10.1016/j.trsl.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 48.Humphrey JA, Sedensky MM, Morgan PG. Understanding anesthesia: making genetic sense of the absence of senses. Hum Mol Genet. 2002;11:1241–1249. doi: 10.1093/hmg/11.10.1241. [DOI] [PubMed] [Google Scholar]
- 49.Eger EI, 2nd, Liao M, Laster MJ, Won A, Popovich J, Raines DE, Solt K, Dutton RC, Cobos FV, 2nd, Sonner JM. Contrasting roles of the N-methyl-D-aspartate receptor in the production of immobilization by conventional and aromatic anesthetics. Anesth Analg. 2006;102:1397–1406. doi: 10.1213/01.ANE.0000219019.91281.51. [DOI] [PubMed] [Google Scholar]
- 50.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penicaud L, Thompson DA, Le Magnen J. Effects of 2-deoxy-D-glucose on food and water intake and body temperature in rats. Physiol Behav. 1986;36:431–435. doi: 10.1016/0031-9384(86)90310-0. [DOI] [PubMed] [Google Scholar]